Abstract
An improved synthesis of vinblastine and vincristine was observed in Catharanthus roseus L. (G). Don by using NaCl as an elicitor. Various in vitro grown embryogenic tissues were cultivated under salinity stress for enhanced synthesis of alkaloids. Different levels of salt [control (0 mM), NT1 (25 mM), NT2 (50 mM), NT3 (75 mM), NT4 (100 mM), and NT5 (125 mM)] were amended in MS and callus biomass growth (fresh- and dry-weight) and biochemical attributes at various embryogenic stages were studied. Maximum callus biomass reduction was observed in 125 mM NaCl amended medium. Antioxidant enzymes i.e. superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase and glutathione reductase activities were assayed as in vitro grown tissues were elicitated with NaCl, causing cellular stress. The antioxidant enzymes activity increased linearly with increasing NaCl level in medium, 4.97 EU min−1 mg−1 SOD and 3.14 EU min−1 mg−1 CAT, both being maximum in proliferating embryos at NT5. Quantitative estimation and comparative yield of alkaloids were made in response to NaCl stress in different cultivated tissues by using HPTLC method. Vinblastine content was observed to be maximum in regenerated leaves (14.17 mg/g dry wt) on 25 mM NaCl amended medium, followed by in vitro raised shoots. Similarly, better accumulation of vincristine (5.12 mg/g dry wt) was also noted in NaCl amended medium especially at low level (NT1). The data presented indicate that the synthesis of Catharanthus alkaloids was growth specific and was influenced by NaCl levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants since time immemorial have been used as medicine and the World Health Organization in a recent report estimates that about 80 %of people still rely on plants as traditional medicines (Abdel-Hady et al. 2008). Plants are also a renewable source of modern medicine as over one-fourth of prescribed drugs contain active ingredients/extracts of plants. The most popular analgesic aspirin was originally obtained from Salix and Spiraea species, similarly paclitaxel and vinblastine; two anti-cancer compounds are primarily derived from Taxus and Catharanthus respectively (Roberts 1988). Catharanthus roseus L. (G). Don is an immensely important anti-cancerous plant belongs to the family Apocynaceae. The plant has widely been used in treating against a number of diseases like diabetes, curing wasp stings; it is also used as an astringent and diuretic, and in recovering cough (Nammi et al. 2003). Two alkaloids vinblastine and vincristine are used in treating leukemias, Hodgkin’s disease, solid tumors and other cancer (Mukherjee et al. 2001). The drugs are produced commercially by extraction of Catharanthus plant; the intact plant however, contains a low concentration of drug (0.0005 % dry weight basis). As an alternative to whole plant extraction, plant cell cultures have been employed in producing and enriching alkaloids in a variety of medicinal plants (Cheruvathur and Thomas 2014) including Catharanthus (Zhao et al. 2001; Junaid et al. 2010; Mujib et al. 2012; Saiman et al. 2014). Various plant parts (shoot, root/hairy roots, callus, suspension etc.) have been used for the establishment of culture and subsequent alkaloids extraction purposes. Several key factors controlling in vitro biosynthesis of alkaloids have also been identified (Moreno et al. 1995; Murthy et al. 2014), of which a number of environmental stresses (adverse temperatures, water deficit, salinity, UV etc.) often referred to as elicitators, have widely been applied to improve secondary metabolite synthesis in cultivated tissues (Dicosmo and Miswa 1985; Moreno-Valenzuela et al. 2003; Zheng and Wu 2004; Junaid et al. 2011). Application of various chemicals like osmotic shock, salt stress, elements has been utilized to improve alkaloid yield in cell cultures of C. roseus (Zhao et al. 2000, 2001; Lovkova et al. 2005; Zahid and Mujib 2012). Some of the enrichment responses of those treatments, although were cell-line specific and often limit their utilization, studies using efficient biotic/abiotic elicitors for improving secondary metabolite synthesis are an important approach in cell cultures (Moreno et al. 1995). The production of indole alkaloids ajmalicine and catharanthine was enhanced by cerium (CeO2 and CeCl3), yttrium (Y2 O3) and neodymium (NdCl3) in C. roseus. The yield of ajmalicine in these treated-cultures reached to 51 mg l−1 (CeO2), 40 mg l−1 (CeCl3), 41 mg l−1 (Y2 O3) and 49 mg l−1 (NdCl3) while catharanthine production reached to 36 mg l−1 (CeO2) and 31 mg l−1 (CeCl3) (Zhao et al. 2001). Beside improving callus biomass growth and secondary metabolite accumulation, in vitro culture serves as an efficient tool to study salt stress response of undifferentiated callus to salinity in controlled, uniform experimental conditions, avoiding complications arising from physiological and structural variability of whole plant, thus offers fast selection and development of salt tolerant lines (Niknam et al. 2006; Elmaghrabi et al. 2013). The plant cell culture studies also allow to elucidate mechanism of tolerance operating at cellular level (Cherian and Reddy 2003; Elkahoui et al. 2005).
In this article, the influence of salinity stress (NaCl) on secondary metabolite production was evaluated in C. roseus. This is, perhaps the first ever vincristine and vinblastine quantification study in somatic embryo derived tissues and plantlets after NaCl elicitation. The callus growth, biochemical differences and the relative association of antioxidant enzymes activity during metabolite synthesis were also investigated and discussed.
