Abstract
As a first step to the establishment of an in vitro conservation protocol for apricot and neem, the effect of several growth retardants on the sprouting and development from nodal buds of both species and meristem development at the histological level was evaluated. Nodal section bud sprouting was affected by growth retardants, ranged from 4 to 100 % in apricot and from 10 to 100 % in neem, depending on the retardant and its concentration. Retardants paclobutrazol (PBZ), flurprimidol (FMD), diniconazole (DIN), and acetyl-salicylic acid (ASA) produced a drastic reduction of shoot length in apricot. PBZ, DIN and ASA decreased the number of leaves, whereas 1-Aminocyclopropane-1-carboxylic acid (ACC) at 10−5 µM improved the number of leaves. Length of neem sprouted buds as well as the number of leaves was affected by all of the growth retardants, with the exception of ACC that only affected the number of leaves. Triiodobenzoic acid (TIBA) at 10−5 µM improved neem shoot length. PBZ, FMD, and DIN produced several effects as very thin, long, and dark green colored leaves in shoots from both species. Once growth retardants were removed from the proliferation medium the recovery of apricot bud growth was good from PBZ and FMD at low and medium concentrations and from ACC and ASA at all concentrations tested. In neem, the recovery was good only from TIBA and ASA at low concentrations; TIBA at 10−5 µM produced an increment of shoot length higher than the control. Histological changes observed in the meristems of apricot and neem buds during growth retardation induced by FMD and ASA could be related to some extent with those observed in the dormant shoot apex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vitro cold storage of crop germplasm provides an alternative means for maintenance and distribution of disease-free propagules with two major methods available, the slow growth storage method and the cryopreservation method. Slow growth storage protocols generally used low temperature, and, sometimes, a modified culture medium with a sugar alcohol or growth retardants to reduce in vitro growth rates and, with this, the frequency of the cyclic subculture of shoots (Jarret 1997; Marino et al. 2010).
Plant growth retardants are synthetic substances that modify a plants growth and development in a typical fashion, including retardation or inhibition of shoot growth, internode elongation and leaf area, and usually with intensified green leaf pigmentation. Because of these properties, retardants are applied in agronomic and horticultural crops to reduce unwanted longitudinal shoot growth without lowering plant productivity, and have also been used for in vitro shoots collection maintenance mainly in vegetable crops (Jarret 1997). Apart from these morphological modifications, physiological alterations as improved resistance to environmental stress conditions, e.g. cold or chilling, has been reported (Baninasab 2009; Eum et al. 2012; Jing-Hua et al. 2008; Lin et al. 2006) that could have the potential for the use of these substances in in vitro cold storage.
The mode of action of these substances depends on the class of growth retardant. Triazoles, e.g. paclobutrazol (PBZ), flurprimidol (FMD), or diniconazole (DIN), interfere with the biosynthesis of gibberellins; inhibit both cell elongation and division at the shoot apex subapical zones and subsequent cell enlargement resulting in reduced stem elongation (Grossmann 1990). Another type, such as 1-Aminocyclopropane-1-carboxylic acid (ACC), is the immediate precursor of ethylene, whose effect reduces stem and internodal elongation predominantly via an inhibition of cell elongation (Sauerbrey et al. 1987). Triiodobenzoic acid (TIBA) is an inhibitor of auxin polar transport by directly competing with indole-acetyl acid for its bounding site on the efflux carrier (Lomax et al. 1995), which inhibits the growth of plants. Acetyl-salicylic acid (ASA), a derivative of the natural metabolite salicylic acid (SA), a phenolic compound considered to be a hormone-like substance, has been described as a growth inhibitor in in vitro potato cultures (López-Delgado and Scott 1997) and also seems to be involved in the plant defense response to biotic and abiotic stresses (Senaratna et al. 2000; Yuan and Lin 2008) by regulating antioxidant enzymes synthesis (Fujita et al. 2006; Zhang et al. 2011).
Since many growth retardants exert their action by inhibiting cell division in the subapical area of the meristem (Sauerbrey et al. 1987) it seems likely that the modification of the cellular structure in that tissue could induce a certain effect of “dormancy”, that could be useful for reduced growth protocols for plant material conservation. Dormancy is the mechanism used by plants to protect sensitive tissue from unfavorable climatic conditions such as chilling temperatures, and is most frequently referred to as ‘absence of visible growth in any plant structure containing a meristem’. In this sense, a dormant meristem is characterized by nuclei with less developed nucleoli, indicating reduced synthesis of ribosomal structures, cessation of cell division, with absence of mitotic figures, starch accumulation and presence of storage vacuoles (Ruttink et al. 2007).
Apricot (Prunus armeniaca L.) is an important cultivated stone fruit in Spain. In our laboratory we are generating many in vitro micropropagated cultures of different cultivars and transgenic lines. We developed a cold storage protocols to storage ‘Helena’ apricot shoots at 3 °C for almost 6 months (Perez-Tornero et al. 1999). More recently, a protocol for cold storage of apricot shoots from cvs. ‘San Castrese’ and ‘Boreale’ for 7 months at 5 °C has also been developed using a sugar alcohol in the storage medium (Marino et al. 2010). Neem tree, Azadirachta indica A. Juss., is an important multipurpose tree, whose products are being globally used in agriculture, medicine, cosmetics, and animal health care (Gahukar 2014; Kumar and Navaratnam 2013; Oh 2003). Neem seeds are recalcitrant, chilling-sensitive, and rapidly lose viability even if stored at relatively high moisture contents (Sacande et al. 2000). This is limiting progress in germplasm collections, ex situ collection, material exchange, and tree improvement in general. Thus, it would be important to develop a method to preserve in vitro culture material of both species for laboratory exchange, and to preserve in vitro collections for longer time, and, so far, any protocol using growth retardants has been developed to preserve in vitro collections of apricot or neem.
