Abstract
Polyamines (PAs) are ubiquitous polycations involved in many physiological processes in plants, including somatic embryogenesis, cellular growth and stress reactions. In the present study, we focus on the consequences in PA metabolism caused by polyethylene glycol (PEG) in proembryogenic Scots pine (Pinus sylvestris L.) liquid cultures. The growth and viability of the cell masses and changes in PA concentrations and phenolic secondary metabolites were investigated under control, 5 and 10 % PEG treatments. The effect of osmotic stress responses was investigated at the gene expression level including stress, cell division, programmed cell death and PA-related genes and PA metabolites. Moreover, the expression of ethylene and proline biosynthesis genes and phenylalanine ammonia lyase and stilbene synthase (psSTS) was analyzed. Under osmotic stress conditions, we found a consistent pattern of endogenous PAs in Scots pine proembryogenic cells. However, accumulation of free spermine (Spm) and methyl putrescine under osmotic stress might indicate their specific role in stress protection. Expression of polyamine oxidase was down-regulated under osmotic stress, suggesting the role of PA catabolism in regulation of Spm levels. Scots pine proliferating proembryogenic cells are in a developmentally undifferentiated stage where the content of secondary metabolites is generally low. However, in the present study the total content of phenolic compounds increased but the biosynthesis of phenylpropanoids and stilbenes, generally considered as stress-protective molecules, was not affected by osmotic stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic stress factors like inadequate water supplies, freezing temperature or salty environment lead to cellular dehydration in plants, which causes osmotic stress and removal of water from the cytoplasm. Dehydration is also an important part of the maturation process of coniferous embryos (Silveira et al. 2004). One of the main mechanisms of plants to tolerate osmotic stress is to accumulate primary and secondary metabolites, which lower the cellular osmotic potential and facilitate water absorption without interfering with the conformation of macromolecules such as proteins or DNA. Reactive oxygen species (ROS) are important molecules in cellular metabolism but stress conditions induce their overaccumulation leading to oxidative stress and cellular damages (Miller et al. 2010). Furthermore, osmotic stress has different impacts on cell cycle progression depending on the severity and the duration of the stress (Skirycz et al. 2011). In the present study, different stress-related metabolic processes were studied by using controllable liquid cultures of Scots pine proembryogenic cell cultures as a coniferous model system.
Elevated levels of PAs have been considered as one of the most remarkable metabolic changes in plants under abiotic stresses (Tiburcio et al. 2014), although the physiological role of the PAs is still under debate. PAs, putrescine (Put), spermidine (Spd) and spermine (Spm) are small, positively charged nitrogenous compounds implicated in pathways regulating cell division, programmed cell death (Moschou and Roubelakis-Angelakis 2013) as well as embryogenesis of pines (Minocha et al. 1999; Vuosku et al. 2006). PA levels are strictly regulated by biosynthesis, degradation, conjugation, back-conversion and transport and by interactions with other pathways in response to stress (Tiburcio et al. 2014). Biosynthesis of the plant hormone ethylene and the amino acid proline have the precursors with PA metabolism and they all are connected to abiotic stress protection in plants (Moschou et al. 2012) (see the figure Online Resource 1). Suppression of proline-synthesizing gene pyrroline-5-carboxylate synthase (P5CS) expression enhanced the sensitivity to salinity stress of tobacco (Nicotiana tabacum L.) (Hong et al. 2000) and was overexpressed in salt tolerant embryogenic cells of Medicago truncatula (Elmaghrabi et al. 2013). Moreover, ethylene is a plant hormone which not only regulates developmental programmed cell death and senescence but also arrests the cell cycle under osmotic stress (Skirycz et al. 2011). Expression of 1-amino-cyclopropane-1-carboxylic acid (ACC) synthase (ACCS) gene is the limiting step of ethylene biosynthesis in plant tissues (Shen et al. 2014) and has been suggested to serve as a potential marker for early stages of maturation of Scots pine somatic embryos (Lu et al. 2011). Transcription of ACC oxidase (ACO) increased under osmotic conditions in Arabidopsis leaves and was linked to ethylene-mediated cell cycle arrestment (Skirycz et al. 2011).
Often in plants under stress conditions, PA synthesis is observed to be activated and then followed by stimulation of PA oxidation, where an important signalling molecule, hydrogen peroxide (H2O2), is released (Cona et al. 2006; Moschu et al. 2012). Depending on the stress magnitude, H2O2 released in PA catabolism may act as a signalling molecule leading to expression of defense genes or to hypersensitive response and programmed cell death (Moschou et al. 2008). Catalase (CAT) is a peroxisomal enzyme and protects the cells from the toxic effects of H2O2 by converting it into water and oxygen (Mhamdi et al. 2012). Gene expression of CAT reflects the H2O2 contents in the plant cells (Luna et al. 2004). Expression of CAT and retinoblastoma-related (RBR) genes has recently been associated with cell death processes in Scots pine zygotic embryogenesis (Vuosku et al. 2015). Phosphorylation of RBR proteins regulates cell cycle by preventing transition from G1 phase to DNA-replicating S phase (Desvoyes et al. 2014). RBR proteins bind to transcription factor E2F which leads to at least partial arrest of the cell cycle in plant cells (Desvoyes et al. 2014). Osmotic stress seems to increase expression of RBR specifically in nucellar layers of Scots pine seeds (Vuosku et al. 2015).
Furthermore, PA conjugates with phenolic acids have been involved in stress adaptation (Bassard et al. 2010). Phenolic acids are components of cell wall structures and provide a significant contribution to the antioxidant activity of plant tissues (Boudet 2007). Recently, transcriptome profiling of Arabidopsis revealed that ethylene signalling and cell wall remodelling via phenyl propanoid pathway have important roles in acclimation to salt stress (Shen et al. 2014). Generally, Scots pine tissues are rich in phenolic compounds (Häggman et al. 2009) including lignins, flavonoids, and stilbenes. In proembryogenic cells (i.e., the cells not yet induced to maturation), the content of secondary metabolites is generally low (Vuosku et al. 2012). However, osmotic stress has been found to induce secondary metabolism also in the proembryogenic cell cultures (Cvikrová et al. 2008; Hatmi et al. 2014) and can be related to, i.e., cell wall stiffening (Boudet 2007). Phenylalanine ammonia lyase (PAL) links primary and secondary metabolism by catalyzing the conversion of l-phenylalanine to cinnamic acid, the initial substrate of phenylpropanoid metabolism (Butland et al. 1998). Further, the phenylalanine is transformed into a variety of phenol molecules, including lignins, lignans, benzoic and hydroxycinnamic acids, flavonoids, and stilbenes (Boudet 2007). The content of hydroxycinnamic acid was decreased by PAL inhibitor in alfalfa (Medicago sativa) suspension cultures, which influenced the balance of free and conjugated PA contents and stimulated cell division activity (Cvikrová et al. 1999). Moreover, in grape vine (Vitis vinifera L.) subjected to osmotic stress, PA homeostasis was recently linked with parallel accumulation of main stilbenes and up-regulation of gene transcripts including stilbene synthases (STS) (Hatmi et al. 2014). psSTS of Scots pine has been characterized as a dihydropinosylvinsynthase (Fliegmann et al. 1992), and psSTS catalyzes biosynthesis of the stilbene backbone from malnoyl-CoA and one CoA-ester of cinnamic acid derivative (Chong et al. 2009) (see the figure Online Resource 2).