Materials and methods
Plant material
Catharanthus roseus L. (G). Don fruits were collected from the Jamia Hamdard (Hamdard University, New Delhi, India) herbal garden and the seeds were used as experimental materials. The establishment of culture was made following procedures as described earlier (Junaid et al. 2006). In short, the seeds were separated from surface disinfected follicles; and twenty to twenty-five seeds were placed in Maganta-7 vessels containing 50 ml of germination medium based on MS (Murashige and Skoog 1962). Germination medium contained all the essential components of MS in half, without any organic compounds and plant growth regulators (PGRs). The seeds started to germinate within 10–14 days of incubation; the seedlings were kept in in vitro conditions until they had attained 2–4 cm length. Later various plant parts (stem, leaf and hypocotyl) were inoculated as explants in full strength MS. The medium pH was adjusted to 5.7 before sterilization. To solidify the medium 8 g l−1 of agar was added, boiled and poured into clean, dry culture tubes (6′ × 1″, Borosil); each tube contained 20 ml of medium. Finally, the medium was sterilized at 121 °C for 15 min at 15 lbs. The cultures were incubated at 25 ± 2 °C under 16-h photo period with cool white fluorescent tubes (100 μmol m−2 s−1).
Embryogenic callus induction
The hypocotyls of germinated seedlings were cultured on MS supplemented with optimized concentration of 2,4-D (1.0 mg l−1) for the induction of embryogenic callus, as was described earlier (Junaid et al. 2006).
Somatic embryo induction and proliferation: The induced embryogenic callus was cultivated on MS, amended with optimized levels of NAA (1.0 mg l−1) and BAP (0.5 mg l−1) for induction and proliferation of embryos (Junaid et al. 2006). The embryos were formed in large numbers in this medium, thus, this stage is henceforth referred to as induction and proliferation stage. The proliferated embryo was processed for alkaloid extraction purposes and parts of the proliferated embryos were cultured for embryo maturation and germination purposes.
Somatic embryo maturation was done in medium containing 3 % maltose and 1.0 mg l−1GA3 (Junaid et al. 2006). Matured embryos were oven-dried for extraction of alkaloids and matured somatic embryos were placed on MS containing 60 g l−1 maltose and optimized level of BAP (0.5 mgl−1) for germination (Junaid et al. 2006). Within 7–10 days the somatic embryos started to germinate and producing plantlets. These stages are called as maturation and germination stages. The in vitro developed tiny shoots and leaves were separately harvested and dried for alkaloid quantification processes.
Different stages of in vitro grown tissues were subject to NaCl treatments ranging from 0 to 200 mM. Callus biomass growth was very poor and was observed toxic at 150 mM (NT6) and onwards, thus, NaCl levels up to 125 mM were only selected for continuation of future sets of investigations to assess the impact of NaCl on cultivated tissues and later on alkaloid quantification processes. The treatments were designated as control, NT1, NT2, NT3, NT4, and NT5 (0, 25, 50, 75, 100 and 125 mM respectively).
Quantification of fresh- and dry-weight
To determine the callus biomass i.e. fresh- and dry-weight, the calli of various stages of growth were taken and analysed. For fresh weights, the calli were taken out from culture medium (with and without NaCl added) and were weighed at a regular interval of 2 weeks (3, 5 and 7 weeks). The calli were dried at 60 °C for 18 h prior to dry weight analysis.
Biochemical analysis
Total soluble protein
The total soluble protein content of embryos was estimated following Bradford’s method (1976). Fifty (50) mg samples fresh weight (FW) were homogenized in 2.0 ml of 0.1 M phosphate buffer (pH 7.5) at 4 °C using pre-chilled mortar and pestle. The homogenate was centrifuged at 5,000 rpm for 10 min at 4 °C. The supernatant was mixed with equal amount of chilled 10 % Trichloro acetic acid, again centrifuged at 3,300 rpm for 10 min. The supernatant was discarded and the pellet was dissolved in 1.0 ml of 0.1 N NaOH after washing with acetone. To 0.1 ml of aliquot, 0.5 ml Bradford’s reagent was added. The absorbance was measured at 595 nm on a spectrophotometer and the protein content was calculated using the standard curve of bovine serum albumin and expressed in mg g−1 FW.
Proline content
Proline content was determined by the method of Bates et al. (1973). About 0.5 g of fresh leaf was homogenized in 10 ml of 3.0 % sulpho-salicylic acid and centrifuged at 5,000 rpm for 10 min. The mixture containing 2.0 ml each of supernatant, acid ninhydrin reagent and glacial acetic acid was boiled at 100 °C for 30 min in a water bath. It was kept in an ice bath for stoppage of reaction and later 4.0 ml of toluene was added to each sample. The toluene (upper) layer was read at 520 nm on a UV–Vis spectrophotometer.
Antioxidant enzyme assays
Superoxide dismutase (SOD) activity
The method of Dhindsa et al. (1981) was followed with slight modification for estimating SOD activity. Tissues from different developmental stages (0.1 g) were homogenized in 2.0 ml of extraction mixture containing 0.5 M of sodium phosphate buffer (pH 7.3), 3.0 mM EDTA, 1.0 % PVP, 1.0 % Triton X 100, and centrifuged at 10,000 rpm at 4 °C. The SOD activity in the supernatant was assayed by its ability to inhibit the photo chemical reduction. The assay mixture contained 1.5 ml reaction buffer, 0.2 ml of methionine, 0.1 ml of enzyme extract with equal amount of 1.0 M NaCO3, 2.25 mM NBT solution, 3.0 mM EDTA, riboflavin; 1.0 ml of double distilled water was taken in test tubes, which was incubated under light (15 W influorescent lamp) at 25 °C for 10 min. Fifty % reduction in the colour was considered as one unit of enzyme activity expressed in enzyme unit (EU) mg−1 protein h−1.