This work describes the effect of growth retardant compounds on the sprouting and development of nodal sections from apricot and neem in vitro cultures, and on their meristem development at histological level. The main objective of this research was to find the best growth retardant for each species to develop a protocol for in vitro cold storage of encapsulated nodal sections.
Materials and methods
Plant materials
Apricot in vitro shoots cultivar `Helena´ were kindly provided by Dr. Craig A. Ledbetter, Horticultural Crops Research Laboratory in Fresno, California, USA, and were maintained by sub-culturing at 3-week intervals onto apricot multiplication medium (AMM) for more than 10 years. Neem cultures were established in vitro from seedlings. Mature fruits of neem were kindly provided by Dr. Elaine Solowey at the Arava Institute for Environmental Studies, Kibbutz Ketura, Israel. Seeds from fresh fruits were extracted and germinated in vitro as described by Salvi et al. (2001). Seeds were sterilized with 70 % (v/v) ethanol for 2–3 min, 0.1 % (w/v) mercuric chloride for 15 min and washed five times with sterile water. Seeds were inoculated on basal MS medium for germination. After 4 weeks, internodes and apical buds of the seedlings were micropropagated. Shoots cultures from seed-derived in vitro plantlets were sub-cultured at 4 weeks intervals onto neem multiplication medium (NMM). In vitro shoots from apricot and neem were isolated and cut into nodal stem sections (5–10 mm) with one axillary bud, and were used as explants in all subsequent experiments.
Culture media and culture conditions
AMM consisted of Quorin and Lepoivre macro and micronutrients (QL; Quoirin and Lepoivre 1977) plus 3 mM CaCl2, Driver and Kuniyuki vitamins and organic components (DKW; Driver and Kuniyuki 1984), plus 29.6 µM adenine and 0.8 mM phloroglucinol, 3 % (v/v) sucrose and 0.7 % agar (Pronadisa, Madrid, Spain). Additional ingredients were 1.12 µM 6-benzyladenine riboside, 0.05 µM indole-3-butyric acid, and 2.1 µM 6-(3-Hydroxybenzylamino)purine (Meta-Topolin). NMM consisted of DKW medium supplemented with 0.022 µM benzyladenine and 10 mg l−1 sequestrene (~12 µM, Syngenta, Basel, Switzerland). Unless otherwise mentioned, all the chemicals were supplied by Duchefa Biochemie B.V. (Haarlem, The Netherlands). The pH of the media was adjusted to 5.7 prior to autoclaving. Medium (12.5 ml) was aliquoted into 150 mm × 25 mm test tubes, covered with Kim-kap caps (Thomas Scientific, New Jersey, USA), and autoclaved for 20 min at 121 °C (1.05 kg cm−2). Growth retardants were previously dissolved in DMSO (PBZ, FMD, DIN), double-distilled H2O (ACC, ASA), or ethanol 96 % (TIBA) before being added to the multiplication medium. One nodal section was cultured per tube. Cultures were maintained at 23 ± 1 °C under cool-white fluorescent tubes (55 µmol s−1 m−2, 400–700 nm photosynthetically active radiation) with a 16-h photoperiod.
Growth retardants experiments
Nodal sections of both species were cultured in their respective proliferation medium with the addition of the following concentrations of growth retardants: PBZ (0, 0.3, 3.4, or 34 µM), FMD (0, 0.3, 3.2, or 32 µM), DIN (0, 1, 3, or 10 µM), TIBA and ACC (0, 10−6, 10−5, or 10−4 µM) and ASA (0, 25, 100, or 400 µM) for 4 weeks and then transferred to recovery medium (proliferation medium without retardants) to study recovery growth. Data on bud sprouting (%), shoot length (cm), number of leaves and fresh weight (mg) were collected after 4 weeks on medium with growth retardants, and again 4 weeks after transfer to recovery medium, with the difference that bud sprouting data reflect only sprouted buds still alive.
Light microscopy
For histological observations, samples of nodal sections from controls and selected treatments were fixed 2.5 h at 4 °C in a 0.1 M cacodylate buffered (pH 7.2) mixture of 2.5 % glutaraldehide and 4 % paraformaldehide, dehydrated in a graded ethanol series and imbibed in LKB historesin according to Yeung and Law (1987). 10 μm-thick sections were stained with 0.05 % toluidine blue and observed with a Leica optical microscope (Leica Instr., Heidelberg, Germany).
Statistical analysis
Data were means of 20 explants per treatment with two replications. We recorded shoot length and number of leaves per shoot after explant incubation with growth retardants for 4 weeks. For each retardant and variable we compared data for each concentration with the control (0 µM). Once the explants were removed to growth retardant-free medium and cultivated for 4 weeks, we compared for each retardant the increments in shoot length and number of leaves obtained for each concentration with the increments observed in the control (0 µM). Data were analysed with the SAS software package version 9 (Littell et al. 2002). Normally distributed variables were analysed by analysis of variance (ANOVA). When significant differences were found treatment means were compared with the control according to the Dunnett´s test. Bud sprouting percentages were compared by a maximum likelihood ANOVA (Littell et al. 2002) and the χ2 test.