Somatic embryogenesis has been described in many coniferous species (Klimazewska et al. 2009) including Scots pine (Häggman et al. 2009; Aronen et al. 2009). The coniferous embryogenic cell mass is proliferated during the proembryogenic phase consisting of elongated suspensor cells with large vacuoles and more condensed embryonal cells (Silveira et al. 2006). Polyethylene glycol (PEG), a polymer, increases osmotic potential of the solution and prevents water uptake of the cells (Michel and Kaufmann 1973). Due to the property of PEG to dehydrate tissues, it is used in somatic embryogenesis to mimic maturation-phase dehydration of the seeds (Stasolla et al. 2003), and it has been included in most of the somatic embryogenesis protocols developed for coniferous species including pine species (reviewed by Häggman et al. 2006). However, little is known about the biochemical and morphological events taking place when the proliferating coniferous proembryogenic cells are exposed to osmotic stress.
In our previous study, we investigated PA metabolism during the transition of Scots pine embryogenic cell mass to the exponential growth phase in a controllable SENBIT® wireless system (teleBITcomGmbH, Teltow, Germany) (Vuosku et al. 2012). In the present study, this system was used to induce osmotic stress with three different PEG (6000) treatments (control, 5 % and 10 % PEG) on pine proembryogenic cells. We hypothesized that PA homeostasis plays a specific role in stress protection and developmental processes in Scots pine. To evaluate the role of PAs in proliferating proembryogenic cell masses subjected to osmotic stress, the transcription and metabolite level regulation of PAs were compared with phenolic biosynthesis, expression of ethylene and amino acid proline-synthesizing genes as well as with stress-, cell death- and cell division-related genes. Moreover, the expression of genes related to the phenyl propanoid pathway and stilbene synthesis was investigated.
Materials and methods
Embryogenic cell line
One-year-old immature seed cones were collected from open-pollinated elite Scots pine (Pinus sylvestris L.) clone K884 in Punkaharju, Finland (61°48′N; 29°17′E) during one growing season as described in detail in Vuosku et al. (2009). The establishment and cultivation of an embryogenic cell line derived from immature embryos has been described in our previous study (Vuosku et al. 2012).
Suspension of embryogenic cultures
Proembryogenic cells, at proliferative phase, were cultured for 2 weeks on solid Douglas-fir cotyledon revised medium (DCR) (Gupta and Durzan 1985) on ten different Petri dishes before transferring them into liquid DCR medium. The pH value of the culture medium was adjusted to 5.8 before autoclaving. In the beginning, 3 g of embryogenic cell mass was mixed with 50 ml of DCR medium and transferred into 35 Erlenmeyer bottles (250 ml) that were sealed with silicone sponge closures (Sigma-Aldrich Co., St. Louis, USA). DCR media contained plant hormones cytokinin (BAP) (0, 5 mg/l) and auxin (2,4-D) (0, 5 mg/l) to maintain the proliferation growth. The suspension cultures were made in Erlenmeyer-type shake flasks with three side necks for the sensors and sampling (Glasgerätebau Ochs GmbH, Bovenden, Germany). Prior to the experiments, the pH electrodes (autoclavable EGA 186-L Meinsberger Elektroden, Germany) were calibrated with standard pH solutions 4 and 7, after which the sensors were autoclaved (121 °C, 20 min). The SENBIT® receiver unit was set to monitor the cultures every 90 s. The data management was made with SENBIT® Control software. The proembryogenic cell suspensions were cultured at room temperature in the dark on two different shaking tables (IKA KS 260 control, IKA-WERKE GMBH & CO. KG and Heidolph Unimax 2010, Heidolph Instruments GmbH & Co. KG) with the speed of 90–110 rpm. After 6 days culturing, 10 ml of DCR medium was added to the bottles to improve the growth of the cell masses. Cell cultures were grown for seven additional days to reach the exponential growth phase.
PEG treatments
Our previous study showed that 14 days of cultivation in liquid culture induces exponential growth of the cells (Vuosku et al. 2012). In the present study, continuous measurement of pH with the SENBIT® wireless system (teleBITcomGmbH, Teltow, Germany) (see the figure Online Resource 3) and preliminary cell mass growth observations were used to assess the favorable time point for the osmotic stress treatments. The cells were adapted for 13 days to the liquid environment before the osmotic stress was induced by replacing 20 ml of the medium in the bottles with a combination of DCR and PEG (with molecular weight 6000) solution to reach 5 % (w/v) or 10 % (w/v) PEG (6000) content. In the control treatment, only liquid DCR medium without PEG (6000) was used. PEG content of the medium was increased stepwise during 3 days to allow the cell masses to adapt to the osmotic environment. The final PEG level was 5 % (ca. −0.5 bar) in 12 bottles, 10 % (ca. −1.5 bar) in 12 bottles, and 11 bottles were used as a control treatment without PEG (i.e., PEG 0 % treatment). The osmotic potential of PEG (6000) was selected according to Michael and Kaufmann (1973).
Harvesting
The samples were harvested one, four and 7 days after the PEG levels (control = no PEG, 5, 10 %) were achieved in the bottles. On each sampling day, four independent samples (bottles) from each treatment were taken, except in the control treatment in which three bottles were sampled on harvesting day one. Total fresh weight of the cell mass per bottle was measured after 10 s vacuum filtering. From the filtered cell mass, 100 mg samples for RNA and PA measurements, 1 g sample for analysis of phenolic compounds and two 200 mg samples for viability analysis were taken.
pH, conductivity and nitrogen sources
After harvesting, the pH values and the conductivity of the culture medium were determined from the filtrates by using a pH meter (Philips PW9422) and YSI 3200 conductivity instrument (YSI, 1700/1725 Brannum Lane, Yellow Springs OH45387 USA) on every sampling date. Also, the concentrations of total nitrogen, ammonium (NH4+) and nitrate (NO3−) were analyzed by flow injection analysis (FIA Star 5020, Tecator, Hillerød, Denmark) from the filtrated medium.