Ascorbate peroxidase (APX) activity
The APX activity was estimated following Nakano and Asada method (1981). Fresh plant material (0.1 g) was ground in 2.0 ml of extraction buffer (0.1 M Na-phosphate, pH 7.0, 3.0 mM EDTA, 1.0 % PVP, 1.0 % Triton X 100) and was centrifuged at 10,000 rpm for 20 min. APX activity was determined in the supernatant by decrease in absorbance at 290 nm, due to its enzymatic breakdown. One ml of reaction buffer contained 0.5 mM ascorbate, 0.1 mM H2O2, 0.1 mM EDTA and 0.05 ml of extract containing enzyme. The reaction was run for 5 min at 25 °C. The APX activity was calculated by using coefficient of absorbance 2.8 mM−1 cm−1. One unit of enzyme determines the amount necessary to decompose 1.0 μmol of ascorbate per min.
Catalase (CAT) activity
CAT activity was determined by the method of Aebi (1984). Fresh plant material (0.1 g) was ground in 2.0 ml of extraction buffer (0.5 M Na-phosphate, pH 7.0, 3.0 mM EDTA, 1.0 % PVP, 1.0 % Triton X 100), centrifuged at 10,000 rpm for 20 min at 4 °C. CAT activity in supernatant was determined by monitoring the disappearance of H2O2, measuring a decrease in absorbance at 240 nm. Reaction was run in a final volume of 2.0 ml of reaction buffer (0.5 M Na-phosphate, pH 7.3) containing 0.1 ml 3.0 mM EDTA, 0.1 ml of enzyme extract and 0.1 ml of 3.0 mM H2O2 for 5 min. The CAT activity was calculated by using coefficient of absorbance of 0.036 mM−1 cm−1. One unit of enzyme determines the amount necessary to decompose 1.0 μmol of H2O2 per min.
Glutathione reductase (GR) activity
GR activity was determined by the method of Foyer and Halliwell (1976), modified by Rao (1992). Fresh leaf material (0.5 g) was ground in 2.0 ml of extraction buffer (0.1 M Na-phosphate, pH 7.0, 3.0 mM EDTA, 1.0 % PVP, 1.0 % Triton X 100) was centrifuged at 10,000 rpm for 10 min. The supernatant was immediately assayed for GR activity through glutathione-dependent oxidation of NADPH at 340 nm. One milli liter reaction mixture contained 0.2 mM NADPH, 0.5 mM glutathione disulfide (GSSG) and 0.05 ml of enzyme extract, kept for 5 min at 25 °C. Corrections were made for any GSSG oxidation in the absence of NADPH. The activity was calculated using coefficient of absorbance of 6.2 mM−1 cm−1. One unit of enzyme determines its amounts necessary to decompose 1.0 μmol of NADPH per min.
Quantification of vincristine and vinblastine by HPTLC
Sample preparation for vinblastine and vincristine estimation
The plant tissue extraction and the quantification of alkaloids described earlier, was followed (Miura et al. 1988; Junaid et al. 2010, 2011). In short, 1 g (1.0) of harvested plant tissue of C. roseus was dried separately at 45 °C for a week and pulverized in a mortar and pestle. Dried materials of powder drug (1 gm) were shaken in 50 ml methanol for 24 h, evaporated up to 2.0 ml; and later 20 ml of (0.5)N H2SO4 (Hi-media Lab. India) was added. The solution was made alkaline by the use of 25 % ammonium hydroxide (Hi-media Lab., India, pH 9–12); extraction was made three times with chloroform (Hi-media Lab., India) and finally evaporated up to dryness. The residue was reconstituted in 4.0 ml methanol (Hi-media Lab., India) and the methanol soluble fraction of each sample was further diluted to 1:10 and processed for HPLC.
Standard curve preparation from standard alkaloids
Two milli gram of vincristine and vinblastine of over 99 % purity available from Sigma Aldrich (St. Louis, MO, USA) were prepared in the same way as that of plant sample. The alkaloids were prepared in methanol and 1.0 ml of it was diluted to 10 ml with methanol to get 100 µg−ml solutions. The UV spectrum of standard vincristine and vinblastine solution in methanol (50 mg−ml) was recorded using a UV spectrophotometer (Shimadzu, Japan). The λmax (220 nm) obtained was matched with that of standard alkaloids reported earlier (Chu et al. 1996). From stock solutions, a dilution of 2–20 µl were taken in duplicates and a standard curve was plotted between concentration and peak area with good linearity. The peak area versus vincristine/vinblastine concentration was calculated by linear least-square regression. The regression equation was used in quantifying vincristine and vinblastine levels in different in vitro cultivated tissue materials.
Application of spots on TLC and development of chromatogram
The various samples (aqueous methanolic extracts of in vitro cultures and aqueous methanolic extract of standard vinblastine and vincristine) were spotted in TLC (4 μl each) with microlitre syringe in triplicate in order to reconfirm the presence of alkaloids in samples. Linomat V spotting device in the form of band (6 mm) was made above 10 mm from the lower edge of plate.
The spotted plates were kept in chromatographic chambers [Twin through chamber (CAMAG) 20 × 10 cm] containing the solvent system. The chambers were carefully covered with glass plates. The solvent system was allowed to ascend up to 7.0 cm after which the plates were taken out, marked and allowed to dry at room temperature. Densitometry scanning was performed on Camag TLC scanner V.
HPTLC instrumentation and conditions
The samples were spotted as bands of width 6.0 mm with CAMAG microlitre syringe on precoated silica gel aluminium plate 60F-254 (20 cm × 10 cm with 0.2 mm thickness, E. Merck, Germany) using a CAMAG Linomat V (Switzerland). A constant 80 ml s−1 application rate was employed and a 5.2 mm space was kept between two bands; a linear ascending development was carried out in twin through glass chamber saturated with mobile phase. The optimized chamber saturation time for mobile phase was 10 min at room temperature. The length of chromatogram run was 65 mm subsequent to the development; TLC plates were dried with air current provided by an air-dryer. Densitometric scanning was performed on CAMAG TLC scanner V in the absorbance mode at 280 nm for vincristine and 300 nm for vinblastine. The source of radiation utilized was tungsten lamp. The slit dimension was kept 6 mm × 0.1 mm, and 10 mm s−1 scanning speed was employed.