Results
Effect of retardants on apricot nodal sections
Nodal sections bud sprouting was not significantly affected by the growth retardants after 4 weeks, with a range from 70 to 100 % of bud sprouting. Only 10−4 µM TIBA, with a 0 % of bud sprouting, was significantly different from the control. However, growth retardants PBZ, FMD, DIN, and ASA produced a significant reduction in shoot length at all concentrations tested (Fig. 1). TIBA only produced a significant reduction in shoot length at 10−5 and 10−4 µM, with the highest concentration tested completely inhibitory (Fig. 1). ACC significantly reduced shoot length only at 10−4 µM (Fig. 1).
Effect of pablobutrazol (PBZ), flurprimidol (FMD), diniconazole (DIN), TIBA, ACC and acetyl-salicylic acid (ASA) on apricot nodal sections shoot length (left) and number of leaves (right) after 4 weeks. The values represented correspond to the mean ± SE. An asterisk means significant differences from the control (P < 0.05) according to the Dunnett’s test
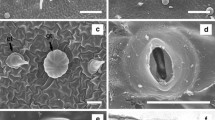
PBZ, DIN and ASA decreased the number of leaves at all concentrations tested, whereas FMD and TIBA had less effect on the number of leaves, since the reduction was more moderated at low concentration and was only drastic and significant at the high concentration (Fig. 1). Buds growing on ACC medium did not show any reduction on the number of leaves, which even was significantly improved at 10−5 µM (Fig. 1). Additional effects of growth retardants were that the leaves of the shoots grown in the presence of PBZ or FMD were long, dark green, and with a slight curved shape (Fig. 2a, b).
Apricot buds growing in a medium with a 0.34 µM paclobutrazol; b 3.2 µM flurprimidol; c growth retardant-free medium: left control, right shoot coming from 3.2 µM flurprimidol medium. d Neem shoots growing in growth retardant-free medium coming from (left to right) 0, 10−6, 10−5, 10−4 µM TIBA. Images were taken after 4 weeks on medium with retardant or on retardant-free medium
Growth recovery in apricot nodal sections
Bud sprouting of explants incubated on medium with PBZ or FMD and later transferred to growth retardant-free medium were significantly affected (P < 0.0001) for both compounds, mainly at the highest concentrations tested. Although intermediate concentrations allowed bud sprouting at quite good percentages, significant differences were observed (Fig. 3). The increase in length and number of leaves during the 4-week recovery period was not different than the control for explants previously treated with low or medium PBZ or low FMD concentrations (Figs. 2c, 4). While explants previously cultured on medium with DIN produced a similar effect regarding bud sprouting (P < 0.001), with significant differences only when the buds come from a 10 µM DIN medium (Fig. 3), shoots did not increase in length at any concentration tested (P < 0.0001) nor produced many new leaves at 1 µM or at 3 µM (P < 0.05) compared with the control (Fig. 4). Bud sprouting in explants that had been previously cultured with TIBA decreased very strongly (P < 0.05) with the increase in TIBA concentration (Fig. 3) which also reduced the growth recovery of shoots, and presented a significant reduction in length and number of leaves (P < 0.0001; Fig. 4). Explants previously cultured on ACC showed a significant decrease in bud sprouting (P < 0.01), mainly due to a reduction in sprouting induced by the highest concentration tested (Fig. 3). This retardant did not affect the recovery of shoot length nor number of leaves, in fact, when explants were previously cultured on medium with 10−6 µM ACC showed an improved number of leaves (Fig. 4) and bud sprouting percentage (Fig. 3) as compared with the control. ASA was the regulator that had less effect on bud sprouting at high concentration during the recovery of explants, although significant differences were obtained for all tested concentrations (Fig. 3). Shoot length and number of leaves increments were similar at all concentrations tested and lower than the control but without significant differences (Fig. 4).