Cell mass growth and viability
The fresh weight of the cell masses in the bottles was weighed straight after removing extra water by 10 s light vacuum filtering. The water content of the cells was approximately 90 %. PEG itself increased the dry weight of the cell masses and therefore the fresh weight values were used for the calculations. A small amount of embryogenic cells was fixed with 1 ml of 4 % paraformaldehyde fixative for microscopic observation. The cell mass was observed under the microscope to see if any osmotic stress-induced morphological changes or cellular aggregation had occurred.
The viability of embryogenic cells was determined by means of the commonly used biochemical marker 2,3,5-triphenyl tetrazolium chloride (TTC) staining (Towill and Mazur 1975), which is based on the reduction of tetrazolium salts to red-colored end products in viable cells. TTC is reduced by mitochondrial dehydrogenases and red color implies mitochondrial activity of the cells (Zapata et al. 1991). The test was done according to Mikula et al. (2006) with slight modifications. Two 0.2 g cell samples from every bottle were collected and washed with 1.5 ml sterilized water for 1 h. Thereafter, cells were centrifuged at 3000g for 5 min and the water was replaced with 1.5 ml of TTC solution (0.6 % TTC in Tris buffer, pH 7.5). Cells were incubated for 24 h at 30 °C in the dark and then washed with distilled water to remove TTC. Red-colored formazan was released from cells by incubating them at 85 °C in 100 % ethanol for 15 min. Cells were spun down and absorbance was measured by a spectrophotometer at 485 nm wavelength (Jenway Genova MK2 Life Science Analyser, Dunmow, Essex, UK).
PA analyses
For the PA analyses, 100 mg fresh weight from the somatic proembryogenic cultures was extracted in 5 % (w/v) perchloric acid (HClO4). PA dansyl derivatives were analyzed with high-performance liquid chromatography (HPLC) (Merck-Hitachi, Darmstadt, Germany) as described in Fornalé et al. (1999). Spm and thermospermine (Tspm) are isomers and in the present study they were included in the same fraction.
RNA isolation and reverse transcriptase polymerase chain reaction
Total RNA was extracted for the gene expression studies from the proembryogenic cell masses (100 mg) using the total RNA purification PureLink™ Plant RNA Reagent (Invitrogen Corporation, California, USA) according to the manufacturer’s instructions. The RNA samples were treated with rDNase set (Magherey-Nagel, Duren, Germany) at room temperature for 10 min to eliminate contaminating genomic DNA. The amount of DNase needed for the DNA-free RNA samples was three times higher than recommended in manufacturer’s instructions, after which the RNA samples were purified with the NucleoSpin® RNA Clean-Up kit (Macherey-Nagel, Duren, Germany). The RNA yields were measured three times with OD260 analysis (Jenway Genova MK2 Life Science Analyser, Dunmow, Essex, UK), and 1 μg of each RNA sample was used for the gene expression analyses. cDNA was reverse-transcribed by SuperScript VILO™ cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA).
Quantitative real-time reverse transcription-PCR analyses
The quantification of the (1) PA metabolism-related enzyme genes: arginine decarboxylase (ADC), spermidine synthase (SPDS), thermospermine synthase (ACL5), S-adenosylmethionine decarboxylase (SAMDC), diamine oxidase (DAO) and polyamine oxidase (PAO); (2) phenylpropanoid pathway genes: phenylalanine ammonia lyase (PAL) and pinosylvin synthase (psSTS); (3) proline synthesis gene; pyrroline-5-carboxylate synthase (P5CS); (4) ethylene synthesizing genes: 1-amino-cyclopropane-1-carboxylic acid (ACC) synthase (ACCS) and ACC oxidase (ACO); (5) stress-related genes: late-embryogenesis abundant protein gene (LEA), catalase (CAT) and S-adenosylmethionine synthase (SAMS); (6) cell division-related genes: retinoblastoma protein gene (RBR), transcriptionfactor (E2F); and (7) cell death-related (TAT-D) mRNA transcription analyses were done with the relative reverse transcription-PCR method. The PCR primers are presented in the table Online Resource 4.
The amplification conditions of the gene fragments were optimized for the LightCycler® 2.0 instrument (Roche Diagnostics, Espoo, Finland), and the subsequent PCR runs showed a single PCR product during the melting curve and electrophoretic analysis. The real-time PCR amplifications were performed using the FastStart DNA Master SYBR Green I real-time PCR kit (Roche Molecular Biochemicals, Mannheim, Germany), 50 nM gene-specific primers and 2 μl cDNA (1:10 dilution) in a reaction volume of 20 μl. The real-time PCR amplification was initiated by incubation at 95 °C for 10 min followed by 40 cycles: 10 s at 95 °C, 10 s at 58 °C and 5 s at 72 °C.
Phenolic compounds
For the analysis of secondary metabolites, 1 g of filtrated proembryogenic cell mass was ground in 6 ml of 100 % methanol, transferred into a test tube and mixed up by vortex. After a15-min incubation on ice, the sample was centrifuged 10 min with 10,400×g (Eppendorf Centrifuge 5804 R). Supernatant was filtered with 0.2 µm filter (Syringe Filter, 25 mm, Nylon Membrane) and divided into 2 ml portions into Eppendorf tubes. The samples were dried with vacuum centrifuge ca. 5 h in +45 °C. The pellets were stored in −80 °C.
Total phenol contents were analyzed by Folin–Ciocalteu procedure (Singleton et al. 1999) modified for use on 96-well microplates (BD Labware, Franklin Lakes, NJ. USA), as described in Viitala et al. (2011). Gallic acid (Sigma-Aldrich) dilutions of 500, 250, 100 and 25 mg/l in deionized water were prepared for standard solutions from a stock solution made by dissolving 500 mg gallic acid in 10 ml of ethanol and diluting with deionized water to 100 ml. From standards and samples, 1:50 dilutions were used for the measurements. The Folin–Ciocalteu assay is also described as an antioxidant capacity method due to its ability to react with phenols but also with any reducing substances, like some vitamins, thiols, glyceraldehyde, inorganic ions, nitrogen-containing molecules, etc. (Everette et al. 2010). Thus, Folin–Ciocalteu reagent gives a rough estimation of the phenolic content and reducing capacity of the samples.
For HPLC analysis, the samples were dissolved to 500 µl of 50 % methanol and filtered with 0.2 µl filter. HPLC analysis was performed using Shimadzu Prominence Liquid Chromatograph system including LC-20AP pumps, UFLC Prominence Communication Bus Module CBM-20A and Prominence Photo diode array SPD-M20A Detector (Shimadzu USA Manufacturing Inc.) and Waters XBridge C18 5 µm 4.6 × 150 mm column (Waters Corporation USA). The wavelengths of the detector were set at 270 and 320 nm. The stepwise HPLC mobile phase conditions were as follows: initial methanol content 2 % increased to 20 % in 10 min, increased to 30 % in 20 min, increased to 45 % in 33 min, increased to 95 % in 35 min and then was held at 95 % for 10 min; injection volume was 25 µl. Phenylalanine (Fluka P1150000), tryptophan (Fluka PHR1176), cinnamic acid (Fluka 97013) and pinosylvin (Arbonova) were used as external standards to quantify the concentrations of compounds in pine cell cultures.