Statistical analysis
The data recorded from different experiments were subjected to statistical analysis to verify the degree of reproducibility of responses. The effects of PGRs, NaCl treatments on callus growth, embryogenesis, differences in biochemical attributes, the activity of antioxidants enzymes and the alkaloids yield were analyzed by one-way analysis of variance (ANOVA). The values are means of three replicates from two experiments, and the presented mean values were separated using Duncan’s Multiple Range Test (DMRT) at P ≤ 0.05.
Results
Effect of NaCl on callus biomass
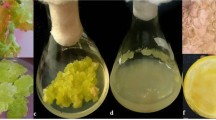
The hypocotyls derived embryogenic calli were subject to various levels of NaCl (Fig. 1a, b). The calli were sub cultured at a regular interval of three, five and 7 weeks and the callus biomass was monitored. The addition of NaCl in medium restricted callus growth in almost all tested concentrations and the influenced was more pronounced on enhanced level of NaCl (Fig. 2). Compared to control, a 10 % reduction in fresh weight and a 9 % in dry weight were observed after 3 weeks of incubation on exposure of embryogenic callus to 25 mM NaCl. Maximum decrease in callus biomass was observed in 125 mM of NaCl amended medium. At high concentration of NaCl (150 mM or above) the embryogenic callus turned brown, became necrotic therefore these high salt treatments were discontinued for future sets of experiments.
Curve plot showing embryogenic callus biomass and growth under various NaCl concentrations. Initial 0.25 gm of embryogenic callus was inoculated on MS, supplemented with optimized 1.0 mg l−1 2,4-D. [C: Control; NT1: 25; NT2: 50; NT3: 75; NT4: 100; NT5: 125 and NT6: 150 mM]. Values are means ± standard errors of three replicates; within each column means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT. FW fresh weight, DW dry weight
NaCl treatments and somatic embryo (SE) formation
The embryogenic calli were cultured on optimized embryo proliferation medium containing varied concentrations of salt to study their effect on embryo proliferation and subsequent embryo development processes. On NaCl free medium, the embryogenic calli demonstrated fairly good embryo forming ability showed an average number of 99.25 SEs per culture. At 25 mM NaCl, the SE numbers were equally high i.e. 102.69/culture Above 25 mM salt stress situations, the embryo numbers gradually decreased (Fig. 3).
Shows the effect of NaCl on embryogenesis and embryo numbers, 50 mg embryogenic callus was inoculated on MS amended with optimized 1.0 mg l−1 NAA and 1.5 mg l−1 BA. Data were scored after 6 weeks of culture. [C: Control; NT1: 25; NT2: 50; NT3: 75; NT4: 100; NT5: 125 mM.] Values are means ± standard errors of three replicates; within each column means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
Salt stress, embryo maturation and plantlets development
White cotyledonary somatic embryos were cultured on 1.0 mg l−1 GA3 added MS. The medium was also amended with varying levels of NaCl. Somatic embryo turned green and elongated in control and low levels of NaCl (Fig. 1c, d), while higher salinity levels were noted to be unfavourable for somatic embryo development. Later, matured somatic embryos were cultivated on MS, amended with optimized BAP (0.5 mg l−1) and varying levels of NaCl for germination. About 70 % somatic embryos germinated and produced plantlets (Fig. 1e, f) in control and low NaCl added MS medium, showing well developed shoot and root system. In NaCl added medium especially at higher levels, the frequency of obtaining plantlets was rather low compared to NaCl free medium.
Salt stress and biochemical attributes
Soluble protein, sugar and proline content
The increasing salt stress induced higher accumulation of soluble protein viz. a 11.9 % protein increase was observed at NT1 as compared to control, while at NT5 the increase was 23 % at SE initiation stage. Similar protein increasing trend was noted in other morphogenetic stages e.g. at embryo germination stage, the protein content was 4.65 mg gm−1 FW in salt free culture, protein accumulation reached to a maximum of 5.10 mg gm−1 FW at NT5 i.e. 125 mM NaCl added medium (Fig. 4a).
Protein (a), sugar (b) and proline (c) content in different stages of somatic embryos in C. roseus treated with different NaCl treatments [C: Control; NT1: 25; NT2: 50; NT3: 75; NT4: 100; NT5: 125 mM]. Values are means ± standard errors of three replicates; within each column means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
The addition of NaCl also improved total sugar level and this increase was more in initiation SE stage compared to matured and germinated embryos. Soluble sugar content increased linearly with increasing salt level up to NT4, further increase however, reduced sugar content (Fig. 4b). In present study, the experiments were designed in order to investigate the influence of salt stress on proline at various embryogenic stages and the data are presented in Fig. 4c. An increase in proline was observed in all morphogenetic stages as the concentration of NaCl increased, being maximum in NT5 and minimum in NT1.
Salt stress and antioxidant enzymes’ activities
SOD, APX, CAT and GR activities
The alterations of enzyme activities under adverse environmental conditions are considered to be an important area of study in assessing oxidative stress in cells. In Table 1, the effect of salt concentrations on SOD activity in different in vitro cultivated tissues was presented as this enzyme is involved in balancing the intracellular concentration of H2O2. The enhancement of SOD activity was nearly linear with increased salinity in medium. At somatic embryo initiation stage, SOD activity was 3.01 EU min−1 mg−1 protein at control while in 25 mM NaCl (NT1) added medium, the level improved marginally (3.12 EU min−1 mg−1 protein). There was almost linear increase of SOD activity with increasing level of NaCl, maximum being at proliferation stage of embryos, which showed a maximum of 4.97 EU min−1 mg−1 proteins in125 mM NaCl added medium.