Bud sprouting on excised apricot (left) and neem (right) nodal sections growing on retardant-free medium previously incubated on growth retardants. Data show sprouted buds still alive after 4 weeks on retardant-free medium. PBZ treatment: explants previously incubated on (columns from left to right) 0, 0.3, 3.4, or 34 µM paclobutrazol medium; FMD treatment: explants previously incubated on (columns from left to right) 0, 0.3, 3.2, or 32 µM flurprimidol medium; DIN treatment: explants previously incubated on (columns from left to right) 0, 1, 3, or 10 µM diniconazole medium; TIBA treatment: explants previously incubated on (columns from left to right) 0, 10−6, 10−5, or 10−4 µM TIBA medium; ACC treatment: explants previously incubated on (columns from left to right) 0, 10−6, 10−5, or 10−4 µM ACC medium; ASA treatment: explants previously incubated on (columns from left to right) 0, 25, 100, or 400 µM acetyl-salicylic acid. Columns with an asterisk mean significant differences to the control (P < 0.05) in each treatment according to χ2 test
Effect of pablobutrazol, flurprimidol, diniconazole, TIBA, ACC and acetyl-salicylic acid on apricot nodal sections shoot length (left) and number of leaves (right) growth recovery on retardant-free medium after 4 weeks. The values represented correspond to the mean ± SE. An asterisk means significant differences from the control (P < 0.05) according to the Dunnett’s test
Effect of growth retardants on neem nodal sections
Growth retardants did not affect neem bud sprouting in a significant way, with a range from 65 to 100 %, and only 3.4 µM PBZ (55 %) and 10−4 µM ACC (20 %) showed significant differences in comparison with their respective controls. Length of neem sprouted shoots as well as the number of new leaves produced during the exposure to the chemical compounds were significantly affected by PBZ, FMD and DIN (P < 0.0001), with the exception of FMD at low concentration that produced a similar number of leaves than the control (Fig. 5). TIBA produced a shoot length decrease only at the highest concentration tested whereas at 10−5 µM improved bud length after 4 weeks of exposure (Fig. 5). Sprouted shoots length from neem nodal sections incubated on ACC medium was similar to the controls and only the number of leaves was significantly affected (P < 0.0001), with less leaves at 10−5 and 10−4 µM ACC (Fig. 5). Shoot buds that had been growing in a medium supplemented with ASA decreased significantly in length and number of leaves with the increase in concentration (Fig. 5). Similar as in apricot, growth retardants produced neem shoots that were very thin (PBZ, FMD), or with small leaf blade (DIN), long (PBZ), bright (FMD) or dark green color leaves (PBZ, FMD, DIN) and stems (DIN) at all concentrations. PBZ and FMD produced callus at all concentrations (up to 30 % of explants) and necrosis at the highest concentration (FMD).
Effect of pablobutrazol, flurprimidol, diniconazole, TIBA, ACC and acetyl-salicylic acid on neem nodal sections shoot length (left) and number of leaves (right) after 4 weeks. The values represented correspond to the mean ± SE. An asterisk means significant differences from the control (P < 0.05) according to the Dunnett’s test
Growth recovery in neem nodal sections
Nodal sections bud sprouting after 4 weeks in medium without growth retardants was significantly decreased by the increase in all retardant concentrations in the previous medium (Fig. 3). The negative effect that the highest concentration tested of all chemicals produced on the explants was observed even once these substances were eliminated from the medium, with the strongest decrease in bud sprouting. PBZ, FMD and ACC were the chemicals that more affected bud sprouting at high concentration, although in all cases bud sprouting percentages at medium and high concentrations were significantly lower than the control (Fig. 3). In the same way, shoot length was significantly affected by all PBZ, FMD, DIN concentration, or ACC at medium and high concentrations (P < 0.0001; Fig. 6). Shoots that were expose to TIBA, once on growth retardant free-medium proceed with their growth with exception of those coming from the higher concentration, which failed to growth. However, those shoots coming from 10−5 µM TIBA continued growing taller than the controls, although in this cases without significant differences (Figs. 2d, 6). Explants previously incubated with PBZ, FMD and DIN, produced a similar number of leaves than the controls, while explants coming from media with TIBA or ASA showed a significant decrease (P < 0.0001 and P < 0.005, respectively) in the number of new leaves only at the highest concentration (Fig. 6), whereas those that had been previously incubated with ACC significantly decreased (P < 0.005) the number of new leaves at all concentrations tested (Fig. 6).
Effect of pablobutrazol, flurprimidol, diniconazole, TIBA, ACC and acetyl-salicylic acid on neem nodal sections shoot length (left) and number of leaves (right) growth recovery on retardant-free medium after 4 weeks. The values represented correspond to the mean ± SE. An asterisk means significant differences from the control (P < 0.05) according to the Dunnett’s test
Histological studies
We used axillary buds from active cultures as controls. In apricot, 0.3 and 3.2 µM FMD and in neem 25 and 100 µM ASA were selected for histological study. The histological structure of meristems in apricot control buds was typical of active tissues, with small cells in L1 and L2 layers (Colin and Verhoyen 1976), evident nuclei and abundant cytoplasm, and no vacuolization [Fig. 7a, b (detail of a)]. Some changes in the anatomy of the apricot meristem could be observed in the buds growing with FMD (0.3 µM), the apical dome (Romberger et al. 1970) appeared enlarged with less developed leaf primordia. Cells in L1 and L2 were enlarged and vacuolated with thicker cell walls [Fig. 7c, d (detail of c)]. In neem, histological structure of meristems from control explants presented buds that appeared to be dormant (Fig. 8a). However, these buds have two morphological types of cells: (1) cells with a large central vacuole, reduced cytoplasm, and small evident nucleus (Fig. 8b-1), and (2) cells with vacuoles of dense content and evident nucleus (Fig. 8b-2). The explants treated with 25 µM ASA presented two type of buds, those that could correspond to a bud partially active (Fig. 8c) and those with a bud probably in dormancy (Fig. 8e). In the first type, L1 cell layer of the meristem presented a large central vacuole and in some cases an evident nucleus (Fig. 8d, L1). The bottom layer L2 presented cells with a less vacuolated cytoplasm and their nuclei were more evident (Fig. 8d, L2), while in the latter, the cells were larger with a prominent vacuole (Fig. 8f). Neem meristems in explants treated with 100 µM ASA, had enlarged apical domes without leaf primordia (Fig. 8g), L2 cells were larger than controls with thicker cell walls, abundant cytoplasm, obvious nuclei, and vacuoles with content (Fig. 8h).