Statistical analyses
The effects of PEG treatments and incubation time, and their interactions on polyamine concentrations and gene expressions were analyzed using linear mixed models (Fitzmaurice et al. 2004). Individual models were estimated for free and soluble conjugated fractions of Put, Spd and Spm. PA levels were log-transformed before the models were estimated. Models were estimated also for the relative expressions of ADC, SPDS, ACL5, DAO, PAO, SAMDC, PAL, psSTS, P5CS, ACCS, ACO, SAMS, LEA, CAT, E2F, RBR and TAT-D,. Expression data of genes were log-transformed when needed to achieve normality.
Both explanatory variables, i.e., treatment (PEG 0, 5 and 10 %) and time (1, 4 and 7 days), were included as factors in the models. At the beginning of the liquid culture, cell mass was taken (and shared) from one Petri dish to more than one bottle, consequently these observations are correlated. Thus, a random factor describing the effect of a Petri dish was added to the models to take this correlation into account. The models were estimated using function lme in package nlme (Pinheiro et al. 2012; R Development Core Team 2012). The estimated regression coefficients and their 95 % confidence intervals (CI) were used to indicate statistical significance. The CI 95 % values not including zero can generally be interpreted as “statistically significant” at significance level 0.05.
Results
Effect of osmotic stress on the viability and growth of the cell mass
The continuous pH measurement in SENBIT® system (see the figure Online Resource 3) indicated a lag phase in cell mass growth after insertion of media, where the pH value increased temporarily. This was also seen in the fresh weight values after PEG treatments (Fig. 1). PEG increased the pH value of the media but did not change the decreasing trend of pH in time function. During the first 4 days after the beginning of the PEG treatments, the cell masses did not grow in the bottles although its viability increased (Fig. 1; Table 1). From day four onwards, the FW of the cell masses increased in the control and 5 % PEG treatments, and decreased in the 10 % PEG treatment. Simultaneously, the viability decreased in the 5 and 10 % PEG-treated bottles and increased in the control ones. Proembryogenic cells treated with 10 % PEG were morphologically not as round-shaped as under other treatments, potentially revealing that osmotic stress caused cellular shrinkage (see the figure Online Resource 5). The content of both nitrogen sources (NO3 − and NH4 +) increased in 10 % PEG filtrate from day four onwards, which also partly increased the pH of the media (see the figures Online Resource 6 and 7) and indicated lower consumption of the nutrients in the cell cultures under osmotic stress. The PEG content itself had an increasing effect on the conductivity and pH of the filtrated media (see the figure Online Resource 7).
Fresh weight and viability of Scots pine embryogenic cells in liquid cultures. Arithmetic mean values of control (dark grey bars), 5 % PEG (light grey bars) and 10 % PEG (open bars) treatments and their 95 % confidence intervals (CI) at incubation time (1, 4, 7 days) are presented. See Table 1
PA metabolism in the Scots pine embryogenic cell cultures under osmotic stress
Osmotic stress had no effect on free and soluble conjugated Put and Spd levels in Scots pine proembryogenic cell cultures (Table 2; Fig. 2). However, the concentration of free Spm increased in the cells under the 10 % PEG treatment, being approximately threefold higher than in the control ones at the end of the experiment. Thus, the ratio of free and conjugated Spm also increased under osmotic stress. A putative methyl putrescine was observed in 5 and 10 % PEG-treated samples but in the control samples in day seven only (Fig. 3).
Free and soluble conjugated polyamine contents of Scots pine embryogenic cells in liquid cultures. Arithmetic mean values of control (dark grey bars), 5 % PEG (light grey bars) and 10 % PEG (open bars) treatments and their 95 % CI at incubation time (1, 4, 7 days) are presented. See Table 2
Expression of ADC slightly decreased towards the end of the experiment under all treatments, whereas expression of SPDS and DAO remained stable throughout the experiment (Table 3; Fig. 4) being in accordance with the stable Put and Spd levels. Furthermore, from day one to four, the expression levels of ACL5 and SAMDC had an increasing trend in control, but decreasing trend in the 10 % PEG treatment (Table 3; Fig. 4), indicating down-regulation of Tspm biosynthesis under osmotic stress. Expression of PAO increased both in the control and 5 % PEG samples (but not significantly), but decreased in the 10 % PEG treatment from day four onwards (Table 3; Fig. 4), potentially affecting the observed increase in free Spm content.
Relative gene expression of PA biosynthesizing (ADC, SPDS, ACL5, SAMDC) and catabolizing genes (DAO, PAO) in Scots pine embryogenic cells in liquid cultures. Arithmetic mean values of control (dark grey bars), 5 % PEG (light grey bars) and 10 % PEG (open bars) treatments and their 95 % CI at incubation time (1, 4, 7 days) are presented. See Table 3
Effects of osmotic stress on phenolic content and expression of PAL and psSTS genes
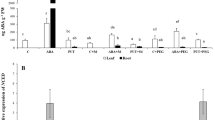
Total phenol content was higher in cells grown under 10 % PEG than in the control treatment (Table 4). Already a one-day-long 10 % PEG treatment increased the total phenol content (detected by Folin–Ciocalteu test) in the cells and the difference between the treatments remained stable throughout the experiment. Furthermore, phenylalanine and cinnamic acid contents were determined and expression of PAL gene analyzed to investigate whether the osmotic stress induces phenyl propanoid production in cell cultures. Phenylalanine content was lower in the 5 % PEG than in the control treatment in days four and seven (Table 4; Fig. 5). Cinnamic acid content also increased towards the end of the experiment under the control treatment but no clear trend was found for PEG 5 and PEG 10 % treatments (Table 4; Fig. 5). Transcription-level investigations showed that expression of PAL had an upward trend in the control treatment and downward trend in the 5 % PEG treatment between days one and seven, being stable in the 10 % PEG treatment (Table 3; Fig. 6). At the beginning of the experiment, the amount of pinosylvin was higher under the control than in other treatments (Table 4; Fig. 5). The expression of pinosylvin-synthesizing gene psSTS had a downward trend in all treatments towards the end of the experiment, being steepest in the 5 % PEG treatment between days one and four (Table 3; Fig. 6). Amino acid tryptophan had decreasing and increasing trends under the control and 10 % PEG treatments, respectively, between days one and seven, being at a higher level in stressed than non-stressed samples at the end of the experiment (Table 4; Fig. 5).