The activity of APX in different in vitro raised tissues of C. roseus was measured under different salt concentrations and was presented in Table 2. An increase in APX activity was observed as a result of external application of NaCl treatments. APX activity increased significantly in embryo initiation stage with maximum of 1.54 EU min−1 mg−1 proteins at NT5. Similar to APX, CAT protects cells against the destructive influence of H2O2 by catalysing its decomposition through oxidation of phenolic and endiolic co-substrates. So CAT activity was examined at different stages of embryo development. As with APX, the CAT activity also increased with enhanced levels of NaCl in medium. The maximum activity (4.89 EU min−1 mg−1 proteins) was observed at initiation stage in NT5 (Table 3). GR regulates the Glutathione/glutathione disulfide (GSH/GSSG) ratio in the cell. GR is involved in recycling of GSH and provides a constant intracellular level of GSH. A similar, though relatively very low GR activity was found in salt free control and in cultures added with low NaCl concentrations. As the salinity increased, a corresponding increase in GR was observed, maximum being at NT5 with 1.20 EU min−1 mg−1 proteins was recorded in SE initiation stage; and a minimum of 0.75 EU min−1 mg−1 proteins at NT1 (Table 4).
Salt stress and alkaloids (vinblastine and vincristine) synthesis
The added NaCl levels on medium induced osmotic stress in cultivated tissues and altered the synthesis of vinblastine and vincristine (Fig. 5a, b). In salt free culture, vinblastine content was low at early initiation stage (1.73 μgm gm−1 DW), while in SE regenerated leaves the yield was relatively high (13.30 μgm gm−1 DW). In salt added medium NT1, the vinblastine yield was even better in leaves (14.17 μgm gm−1 DW), followed by NT2, the yield was 13.45 μgm gm−1DW (Fig. 6a). The level dropped significantly at higher concentrations of NaCl. Among the different SE stages, maximum vinblastine content was noted in germinating embryos. The yield was below detectable range at early SE stage in NT4 and NT5.
a Vinblastine content (μg gm−1 dry weight) and b vincristine content (μg gm−1 dry weight) in NaCl treated cultures in C. roseus. C: Control; NT1: 25; NT2: 50; NT3: 75; NT4: 100; NT5: 125 mM. Values are means ± standard errors of three replicates; within each column means followed by the same letter are not significantly different at p ≤ 0.05 according to DMRT
The in vitro cultivated tissues amended with NaCl showed impact on vincristine accumulation and a comparative account of yield obtained in different tissues is presented in Fig. 6b. The leaves were noted to be the most important source of alkaloid; and in NT1 maximum i.e. 5.12 μg gm−1 DW of vincristine was obtained compared to salt-free medium (4.69 μgm gm−1 DW). Increased salt concentrations (NT4 and NT5) were noted to be inhibitory especially at initiation and proliferation stage of tissues.
Discussion
Cultivation of plant cells is a useful technique for the study of responses to environmental stress at cellular level (Zhu 2001). In the present investigation, the influence of salt (NaCl) on callus biomass and in vitro embryogeny was conducted and evaluated in C. roseus. The alkaloid (vinblastine and vincristine) yield was also quantified in response to stress in different in vitro grown tissues. Study revealed that the addition of high concentrations of NaCl inhibited cell growth and reduced callus biomass. This reduction of growth may be due to excess availability of Na+ ion, which created osmotic imbalance in medium (Niknam et al. 2006). Similar responses were earlier reported in other plants like Suaeda nudiflora (Cherian and Reddy 2003), Trigonella species (Niknam et al. 2006), Lycopersicon species (Shibli et al. 2007), Medicago truncatula (Elmaghrabi et al. 2013) and even in C. roseus (Elkahoui et al. 2005: Saiman et al. 2014). The soluble salts perhaps decreased the availability of water to plants by diminishing free energy or high levels of salts showed negative influence on plant growth through the toxicity of one or more specific ions (Arshi et al. 2002). More often, plant biomass is inhibited by an excess of solute taken up by plants from the saline growth medium. Sodium accumulation in tissues is considered to be a major reason behind the adverse effect of salinity on nutrient uptake and growth (Shibli et al. 2001). Higher salinity levels in external medium are known to affect various physiological and metabolic processes, cause osmotic stress and water removal from the cytoplasm resulting in a reduction of cytosolic and vacuolar volume of cells (Ashraf and Harris 2004; Ramakrishna and Ravishankar 2011). Growth reduction has been described in several other NaCl-treated cell lines, for instance in P. sativum calli, adapted to 85.5 mM NaCl, a 65 % reduction of dry weight was registered compared with sensitive calli (Olmos et al. 1994). In NaCl tolerant citrus lines, addition of 170 mM NaCl reduced growth by fivefold when compared to control (Piqueras et al. 1996). Osmotic stress has been known to be a good trigger for the induction of embryogenic tissues in several studied plant materials (Benkirane et al. 2000). In Ipomea batatas, the addition of NaCl improved multiplication by producing quality, hardy somatic embryos (Mukherjee 2002). Here, lower concentration of NaCl (25 mM) improved embryo proliferation in medium. This observation is in accordance with results obtained in a number of previous reports dealing with salt-stress (Kawana and Sasamoto 2008). Different reports suggest that salt treatments had positive effects on plant regeneration of select callus lines, developed on a salt-free medium (Ghosh et al. 2006) and salt stress also showed a positive stimulatory role on embryogenic process (D’onofrio and Morini 2002).