Histological sections showing buds meristems from apricot. a Control—axillary buds of shoots in proliferation medium. b Detail of (a), bud with layers L1 and L2 with small cells, clear nuclei and abundant cytoplasm, vacuolization is not observed. c Axillary buds of apricot incubated for 4 weeks in proliferation medium with the addition of 0.3 µM flurprimidol. d Detail of (c), cells in L1 and L2 were enlarged and vacuolated with thicker cell walls
Histological sections showing axillary buds meristems of neem. a Control—axillary bud prior to treatment and in a state of latency. There is two morphological types of cells; (1) Cells with a large central vacuole, little cytoplasm and little evident nucleus (b-1); (2) cells with vacuoles containing possible compounds, phenolics, and evident nucleus (b-2). c, e Buds incubated in proliferation medium with 25 µM ASA—observed two types of buds, some more active (c), an another kind of bud in latency (e). d Detail of (c) the cell layer L1 has cells with a large central vacuole and in some cases the nucleus is evident. The bottom layer L2 show less vacuolated cells and their nuclei are more evident. f Detail of (e) the cells are unique type, presenting a large cell size occupied mainly by a single vacuole. g Buds incubated in proliferation medium with 100 µM ASA—possibly active buds showing only one cell type. h Detail of (g), presents a large cell size, abundant cytoplasm, obvious nuclei and vacuoles with content. ASA acetylsalicylic acid
Discussion
In a previous work, we developed a protocol for apricot and neem nodal section encapsulation but we observed that the viability of the encapsulated material when storage at low temperature decreased with the storage time (Padilla et al. 2008). Here we studied the growth-inhibiting effect of various growth retardants on the development of apricot and neem nodal buds to evaluate the efficacy and usability of these substances to subsequently extend the cold storage period of encapsulated nodal sections of these species.
None of the retardants completely inhibited bud sprouting in apricot, although the highest concentration tested of PBZ and FMD had clearly toxic effect that completely inhibited the recovery of the cultures. In neem, although the percentage of bud sprouting was scarcely affected by these retardants, the effect produced on the length of the shoots that did not recover in any of the tested concentrations, indicates a differential sensibility to these compounds depending on the species.
The reduction in growth produced by triazoles, in cultured nodal sections of apricot and neem with a reduced shoot length and with fewer leaves compared with controls, has been widely described since these regulators have been used extensively in agriculture and horticulture both as plant growth regulators and fungicides, mainly to control the growth of many crop species (Espindula et al. 2009; Fair et al. 2012) and ornamental pot-plants (Eum et al. 2011; Currey and López 2011), but also to control the growth and to improve the rooting of Prunus rootstocks in vitro (Krizan et al. 2007) or to preserve in vitro collections (Lisek and Orlikowska 2004). The reduction in shoot length produced by triazoles at the lowest retardant concentration tested was probably a result of shorter internodes since the number of leaves was similar to the controls, corroborating their effect interfering with the biosynthesis of gibberellins (Grossmann 1990). Similar effects to those observed in our experiments with neem and apricot in relation to shoot development and morphology or color of leaves has been widely described in the literature in vivo (Eum et al. 2012; diniconazole-50 mg l−1; Won and Jeong 2007; paclobutrazol-0.5–1.5 mg l−1) and in vitro (Snir 1988; paclobutrazol-; Ilczuk et al. 2005; flurprimidol 0.1–1 mg l−1), where particular attention was paid to the increase of pigments such as chlorophyll a + b. Thus, several studies have shown the effect of triazole retardants on the concentration of chlorophylls a + b, which was increased by the action of these compounds (Hwang et al. 2008; Yiu et al. 2008; Won and Jeong 2007). It has been suggested that this increase in the synthesis of chlorophyll could be mediated by the effect that triazoles exert increasing plant endogenous cytokinin concentration (Yiu et al. 2008).
In our study, except in concentrations that were toxic of the three studied triazoles compounds, the effect of these retardants disappeared over time (data not shown), as has been described in P. avium or in sweet potato (Snir 1988; Jarret 1997). However, with in vivo applications Pobudkiewicz and Treder (2006) observed that the effect of FMD reduced the size of lily pot-plants to 45 % and increased the intensity of their color, which persisted in the year after treatment.
Regardless of its function by reducing the growth and development of the aerial parts of plants, triazoles have been used to protect plants against abiotic stresses, such as low temperatures (Lin et al. 2006; Baninasab 2009), flood (Yiu et al. 2008), or reducing stress response for ex vitro transplanting (Ziv 2008). It has been shown that plants treated with these retardants have a higher content of endogenous cytokinins and abcisic acid (ABA; Suttle et al. 2012; Hsu and Kao 2005) and auxins in bulbs (Zheng et al. 2012). It has also been indicated that different antioxidative systems in the plant have been induced by PBZ treatment defending the plant in case of stress (Yiu et al. 2008).