Contents of phenylalanine, tryptophan, cinnamic acid and pinosylvin in Scots pine embryogenic cells in liquid cultures. Arithmetic mean values of control (dark grey bars), 5 % PEG (light grey bars) and 10 % PEG (open bars) treatments and their 95 % CI at incubation time (1, 4, 7 days) are presented. See Table 4
Relative expression of genes related to proline synthesis (P5CS), phenolic biosynthesis (PAL, psSTS), cell division (E2F, RBR) and programmed cell death (TAT-D) in Scots pine embryogenic cells in liquid cultures in SENBIT system. Arithmetic mean values of control (dark grey bars), 5 % PEG (light grey bars) and 10 % PEG (open bars) treatments and their 95 % CI at incubation time (1, 4, 7 days) are presented. See Table 3
Gene expression related to stress (LEA, CAT, SAMS), proline (P5CS), ethylene synthesis (ACO and ACCS), cell division (RBR, E2F), and cell death (TAT-D)
LEA, CAT and SAMS were used to indicate stress responses at the transcription level. However, proembryogenic cells did not show any severe stress responses to be recognized as significant expression changes of these genes (Table 3). The expression of LEA gene had a slightly decreasing trend towards the end of the experiment under all treatments (Table 3), whereas the decrease in the expression of CAT was stronger in the 10 % PEG compared to the control treatment between days one and four (Table 3). The expression of proline-synthesizing gene P5CS had a decreasing trend towards the end of the experiment under the control treatment, while slightly increasing under the 10 % PEG treatment (Table 3; Fig. 6). The expression of ethylene-producing ACCS gene decreased more strongly under the 5 % PEG than control treatment between days one and four. Moreover, ACO had a peak on day four in the control treatment, being lower in the 10 % PEG than control treatment at the end of the experiment (Table 3).
The gene expression of E2F had a downward and RBR an upward trend towards the end of the experiment under all treatments (Table 3; Fig. 6). The expression of RBR increased especially in the 10 % PEG treatment at the end of the experiment, being clearly higher in two out of four 10 % PEG-treated samples (Fig. 6). However, the PEG treatments had no significant effect on the cell division-related RBR and E2F genes. Furthermore, the expression of programmed cell death-related TAT-D gene remained stable during the experiment under all treatments, but similarly the variation increased in the 10 % PEG treatment at the end of the experiment, being higher in two out of four bottles (Table 3; Fig. 6).
Discussion
Usually, the growth kinetics of suspension cells follows an exponential curve, consisting of an initial lag phase where the cells adapt to the liquid environment, an exponential phase with cell growth and divisions, and finally a stationary phase (Astarita and Guerra 2000). Inactivation processes during growth can cause intermediate lag phases (Swinnen et al. 2004). Also in our study, insertion of new media into the bottles caused a lag phase in cell mass growth which could be seen as constant fresh weight values between days one and four under all treatments. Compared to the control and 5 % PEG treatments, the 10 % PEG treatment decreased cell mass growth. Both nitrogen sources NO3 − and NH4 + were used during cell mass growth. However, NO3 − and NH4 + content increased in the media under the 10 % PEG treatment, hence indicating lower nutrient consumption under osmotic stress conditions and maybe leaching of nutrients from the dying cells. In our previous study, a negative correlation between pH and the FWs of the embryogenic cell masses was evident (Vuosku et al. 2012). Accordingly, in the present study, the pH value was higher in the filtrate of 10 % PEG than in others partly due to the lower growth rate of the cell masses. Thus, osmotic stress restrained cell mass proliferation in Scots pine proembryogenic cell cultures.
E2F is a transcription factor regulated by RBR proteins, which are negative regulators of cell division (Desvoyes et al. 2014). In Scots pine, the RBR/E2F pathway is functioning in embryogenic cells during proliferation growth (Vuosku et al. 2012). In the present study, expression of RBR had a general increasing and E2F a decreasing trend in all treatments. However, the variance of RBR gene expression between the bottles increased markedly in the 10 % PEG treatment, which induced an increase in RBR gene in some of the bottles. Although the RBR protein is known to be abundant during all phases of the cell cycle and exhibits only slight variation at the gene expression level (Miskolczi et al. 2007), intense RBR expression was found in dying cells in nucellar layers of Scots pine seeds (Vuosku et al. 2015). In the present study, the increased variance of RBR gene expression might indicate inhibitory regulation of cell division under the 10 % PEG treatment in some bottles. Oxidative stress possibly leads to cell cycle arrest and prevents the replication of damaged DNA by up-regulation of RBR expression (Vuosku et al. 2015). Moreover, TAT-D gene had a similar response as RBR gene to the PEG treatment by showing increased variance between the bottles under the 10 % PEG treatment. The increase in TAT-D gene expression might indicate PEG-induced apoptotic DNA degradation and cell death (Qiu et al. 2005).
In our previous study, we have shown that severe drought stress increases expression of LEA gene in root, needle and stem tissues of Scots pine seedlings (Muilu-Mäkelä et al. 2015). LEA genes are expressed also in somatic coniferous embryos at the maturation phase (Stasolla et al. 2003). Furthermore, expression of CAT is induced under drought stress in loblolly pine (Lorenz et al. 2005) and Scots pine roots (Muilu-Mäkelä et al. 2015). S-adenosyl methionine (SAM) is a common precursor of ethylene and PA biosynthesis (Moschou et al. 2012), and SAM synthase (SAMS) is shown to be up-regulated under drought stress in pine tissues (Lorenz et al. 2005). Still, in the present study, the stress-related genes LEA, CAT and SAMS were not up-regulated under PEG-induced stress, suggesting that the proembryogenic cells of pine are tolerant to osmotic stress. Furthermore, the transcription-level regulation of ethylene synthesis was not induced under the treatments. Expression of SAMS remained stable but expression of SAMDC, ACCS and ACO decreased or remained stable in the cells under osmotic stress, therefore suggesting neither antagonism nor up-regulation of ethylene and PA biosynthesis in the cell cultures under osmotic conditions. In Arabidopsis, ethylene biosynthesis was down-regulated after acclimation to salt stress (Shen et al. 2014), which is also possibly the case in the present study. Accordingly, the expression of proline-synthesizing gene P5CS indicated slight up-regulation under the PEG treatment in Scots pine proembryogenic cells, and this was also the case in salt-acclimated Arabidopsis with an increased transcription level of P5CS1 (Shen et al. 2014), and in salt resistant cells of Medicago truncatula (Elmaghrabi et al. 2013) where P5CS expression was significantly higher at the point of Na+/K+ homeostasis.