The effect of NaCl stress on various biochemical parameters was evaluated in C. roseus. Increase in soluble protein content in response to increased salt stress was reported in culture in almost all investigated plant species (Cusido et al. 1987; D’Souza and Devaraj 2010). The alteration of soluble N-containing compounds such as amino acids, polyamines and soluble proteins in response to salt could be a crucial mechanism in adaptation of plant tissues against stress (Rai 2002; Mittler 2002). In the present study, extra proline accumulation was noted in salt amended embryogenic tissues. Similar proline accumulation in response to stress was earlier reported in cytosol in several investigated observations (Yamaguchi and Blumwald 2005; Hariadi et al. 2011) and is reported to act as osmo-regulator in protecting cells against osmotic perturbation (Mattioni et al. 1997; Elmaghrabi et al. 2013). Proline is produced from a precursor glutamate by the active participation of two enzymes, c-glutamyl kinase and glutamate-5-semialdehyde dehydrogenase (Chen et al. 2009). The over accumulation of proline during stress is due to up-regulation of these biosynthetic genes, thus the measurement of proline level and the expression of proline synthesis gene (P5CS) have been suggested to be a good indicator for monitoring stress condition (Silva-Ortega et al. 2008). In this study, salt added tissues accumulated more sugar than the salt free cultures and there was a gradual increase in soluble carbohydrate in cultured tissues as the NaCl concentration increased. The accumulation of soluble carbohydrates with salinity was previously reported in other plants where sugar seems to be associated with osmotic adjustment (Hamada and Khulaef 1995; Watanabe et al. 2000; Daneshmand et al. 2010). This observation of more soluble sugar accumulation in response to stress is in sync with previous reports (Nunez et al. 2003; Jaleel et al. 2007; Vinayak et al. 2011; Elmaghrabi et al. 2013). Beside the accumulation of physiological reserves (protein, proline and soluble sugar), recent molecular studies reveal increased Salt Overly Sensitive 1 (SOS1) expression is an important element in stress adaptation, which is perhaps more active at low 25/50 mM NaCl levels. It is also known that the external NaCl (high or low) application has a direct effect on endogenous Na+ level, which is necessary to maintain an optimal cytosolic Na+/K+ homeostasis (Türkan and Demiral 2009; Misic et al. 2012) helped in accelerating callus growth under salt stress (Elmaghrabi et al. 2013).
NaCl induced a dose dependent increase in SOD activity in C. roseus callus which could represent a defense mechanism against NaCl induced generation of superoxide anion (O−), hydroxyl radical (.OH), singlet oxygen (O2). Increased activity of SOD under stress condition was earlier reported in many other plants (Cherian and Reddy 2003; Elkahoui et al. 2005; Samar et al. 2011). The CAT and APX activity also showed a progressive increase with increasing salinity in medium. NaCl increased APX and CAT activity in embryogenic callus in C. roseus, indicates that these cells have a higher efficiency to scavenge H2O2 generated by SOD, which may be required for preventing the peroxidation of membrane lipids, generated by salt stress (Hernandez et al. 2000; Cherian and Reddy 2003; Niknam et al. 2006).
Low salt levels (NT1 and NT2), especially NT1 had a great impact on vinblastine and vincristine yield where maximum alkaloid yield was recorded whilst high level induced poor alkaloids accumulation. The same 25/50 mM NaCl levels were also observed to be very responsive for increased callus growth. This fast growth of callus may be due to rapid mitosis of cells caused by over/up-regulation of cell cycle gene, WEE1 as was observed in Arabidopsis (Sorrell et al. 2002). De Schutter et al. (2007) noted that this gene is expressed strongly in rapidly dividing cells at DNA replication and DNA damage checkpoints, the concept is in contrast to opposite perception i.e. WEE1 encoded protein kinase is a negative regulator, suppressing cell division by inhibiting CDK/cyclin complexes (Rhind and Russell 2000; Bourdon et al. 2010). Very similar to this, increased solasodine synthesis was reported from in vitro grown Solanum nigrum culture in response to salt stress (Bhat et al. 2008). The increase of alkaloids may be due to perceived stress signals, created by NaCl (at low levels) activates signal transduction pathways by promoting transcriptional activation of cascade genes like SOS1, somatic embryogenesis receptor-like kinase (SERK) whose expression are up-regulated in embryogenenic calli (Hu et al. 2005; Zhang et al. 2010; Elmaghrabi et al. 2013). Molecular mechanisms reveal that the coordinated expression of genes (stress-related-, somatic embryogenesis related- and biosynthetic genes), often at its maximum in stress (NaCl amended) conditions, emerges as a major regulating mechanism for over accumulation of secondary metabolites (Nimchuk et al. 2003; Dutta et al. 2007; Ma et al. 2012; Elmaghrabi et al. 2013). The molecular role of these encoded proteins though not known fully NaCl-mediated enhancement would be a good approach in secondary metabolite synthesis.