Regarding TIBA, the highest concentration tested (10−4 µM) was toxic in both apricot and neem, which agrees with previous work with in vitro shoots of sweet potato (Jarret 1997). In neem, the promoting effect on the growth of shoots and the number of leaves at 10−5 µM was observed in sweet potato at 10−8 µM, and it has also been described in avocado plants in vivo (McCarty et al. 1971). In apricot, shoots with a rosette-like appearance were observed with TIBA at 10−5 µM as it has been previously reported in sweet potato (Jarret 1997), indicating that TIBA also produced a reduction of internodal elongation. We did not observe morphologically aberrant shoots as it has been observed in sweet potato and avocado (McCarty et al. 1971; Jarret 1997). TIBA has been used in vivo to break the apical dominance in fruit trees such as avocado, apple, or citrus and induces the production of axillary shoots (McCarty et al. 1971). As a result, it has been used in vitro to promote axillary multiplication in jojoba (Prakash et al. 2003), but we have not observed this effect in apricot or neem.
ACC has been used in vitro as a way to increase ethylene concentration, without having to supply exogenously, thus increasing the regeneration of shoots and bud development (Trujillo-Moya and Gisbert 2012). We did not observe a marked effect of ACC in reducing the growth of apricot buds at low concentration, as it has been described in sweet potato (Jarret 1997), but a greater number of leaves was found. In our case, the addition of ACC to the medium stimulated the growth of apricot buds at 10−5 µM, as it has also been described in sweet potato at 10−8 µM (Jarret 1997). In neem, the inhibitory effect on growth was stronger in terms of percentage of bud sprouting and bud growth recovering. Regardless of the shoot growth effect, ACC seems to play an important role in the response of plant to stress. Thus, it has been suggested that the increase of ethylene produced by the addition of ACC to the medium could produce an increase of ABA, which would result in a reduction in shoot growth and in turn a protection of plants to abiotic stress (Dong et al. 2011).
We have shown that ASA acts as a growth retardant in apricot and neem, as was observed by López-Delgado and Scott (1997) in sweet potato, although to a lesser extent than the other growth retardants tested. In some species, it has been described that SA promotes shoot growth at low concentrations and inhibits the growth at higher levels (López-Delgado and Scott 1997; Kovacik et al. 2009) however, we did not see this growth promoting effect but a toxic effect at high concentration, with high necrosis percentages and chlorotic shoots, which could indicate an effect of ASA on chlorophyll synthesis. This would agree with several works describing a decrease in leaf chlorophyll content after exogenous application (Moharekar et al. 2003; Kovacik et al. 2009). However, exogenous SA has been used in vitro to avoid shoot hyperhydricity and to increase shoot chlorophyll content (Hassannejad et al. 2012; Andarwulan and Shetty 1999). In addition, exogenous SA has been shown to confer tolerance against biotic and abiotic stresses, and freezing or chilling stress (Kirillova et al. 2011; Karlidag et al. 2009; Jing-Hua et al. 2008). Apricot, as a temperate fruit tree species, can tolerate low temperature (Perez-Tornero et al. 1999; Marino et al. 2010), but neem explants probably would have more problems to resist temperatures below 12 °C (Padilla et al. 2008). For this reason, ASA could be a good pretreatment for neem nodal sections. This effect of SA protecting plants from cold stress performed probably through modulating the antioxidant system (Kirillova et al. 2011) but, Pal et al. (2011) suggested that there might be an overlap between cold acclimation induced by ABA and SA-related stress response. However, other authors have indicated that the role of SA was still ambiguous and the induction of stress tolerance appears to depend greatly on the mode of application (Horvath et al. 2007; Eraslan et al. 2008).
The histological changes observed in the meristems of apricot and neem buds during growth retardation induced by FMD and ASA could be related to some extent with those observed in the dormant shoot apex. The shoot apical meristem of woody plants transitions from active to a dormant state that can be regulated by external and endogenous signals and induce characteristic modifications in their anatomy and cytology (Van der Schoot and Rinne 2011; Paul et al. 2014). Enlarged apical domes, thick cell walls, and vacuolated cytoplasm as we have observed in growth retarded cultures of apricot and neem have also been described in dormant meristems of peach (Boonprakob et al. 1996), or pines (Jordy 2004).
In summary, in this work we have attempted to find those growth retardants that would reduce apricot and neem bud development without toxicity, hoping to induce a certain degree of dormancy to protect the buds from the cold during storage. In this sense, it could be useful to test PBZ, FMD at low or medium concentrations in apricot, and TIBA or ASA at low concentration in neem. However, we also have to consider that these retardants used at concentrations that did not produce a delay in bud growth could protect the buds from the cold because of its action promoting the response of buds to stress, and in this regard it could be interesting to use ACC in apricot at low and medium concentrations.