Decrease in PA catabolism has recently been shown to be involved in PA homeostasis under stress conditions in Arabidopsis (Wi et al. 2014). This was also confirmed in our previous study with Scots pine seedlings under drought stress (Muilu-Mäkelä et al. 2015) and likewise the results of the present study support this finding. The transcription and metabolite-level investigation suggests that PA metabolism is maintained at a constant level under osmotic stress conditions in Scots pine cells by decreasing expression of catabolizing enzyme genes rather than activating PA biosynthesis. Accordingly, in tobacco normal PA biosynthesis was restored in a background with redistributed protein synthesis due to stress conditions (Moschou et al. 2008). If PAs are synthesized more than they are catabolized, programmed cell death does not occur. Thus, an increase in the PA anabolism-to-catabolism ratio is necessary for programmed cell death inhibition/defense as a response to abiotic stress (Moschou et al. 2008). H2O2, which is released in PA catabolism, has been shown to activate antioxidant enzymes like CAT (Moschou et al. 2012) also at the transcription level (Luna et al. 2004). H2O2 behaves as an important signaling molecule being involved in growth and developmental processes in plants (Cona et al. 2006), but overly high contents are eliminated by the CAT activity. In the present study, potentially, the stable PA levels and thus stable anabolism-to-catabolism ratio indicate that the H2O2 level might not be sufficient to induce expression of CAT. In addition, in zygotic embryogenesis of Scots pine, CAT seems to be involved in active metabolism rather than in dehydration protection during the maturation drying (Vuosku et al. 2015).
We have previously shown that during the transition to the exponential growth phase, the accumulation of Put seemed to be a consequence of the decrease in the biosynthesis of Spd and Spm, and possibly the Spd was converted back into Put for proliferative growth (Vuosku et al. 2012). The predicted Scots pine PAO protein shows 42 % identity with Arabidopsis AtPAO1 (Vuosku 2011), which is reported to be involved in a PA back-conversion pathway (Tavladoraki et al. 2006). In the present study, the expression of PAO slightly increased, but not significantly, towards the end of the experiment in proliferating cells under the control treatment. This is in accordance with the idea (Vuosku et al. 2012) that Spd may be converted back into Put during the transition to exponential growth. However, this was not clearly seen from the PA contents in the present study.
Osmotic stress induced accumulation of free Spm (and potentially also Tspm) in the cells. Expression of SAMDC and ACL5 decreased in four and catabolizing gene PAO in 7 days under the 10 % PEG treatment. Thus, the increase in Spm/Tspm levels under osmotic stress is at least partly regulated by down-regulation of PAO transcription. The possible decrease in available aminopropyl groups due to decreased transcription of SAMDC could explain the decreased expression levels of ACL5 as well. However, the decrease in SAMDC transcription was not accompanied by lower expression levels of SPDS. Spd and Spm accumulation induced by SAMDC over-expression has been shown to result in drought stress tolerance in Arabidopsis (Wi et al. 2014). Moreover, exogenous Spm reduced cellular growth and affected the physiology of Brazilian pine (Araucaria angustifolia) embryogenic cultures, which was associated with a maturation process via reduction in activities of proton pumps (Dutra et al. 2013). Similarly, in the present study, Spm/Tspm accumulated in bottles, where cell proliferation was arrested due to osmotic stress, suggesting the possible role of Spm in inhibition of cell mass growth. Transgenic tobacco plants with decreased PAO activity and increased Spd and Spm titers were more tolerant to salinity stress than genotypes with increased PAO activity (Moschou et al. 2008b). PAs have been shown to modulate the activity of a certain set of ion channels to adapt ionic fluxes in response to environmental changes (Pottosin and Shabala 2014). Spm with three positive charges in its molecule has been viewed as the most effective PA in regulating ion channels (Yamaguchi et al. 2007).
Furthermore, putative methyl-Put was present in the tissues under the 5 and 10 % PEG treatments but not in the control treatment, indicating that osmotic stress induced methyl-Put accumulation in Scots pine proembryogenic cells. The methyl moiety of SAM is transferred to Put by methyl transferase, forming N-methyl-Put. In the Solanacea family, the methyl-Put is a limiting step of biosynthesis of tropane alkaloids which accumulates under osmotic stress (Godoy-Hernández et al. 2000). However, the role of methyl-Put in pines under stress conditions remains to be evaluated. The results here suggest that under osmotic conditions the methyl group may be transferred from SAM to Put rather than to the synthesis of higher PAs or ethylene via SAMDC or ACCS, respectively.
Conjugation of PAs with phenolic acids reduces polarity and hydrophilicity of PAs thus favoring their translocation and stability (Bassard et al. 2010). Furthermore, the ratio of free and conjugated PAs has been shown to be affected by the content of phenolics in suspension cultures of alfalfa (Cvikrová et al. 1999) and in somatic embryogenesis of oaks (Quercus sp.) (Cvikrová et al. 2003) where hydroxycinnamic acid content and mitotic activation were negatively correlated. In the present study, the 10 % PEG treatment increased the total phenol content in the cells. However, the upward trend in expression of PAL gene as well as increasing phenylalanine and cinnamic acid contents towards the end of the experiment in the control treatment suggest the role of phenyl propanoid pathway in cell proliferation rather than in stress protection. The increase in total phenolic compounds under osmotic stress suggests accumulation of compounds such as condensed and hydrolysable tannins (Romani et al. 2006), which are not synthesized from phenylpropanoid precursors. Furthermore, Folin–Ciocalteu reagent does not only measure phenols, but reacts with any reducing substances thus actually indicating the total reducing capacity of the samples (Everette et al. 2010). Hence, the osmotic stress under the 10 % PEG treatment might induce antioxidant capacity of the proembryogenic cells. Moreover, the ratio of free or conjugated Put and Spd was not affected by the PEG treatments but the ratio of free and conjugated Spm increased under osmotic stress.
Osmotic stress did not activate defense reactions via the phenylpropanoid pathway in Scots pine proembryogenic cell cultures. In the present study, the embryogenic cell cultures were at a proliferative phase induced by cytokinin and auxin, i.e., not induced to further embryo development, therefore the secondary metabolite production or secondary cell wall formation was not expected to be developmentally activated. Thus, although in plants the first defense against environmental stress is the cell wall, in Scots pine proembryogenic cell cultures osmotic stress tolerance seems to be more related to the accumulation of osmolytes than to remodeling of the cell wall components. Similarly, stilbene synthesis was not induced in embryogenic cells by osmotic stress. This is also supported by the expression results of psSTS, which decreased under all treatments towards the end of the experiment.
Conclusion
Low fresh weight and viability values indicated demoted cell division intensity and cell death in Scots pine proembryogenic cell cultures under osmotic stress conditions. This was supported by the gene expression-level investigations of cell division (RBR) and cell death-related (TAT-D) genes. However, different osmotic stress-related genes LEA, CAT and SAMS and ethylene synthesizing ACCS and ACO were not up-regulated by osmotic stress conditions, suggesting that viable embryogenic cells under the PEG treatments were able to maintain their developmental metabolism. Transcription-level regulation of PA catabolism, instead, seemed to play a specific role in PA homeostasis during stress. Transcription of PAO was down-regulated under osmotic stress leading to accumulation of free Spm, which can also be a consequence of the demoted cell division intensity. As expected, the amount of phenolic compounds was low in Scots pine proembryogenic cell cultures. Phenylalanine and cinnamic acid contents and PAL gene expression indicated that osmotic stress did not activate production of phenylpropanoids but their content was instead increased in proliferating cells.