References
Andarwulan N, Shetty K (1999) Influence of acetyl salicylic acid in combination with fish protein hydrolysates on hyperhydricity reduction and phenolic synthesis in oregano (Origanum vulgare) tissue cultures. J Food Biochem 23(6):619–635
Baninasab B (2009) Amelioration of chilling stress by paclobutrazol in watermelon seedlings. Sci Hortic 121(2):144–148
Boonprakob U, Byrne DH, Mueller DMJ (1996) Anatomical differences of axillary bud development in blind nodes and normal nodes in peach. HortScience 31(5):798–801
Colin J, Verhoyen M (1976) Anatomical study of Prunus meristematic tips. Acta Hortic 67:87–95
Currey ChJ, Lopez RG (2011) Early flurprimidol drench applications suppress final height of four poinsettia cultivars. HortTechnology 21(1):35–40
Dong H, Zhen ZQ, Pen JY, Chang L, Gong QQ, Wang NN (2011) Loss of ACS7 confers abiotic stress tolerance by modulating ABA sensibility and accumulation in Arabidopsis. J Exp Bot 62(14):4875–4887
Driver JA, Kuniyuki A (1984) In vitro propagation of paradox walnut rootstock. HortScience 19(4):507–509
Eraslan F, Inal A, Pilbeam DJ, Gunes A (2008) Interactive effects of salicylic acid and silicon on oxidative damage and antioxidant activity in spinach (Spinacia oleracea L. Cv. ‘Matador’) grown under boron toxicity and salinity. Plant Growth Regul 55(3):207–219
Espindula MC, Rocha VS, Grossi JAS, Souza MA, Souza LT, Favarato LF (2009) Use of growth retardants in wheat. Planta Daninha 27(2):379–387
Eum SJ, Park KI, Choi YJ (2012) Effects of diniconazole application on anatomical and biochemical characteristics related to stress tolerance in Lilum davuricum. Korean J Hortic Sci Technol 30(3):229–235
Eum SJ, Park KI, Lee IJ (2011) Effects of foliar-sprayed diniconazole on contents of endogenous gibberellic acids and abscisic acid in Lilium davuricum. Korean J Hortic Sci Technol 29(3):165–171
Fair BA, Whipker B, Mccall I, Buhler W (2012) Height control of ‘hot lips’ hybrid sage to flurprimidol substrate drench. HortTechnology 22(4):539–541
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9(4):436–442
Grossmann K (1990) Plant-growth retardants as tools in physiological research. Physiol Plant 78(4):640–648
Gahukar RT (2014) Factors affecting content and bioefficacy of neem (Azadirachta indica A. Juss.) phytochemicals used in agricultural pest control: a review. Crop Prot 62:93–99
Hassannejad S, Bernard F, Mirzajani F, Gholami M (2012) SA improvement of hyperhydricity reversion in Thymus daenensis shoots culture may be associated with polyamines changes. Plant Physiol Biochem 51:40–46
Horvath E, Pal M, Szalai G, Paldi E, Janda T (2007) Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol Plant 51(3):480–487
Hsu YT, Kao CH (2005) Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiol Plant 124(1):71–80
Hwang SJ, Lee MY, Sivanesan I, Jeong BR (2008) Growth control of kalanchoe cultivars Rako and Gold Strike by application of paclobutrazol and uniconazole as soaking treatment of cuttings. Afr J Biotechnol 7(22):4212–4218
Ilczuk A, Winkelmann T, Richartz S, Witomska M, Serek M (2005) In vitro propagation of Hippeastrum × chmielii Chm.—influence of flurprimidol and the culture in solid or liquid medium and in temporary immersion systems. Plant Cell Tissue Organ Cult 83(3):339–346
Jarret RL (1997) Effects of chemical growth retardants on growth and development of sweetpotato (Ipomoea batatas (L.) Lam.) in vitro. J Plant Growth Regul 16(4):227–231
Jing-Hua Y, Yuan G, Yan-Man L, Xiao-Hua Q, Ming-Fang Z (2008) Salicylic acid-induced enhancement of cold tolerance through activation of antioxidative capacity in watermelon. Sci Hortic 118(3):200–205
Jordy MN (2004) Seasonal variation in organogenetic activity and reserves allocation in the shoot apex of Pinus pinaster Ait. Ann Bot 93:25–37
Karlidag H, Yildirim E, Turan M (2009) Exogenous applications of salicylic acid affect quality and yield of strawberry grown under anti-frost heated greenhouse conditions. J Plant Nutr Soil Sci 172(2):270–276
Kirillova NV, Belykh YV, Spasenkov AI (2011) Influence of salicylic acid on biosynthesis of nucleic acids in Polyscias filicifolia cell culture under the action of unfavorable temperatures. Appl Biochem Microbiol 47(4):431–434
Kovacik J, Klejdus B, Hedbavny J, Backor M (2009) Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 18(5):544–554
Krizan B, Ondrusikova E, Trckova K, Benedikova D (2007) Effects of paclobutrazol and indole-3-butyric acid on in vitro rooting and growth of some rootstocks of the genus Prunus L. Eur J Hortic Sci 72(5):198–201
Kumar VS, Navaratnam V (2013) Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed 3(7):505–514
Lin KH, Pai FH, Hwang SY, Lo HF (2006) Pre-treating paclobutrazol enhanced chilling tolerance of sweetpotato. Plant Growth Regul 49(2–3):249–262
Lisek A, Orlikowska T (2004) In vitro storage of strawberry and raspberry in calcium-alginate beads at 4 °C. Plant Cell Tissue Organ Cult 78(2):167–172
Littell RC, Stroup WW, Freund RJ (2002) SAS for linear models, 4th edn. SAS Institute Inc., Cary, p 466