References
Aronen T, Pehkonen T, Ryynänen L (2009) Enhancement of somatic embryogenesis from immature zygotic embryos of Pinus sylvestris. Scan J For Res 24:372–383
Astarita LV, Guerra MP (2000) Conditioning of culture medium by suspension cells and formation of somatic proembryo in Araucaria angustifolia (Conifereae). In Vitro Cell Dev Biol Plant 36:194–200
Bassard JE, Ullmann P, Bernier F, Werck-Reichhart D (2010) Phenolamides: bridging polyamines to phenolic metabolism. Phytochemistry 71:1808–1824
Boudet AM (2007) Evolution and current status of research in phenolic compounds. Phytochemistry 68:2722–2735
Butland SL, Chow ML, Ellis BE (1998) A diverse family of phenylalanine ammonia-lyase genes expressed in pine trees and cell cultures. Plant Mol Biol 37:15–24
Chong J, Poutaraud A, Hugueney P (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177:143–155
Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2006) Functions of amine oxidases in plant development and defense. Trends Plant Sci 11:80–88
Cvikrová M, Binarová P, Eder J, Vágner M, Hrubcová M, Zoń J, Machácˇková I (1999) Effect of inhibition of phenylalanine ammonia-lyase activity on growth of alfalfa cell suspension culture: alterations in mitotic index, ethylene production, and contents of phenolics, cytokinins, and polyamines. Physiol Plant 107:329–337
Cvikrová M, Malá J, Hrubcová M, Eder J, Zoń J, Machácˇková I (2003) Effect of inhibition of biosynthesis of phenylpropanoids on sessile oak somatic embryogenesis. Plant Physiol Biochem 41:251–259
Cvikrová M, Malá J, Hrubcová M, Eder J, Foretová S (2008) Induced changes in phenolic acids and stilbenes in embryogenic cell cultures of Norway spruce by culture filtrate of Ascocalyx abietina. J Plant Dis Prot 115:57–62
Desvoyes B, de Mendoza A, Ruiz-Trillo I, Gutierrez C (2014) Novel roles of plant Retinoblastoma-Related (RBR) protein in cell proliferation and asymmetric cell division. J Exp Bot 65:2657–2666. doi:10.1093/jxb/ert411
Dutra NT, Silveira V, Azevedo IG, Gomes-Neto LR, Façanha AR, Steiner N, Guerra MP, Floh EIS, Santa-Catarina C (2013) Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol Plant 148:121–132
Elmaghrabi AM, Ochatt S, Rogers H, Francis D (2013) Enhanced tolerance to salinity following cellular acclimation to increase NaCl levels in Medicago truncatula. Plant Cell Tissue Organ Cult 114:61–70. doi:10.1007/s11240-013-0306-2
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB (2010) A thorough study of reactivity of various compound classes towards the Folin–Ciocalteu reagent. J Agric Food Chem 58:8139–8144. doi:10.1021/jf1005935
Fitzmaurice GM, Laird N, Ware JH (2004) Applied longitudinal analysis. Wiley, Hoboken
Fliegmann J, Schröder G, Schanz S, Britsch L, Schröder J (1992) Molecular analysis of chalcone synthase and dihydropinosylvin synthase from Scots pine (Pinus sylvestris), and differential regulation of these and related enzyme activities in stressed plants. Plant Mol Biol 18:489–503
Fornalé S, Sarjala T, Bagni N (1999) Endogenous polyamine content and metabolism in the ectomycorrhizal fungus Paxillus involutus. New Phytol 143:581–587
Godoy-Hernández GC, Vázquez-Flota F, Loyola-Vargas V (2000) The exposure to trans-cinnamic acid of osmotically stressed Catharanthus roseus cells cultured in a 14 L bioreactor increases alkaloid accumulation. Biotechnol Lett 22:921–925
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–186
Häggman H, Vuosku J, Sarjala T, Jokela A, Niemi K (2006) Somatic embryogenesis of pine species—from functional genomics to plantation forestry. In: Mujib A, Samaj J (eds) Somatic embryogenesis. In series: plant cell monographs, vol 2. Springer, Berlin, pp 119–140
Häggman H, Pirttilä AM, Niemi K, Sarjala T, Julkunen-Tiitto R (2009) Medicinal properties, in vitro protocols and secondary metabolite analyses of Scots Pine. Methods Mol Biol 547:35–52
Hatmi S, Trotel-Aziz P, Villaume S, Couderchet M, Clément C, Aziz A (2014) Osmotic stress-induced polyamine oxidation mediates defence responses and reduces stress-enhanced grapevine susceptibility to Botrytis cinerea. J Exp Bot 65:75–88
Hong Z, Lakkinen K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of delta(1)-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Klimaszewska K, Noceda C, Pelletier G, Label P, Rodriguez R, Lelu-Walter MA (2009) Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). In Vitro Cell Dev Biol Plant 45:20–33
Lorenz WW, Sun F, Liang G, Kolychev D, Wang H, Zhao X, Cordonnier-Pratt MM, Pratt LH, Dean JFD (2005) Water stress-responsive genes in loblolly pine (Pinus taeda) roots identified by analyses of expressed sequence tag libraries. Tree Physiol 26:1–16
Lu J, Vahala J, Pappinen A (2011) Involvement of ethylene in somatic embryogenesis in Scots pine (Pinus sylvestris L.). Plant Cell Tiss Organ Cult 107:25–33. doi:10.1007/s11240-011-9952-4
Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH (2004) Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot 56:417–423
Mhamdi A, Noctor G, Baker A (2012) Plant catalases: peroxisomal redox guardians. Arch Biochem Biophys 525:181–194
Michel RE, Kaufmann MR (1973) The osmotic potential of polyethylene glycol 6000. Plant Phys 51:914–916
Mikula A, Niedzielski M, Rybczynsky JJ (2006) The use of TTC reduction assay for assessment of Gentiana spp. cell suspension viability after cryopreservation. Acta Physiol Plant 28:315–324
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stress. Plant Cell Environ 33:453–467
Minocha R, Smith DR, Reeves C, Steele KD, Minocha SC (1999) Polyamine levels during the development of zygotic and somatic embryos of Pinus radiata. Physiol Plant 105:155–164
Miskolczi P, Lendvai Á, Horváth GV, Pettkó-Szandtner A, Dudits D (2007) Conserved functions of retinoblastoma proteins: from purple retina to green plant cells. Plant Sci 172:671–683
Moschou PN, Roubelakis-Angelakis K (2013) Polyamines and programmed cell death. J Exp Bot. doi:10.1093/jxb/ert373