Lomax TL, Mehlhorn RJ, Briggs WR (1995) Active auxin uptake by zucchini membrane-vesicles: quantitation using electron-spin-resonance volume and delta-PH determinations. Proc Natl Acad Sci USA 82:6541–6545
López-Delgado H, Scott IM (1997) Induction of in vitro tuberization of potato microplants by acetylsalicylic acid. J Plant Physiol 151(1):74–78
Marino G, Negri P, Cellini A, Masia A (2010) Effect of carbohydrate on in vitro low-temperature storage of shoot culture of apricot. Sci Hortic 126(4):434–440
McCarty CD, Burns RM, Boswell SB (1971) Chemically induced sprouting of axillary buds in avocados. Calif Agric 25(12):4–6
Moharekar ST, Lokhande SD, Hara T, Tanaka R, Tanaka A, Chavan PD (2003) Effect of salicylic acid on chlorophyll and carotenoid contents of wheat and moong seedlings. Photosynthetica 41(2):315–317
Oh SG (2003) Cosmetic composition containing antimicrobial neem oil. Patent number KR2003002004-A
Padilla IMG, Alburquerque N, Burgos L, Piqueras A (2008) Germplasm conservation in apricot and neem by encapsulation-refrigeration of internodes from micropropagated shoots. In Vitro Cell Dev Biol-Anim 44:S79–S80
Pal M, Janda T, Szalai G (2011) Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. J Agron Crop Sci 197(5):368–377
Paul LK, Rinne PLH, Van der Schoot Ch (2014) Shoot meristems of deciduous woody perennials: self-organization and morphogenetic transitions. Curr Opin Plant Biol 14:86–95
Perez-Tornero O, Ortin-Parraga F, Egea J, Burgos L (1999) Medium-term storage of apricot shoot tips in vitro by minimal growth method. HortScience 34(7):1277–1278
Pobudkiewicz A, Treder J (2006) Effects of flurprimidol and daminozide on growth and flowering of oriental lily ‘Mona Lisa’. Sci Hortic 110(4):328–333
Prakash S, Agrawal V, Gupta SC (2003) Influence of some adjuvants on in vitro clonal propagation of male and female jojoba plants. In Vitro Cell Dev Biol-Plant 39(2):217–222
Quoirin M, Lepoivre P (1977) Improved media for in vitro culture of Prunus sp. Acta Hortic 78:437–442
Romberger JA, Verneell RJ, Tabor CA (1970) Culture of apical meristems and embryonic shoots of Picea abies approach and techniques. USDA Tech Bull 1409:1–30
Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19(8):2370–2390
Sacande M, Buitink J, Hoekstra FA (2000) A study of water relations in neem (Azadirachta indica) seed that is characterized by complex storage behaviour. J Exp Bot 51(344):635–643
Salvi ND, Singh H, Tivarekar S, Eapen S (2001) Plant regeneration from different explants of neem. Plant Cell Tissue Org Cult 65:159–162
Sauerbrey E, Grossmann K, Jung J (1987) Influence of growth-retardants on the internode elongation and ethylene production of sunflower plants. Physiol Plant 70(1):8–12
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul 30(2):157–161
Snir I (1988) Influence of paclobutrazol on in vitro growth of sweet cherry shoots. HortScience 23:304–305
Suttle JC, Abrams SR, De stefano-Beltrán L, Huckle LL (2012) Chemical inhibition of potato ABA-8′-hydroxylase activity alters in vitro and in vivo ABA metabolism and endogenous ABA levels but does not affect potato microtuber dormancy duration. J Exp Bot 63(15):5717–5735
Trujillo-Moya C, Gisbert C (2012) The influence of ethylene and ethylene modulators on shoot organogenesis in tomato. Plant Cell Tissue Organ Cult 111(1):41–48
Van der Schoot Ch, Rinne PLH (2011) Dormancy cycling at the shoot apical meristem: transition between self-organization and self-arrest. Plant Sci 180:120–131
Won EJ, Jeong BR (2007) Effect of plant growth retardants on the growth characteristics of potted Spathiphyllum in an ebb and flow system. Korean J Hortic Sci Technol 25(4):443–450
Yeung EC, Law SK (1987) Serial sectioning techniques for a modified LKB historesin. Stain Technol 62:147–153
Yiu JC, Liu CW, Kuo CT, Tseng MJ, Lai YS, Lai WJ (2008) Changes in antioxidant properties and their relationship to paclobutrazol-induced flooding tolerance in Welsh onion. J Sci Food Agric 88(7):1222–1230
Yuan S, Lin HH (2008) Role of salicylic acid in plant abiotic stress. Z Naturforsch C 63(5–6):313–320
Zhang WP, Jiang B, Lou NL, Lu MH, Yang M, Chen JF (2011) Impact of salicylic acid on the antioxidant enzyme system hydrogen peroxide production in Cucumis sativus under chilling stress. Z Naturforsch C 66(7–8):413–422
Zheng R-R, Wu Y, Xia Y-P (2012) Chlorocholine chloride and pacrobutrazol treatments promote carbohydrate accumulation in bulbs of Lilium Oriental hybrid ‘Sorbonne’. J Zhejiang Univ Sci B 13(2):136–144
Ziv M (2008) Paclobutrazol and xanthan gum involvement in proliferation and stress response of Ornithogalum dubium Houtt. bud clusters cultured in bioreactors. Propag Ornam Plants 8(1):28–32
Acknowledgments
IMGP and NFG thank The Spanish National Research Council-European Social Fund I3P program for a postdoctoral contract.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padilla, I.M.G., Fernández-García, N., Olmos, E. et al. Effects of growth retardants on sprouting and development of apricot (Prunus armeniaca L.) and neem (Azarchta indica A. Juss.) nodal buds. Plant Cell Tiss Organ Cult 122, 285–297 (2015). https://doi.org/10.1007/s11240-015-0765-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0765-8