Moschou PN, Paschalidis KA, Delis ID, Andriopoulou AH, Lagiotis GD, Yamakoumakis DI, Kalliopi A, Roubelakis-Angelakis KA (2008) Spermidine exodous and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerances in tobacco. Plant Cell 20:1708–1724
Moschou PN, Wu J, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63:5003–5015. doi:10.1093/jxb/ers202
Muilu-Mäkelä R, Vuosku J, Läärä E, Häggman H, Saarinen M, Heiskanen J, Sarjala T (2015) Water availability influence morphology, mycorrhizal associations, PSII efficiency and polyamine metabolism at early growth phase of Scots pine seedlings. Plant Phys Biochem 88:70–81
Pinheiro J, Bates D, DebRoy S, Sarkar D and the R Development Core Team (2012) nlme: linear and nonlinear mixed effects models. R package version 3.1-103
Pottosin I, Shabala S (2014) Polyamines control of cation transport across plant membranes: implications for ion homeostasis and abiotic stress signaling. Front Plant Sci 5:1–15. doi:10.3389/fpls.2014.00154
Qiu J, Yoon JH, Shen B (2005) Search for apoptotic nucleases in yeast: role of Tat-D nuclease in apoptotic DNA degradation. J Biol Chem 280:15370–15379
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. http://www.R-project.org/. ISBN 3-900051-07-0
Romani A, Ieri F, Turchetti B, Mulinacci N, Vincieri FF, Buzzini P (2006) Analysis of condensed and hydrolysable tannins from commercial plant extracts. J Pharm Biomed Anal 41:415–420
Shen X, Wang Z, Song X, Xu J, Jiang C, Zhao Y, Ma C, Zhang H (2014) Transcriptomic profiling revealed an important role of cell wall remodeling and ethylene signaling pathway during salt acclimation in Arabidopsis. Plant Mol Biol 86:303–317
Silveira V, Balbuena TS, Santa-Catarina C, Floh EIS, Guerra MP, Handro W (2004) Biochemical changes during seed development in Pinus taeda L. Plant Growth Regul 44:147–156
Silveira V, Santa-Catarina C, Tun N, Scherer G, Handro W, Guerra M, Floh E (2006) Polyamine effects on the endogenous polyamine contents, nitric oxide release, growth and differentiation of embryogenic suspension cultures of Araucaria angustifolia (Bert.) O. Ktze. Plant Sci 171:91–98
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol 299:152–178
Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Eloy NB, Coppens F, Yoo SD, Saito K, Inze D (2011) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23:1876–1888
Stasolla C, van Zyl L, Egertsdotter U, Craig D, Liu W, Sederoff RR (2003) The effects of polyethylene glycol on gene expression of developing white spruce somatic embryos. Plant Phys 131:49–60
Swinnen IAM, Bernaerts K, Dens EJJ, Geeraerd AH, Van Impe JF (2004) Predictive modelling of the microbial lag phase: a review. Int J Food Microbiol 94:137–159
Tavladoraki P, Rossi MN, Saccuti G, Perez-Amador MA, Polticelli F, Angelini R, Federico R (2006) Heterologous expression and biochemical characterization of a polyamine oxidase from Arabidopsis involved in polyamine back conversion. Plant Phys 141:1519–1532
Tiburcio AF, Altabella T, Bitrián M, Alcázar R (2014) The roles of polyamines during the lifespan of plants: from development to stress. Planta. doi:10.1007/s00425-014-2055-9
Towill LE, Mazur P (1975) Studies on the reduction of 2,3,5-triphenyl tetrazolium chloride as a viability assay for plant tissue cultures. Can J Bot 53:1097–1102
Viitala K, Potila H, Savonen E-M, Sarjala T (2011) Health from forest-antioxidative properties of endophytic fungi from Scots pine roots. Proceedings of the 7th international conference on mushroom biology and mushroom products (ICMBMP7)
Vuosku J (2011) A matter of life and death—polyamine metabolism during zygotic embryogenesis of pine. PhD thesis, Acta Universitatis Ouluensis, Scientiae rerum naturalium 573, Oulu, Finland
Vuosku J, Jokela A, Läärä E, Sääskilahti M, Muilu R, Sutela S, Altabella T, Sarjala T, Häggman H (2006) Consistency of polyamine profiles and expression of arginine decarboxylase in mitosis during zygotic embryogenesis of Scots pine. Plant Phys 142:1027–1038
Vuosku J, Sarjala T, Jokela A, Sutela S, Sääskilahti M, Suorsa M, Läärä E, Häggman H (2009) One tissue, two fates: different roles of megagametophyte cells during Scots pine embryogenesis. J Exp Bot 60:1375–1386. doi:10.1093/jxb/erp020
Vuosku J, Suorsa M, Ruottinen M, Sutela S, Muilu-Mäkelä R, Julkunen-Tiitto R, Sarjala T, Neubauer P, Häggman H (2012) Polyamine metabolism during exponential growth transition in Scots pine embryogenic cell culture. Tree Phys 32:1247–1287
Vuosku J, Sutela S, Kestilä J, Jokela A, Sarjala T, Häggman H (2015) Expression of catalase and retinoblastoma-related protein genes associated with cell death processes in Scots pine zygotic embryogenesis. BMC Plant Biol 15:88. doi:10.1186/s12870-015-0462-0
Wi SJ, Kim SJ, Kim WT, Park KY (2014) Constitutive S-adenosylmethionine decarboxylase gene expression increases droughty tolerance through inhibition of reactive oxygen species accumulation in Arabidopsis. Planta. doi:10.1007/s00425-014-2027-0
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Zapata JM, Salinas C, Calderón AA, Muñoz R, Ros Barceló A (1991) Reduction of 2,3,5-triphenyltetrazolium chloride by KCN-insensitive salicylhydroxamic acid-sensitive alternative pathway of mitochondria from cultured grapevine cells. Plant Cell Rep 10:579–582
Acknowledgments
We are grateful to Ms. Eeva Pihlajaviita, Ms. Anneli Käenmäki and Ms. Hanna Leppälammi for their skillful technical help. We thank Meeri Pearson for proofreading the manuscript. This research was funded by the Thule Institute (2010–2013) (to HH), Academy of Finland (Project 121994 to TS) and Graduate School of Forest Sciences (to RMM).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muilu-Mäkelä, R., Vuosku, J., Hamberg, L. et al. Osmotic stress affects polyamine homeostasis and phenolic content in proembryogenic liquid cell cultures of Scots pine. Plant Cell Tiss Organ Cult 122, 709–726 (2015). https://doi.org/10.1007/s11240-015-0805-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0805-4