References
Abdel-Hady MS, Okasha EM, Solimaan SSA, Talaat M (2008) Effect of gamma radiation and gibberellic acid on germination and alkaloid production in Atropa belladonna L. Aust J Basic Appl Sci 2:401–405
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Arshi A, Abdin MZ, Iqbal M (2002) Growth and metabolism of senna as affected by salt stress. Biol Plant 45:295–298
Ashraf M, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166:3–16
Bates LS, Waldron RP, Teare LD (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benkirane H, Sabounji K, Chlyah A, Chlyah H (2000) Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell Tissue Org Cult 61:107–113
Bhat MA, Ahmed S, Aslam J, Mujib A, Mahmooduzzafar (2008) Salinity stress enhances production of solasodine in Solanum nigrum L. Chem Pharm Bull 56:17–21
Bourdon M, Frange N, Nafati M, Mathieu E, Cheniclet C, Renaudin J-P, Chevalier C (2010) Endoreduplication and growth of fleshy fruits. In: Lu¨tgge U, Beyschlag W, Bu¨del B, Francis D (eds) Progress in botany. Springer, Heidelberg, pp 101–132
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein dye binding. Anal Biochem 72:248–252
Chen J-B, Wang S-M, Jing R-L, Mao X-G (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166:12–19
Cherian S, Reddy MP (2003) Evaluation of NaCl tolerance in callus cultures of Suaeda nudiflora Moq. Biol Plant 46:193–198
Cheruvathur MK, Thomas TD (2014) High frequency multiple shoot induction from nodal segments and rhinacanthin production in the medicinal shrub Rhinacanthus nasutus (L.) Kurz. Plant Growth Reg 74:47–54
Chu I, Bodnar JA, White EL, Bowman RN (1996) Quantification of vincristine and vinblastine in Catharanthus roseus plants by capillary zone electrophoresis. J Chromatogr 755(2):281–288
Cusido RM, Pelazon J, Altabella T, Moralles C (1987) Effect of salinity on soluble protein, free amino acid and nicotine content in Nicotaina rustica L. Plant Soil 102:55–60
D’Onofrio C, Morini S (2002) Increasing NaCl and CaCl2 concentrations in the growth medium of Quince leaves; I. Effects on somatic embryo and root regeneration. In Vitro Cell Dev Biol Plant 38:366–372
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353
Daneshmand F, Arvin MJ, Kalantari KM (2010) Physiological responses to NaCl stress in three wild species of potato in vitro. Acta Physiol Plant 32:91–101
De Schutter K, Joubes J, Cools T, Verkest A, Corellou F, Babiychuk E, Der Schueren E-V, Beeckman T, Kushnir S, Inze D, De Veylder L (2007) Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19:211–225
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Dicosmo F, Misawa M (1985) Eliciting secondary metabolism in plant cell cultures. Trends Biotechnol 3:318–322
Dutta A, Sen J, Deswal R (2007) Down regulation of terpenoid indole alkaloid biosynthetic pathway by low temperature and cloning of a AP2 type C-repeat binding factor (CBF) from Catharanthus roseus (L). G. Don. Plant Cell Rep 26:1869–1878
Elkahoui S, Hernandez JA, Abdelly C, Ghrir R, Limam F (2005) Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci 168:607–613
Elmaghrabi AM, Ochatt S, Rogers HJ, Francis D (2013) Enhanced tolerance to salinity following cellular acclimation to increasing NaCl levels in Medicago truncatula. Plant Cell Tiss Organ Cult 114:61–70
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Ghosh S, Ghosh B, Jha S (2006) Aluminium chloride enhances colchicine production in root cultures of Gloriosa superba. Biotechnol Lett 28:497–503
Hamada AM, Khulaef EM (1995) Effects of salinity and heat-shock on wheat seedling growth and content of carbohydrates, proteins and amino acids. Biol Plant 37:399–404
Hariadi Y, Marandon K, Tian Y, Jacobsen S-E, Shabala S (2011) Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Bot 62:1–9
Hernandez JA, Jimenez A, Mullineaux PM, Sevilla F (2000) Tolerance of pea (Pisum sativum L.) to long term salt stress is associated with induction of antioxidant defenses. Plant Cell Env 23:853–862
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Jaleel CA, Gopi RP, Panneerselvam R (2007) Antioxidative potentials as a protective mechanism in Catharanthus roseus (L.) G. Don. plants under salinity stress. Turk J Bot 31:245–251
Junaid A, Bhat MA, Mujib A, Sharma MP (2006) Somatic embryo proliferation, maturation and germination in Catharanthus roseus. Plant Cell Tissue Org Cult 84:325–332
Junaid A, Mujib A, Sharma MP (2010) Variations in vinblastine production at different stages of somatic embryogenesis, embryo and field grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. In Vitro Cell Dev Biol Plant 46:348–353
Junaid A, Mujib A, Sharma MP (2011) Influence of freezing and non-freezing temperature on somatic embryogenesis and vinblastine production in Catharanthus roseus (L.) G. Don. Acta Physiol Plant 33:473–480
Kawana Y, Sasamoto H (2008) Stimulation effects of salts on growth in suspension culture of a mangrove plant, Sonneratia alba, compared with another mangrove, Bruguiera sexangula and non-mangrove tobacco BY-2 cells. Plant Biotechnol 25:151–155
Lovkova MY, Buzuk GN, Sokolova SM, Buzuk LN (2005) Role of elements and physiologically active compounds in the regulation of synthesis and accumulation of indole alkaloids in Catharanthus roseus L. Appl Biochem Microbiol 41:299–305
Ma J, He Y, Hu Z, Xu W, Xia J, Guo C, Lin S, Cao L, Chen C, Wu C, Zhang J (2012) Characterization and expression analysis of AcSERK2, a somatic embryogenesis and stress resistance related gene in pineapple. Gene 500:115–123
Mattioni C, Lacerenza NG, Troccoli N, De Leonardis AM, Di Fonzo N (1997) Water and salt stress-induced alterations in proline metabolism of Triticum durum seedlings. Physiol Plant 101:787–792
Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Durickovic M, Nikolic M (2012) Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tissue Org Cult 108:389–400
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 9:405–410
Miura Y, Hirata K, Miyamoto K, Uchida K (1988) Formation of vinblastine from multiple shoot culture of Catharanthus roseus. Planta Med 54:18–20
Moreno PRH, Van der Heijden R, Verpoorte R (1995) Cell and tissue cultures of Catharanthus roseus: a literature survey. II. Updating from1988 to 1993. Plant Cell Tissue Org Cult 42:1–25
Moreno-Valenzuela OA, Minero-Gracia Y, Chan W, Mayer-Geraldo E, Carbajal E, Loyola-Vargas VM (2003) Increase in the indole alkaloid production and its excretion into the culture medium by calcium antagonists in Catharanthus roseus hairy roots. Biotechnol Lett 25:1345–1349
Mujib A, Ilah A, Aslam J, Fatima S, Siddiqui ZH, Maqsood M (2012) Catharanthus roseus alkaloids: application of biotechnology for improving yield. Plant Growth Reg 68:111–127
Mukherjee A (2002) Effect of NaCl on in vitro propagation of sweet potato (Ipomoea batatas L.). Appl Biochem Biotechnol 102:431–441
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Org Cult 118:1–16
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nammi S, Boini MK, Lodagala SD, Behara RBS (2003) The juice of fresh leaves of Catharanthus roseus Linn. reduces blood glucose in normal and alloxan diabetic rabbits. BMC Compl Alter Med 3:1–4
Niknam V, Razavi N, Ebrahimzadeh H, Sharifizadeh B (2006) Effect of NaCl on biomass, protein and proline contents, and antioxidant enzymes in seedling and calli of two Trigonella species. Biol Plant 50:591–596
Nimchuk Z, Eulgem T, Holt BF III, Dangl JL (2003) Recognition and response in the plant immune system. Ann Rev Gen 37:579–609
Nunez M, Mazzafera P, Mazorra LM, Siqueira WJ, Zullo MAT (2003) Influence of a brassinosteroid analog on antioxidant enzymes in rice grown in culture medium with NaCl. Biol Plant 47:67–70
Olmos E, Hernandez JA, Sevilla F, Hellın E (1994) Induction of several antioxidant enzymes in the selection of a salt-tolerant cell line of Pisum sativum. J Plant Physiol 144:594–598
Piqueras A, Hernandez JA, Olmos E, Hellin E, Sevilla F (1996) Changes in antioxidant (CT) enzymes and organic solutes associated with adaptation of citrus cells to salt stress. Plant Cell Tissue Org Cult 45:53–60
Rai VK (2002) Role of amino acids in plant responses to stresses. Biol Plant 45:481–487
Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6:1720–1731
Rao MV (1992) Cellular detoxifying mechanisms determine age dependent injury in tropical plants exposed to SO2. J Plant Physiol 140:733–740
Rhind N, Russell P (2000) Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci 113:3896–3899
Roberts MF (1988) Medicinal products through plant biotechnology. In: Robins RJ, Rhodes MJC (eds) MJC Manipulating Secondary Metabolism in Culture. Cambridge University Press, Cambridge, pp 201–216
Saiman MZ, Mustafa NR, Pomahocova B, Verberne M, Verpoorte R, Choi YH, Schulte AE (2014) Analysis of metabolites in the terpenoid pathway of Catharanthus roseus cell suspensions. Plant Cell Tissue Org Cult 117:225–239
Samar F, Mujib A, Samaj J (2011) Anti-oxidant enzyme responses during in vitro embryogenesis in Catharanthus roseus. J Hort Sci Biotechnol 86(6):569–574
Shibli RA, Sawwan J, Swaidat I, Tahat M (2001) Increased phosphorus mitigates the adverse effects of salinity in tissue culture. Comm Soil Sci Plant Anal 32:429–440
Shibli RA, Kushad M, Yousef GG, Lila MA (2007) Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Reg 51:159–169
Silva-Ortega CO, Ochoa-Alfaro AE, Reyes-Aguero JA, Aguado-Santacruz GA, Jime´nez-Bremont JF (2008) Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Physiol Biochem 46:82–92
Sorrell DA, Marchbank A, McMahon K, Dickinson JR, Rogers HJ, Francis D (2002) A WEE1 homologue from Arabidopsis thaliana. Planta 215:518–522
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Vinayak H, Lokhande TD, Nikam VY, Patade ML, Ahire PS (2011) Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L. Plant Cell Tissue Org Cult 104:41–49
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Org Cult 63:199–206
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 12:615–620
Zahid HS, Mujib A (2012) Accumulation of vincristine in calcium chloride elicitated Catharanthus roseus cultures. Nat Prod J 2:307–315
Zhang SG, Zhou J, Han SY, Yang WH, Li WF, Wei HL, Li XM, Qi LW (2010) Four abiotic stress-induced miRNA families differentially regulated in the embryogenic and non-embryogenic callus tissues of Larix leptolepis. Biochem Bioph Res Commun 398:355–360
Zhao J, Zhu WH, Hu Q (2000) Promotion of indole alkaloid production in Catharanthus roseus cell cultures by rare earth elements. Biotechnol Lett 22:825–828
Zhao J, Hu Q, Zhu WH (2001) Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enz Microbiol Technol 28:673–681
Zheng Z, Wu M (2004) Cadmium treatment enhances the production of alkaloid secondary metabolites in Catharanthus roseus. Plant Sci 166:507–514
Zhu JK (2001) Cell signaling under salt, water, and cold stresses. Curr Opin Plant Biol 4:401–406
Acknowledgments
The authors are thankful to Department of Botany and Central Instrumental Facility (CIF), Jamia Hamdard (Hamdard University) for providing laboratory, instruments and other facilities. The authors are also thankful to anonymous reviewers and editor-in-chief, Sergei Ochatt for their comments and suggestions on manuscript. The work, sponsored by University Grants Commission (UGC), New Delhi by providing a Doctoral Fellowship to S.F. is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fatima, S., Mujib, A. & Tonk, D. NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tiss Organ Cult 121, 445–458 (2015). https://doi.org/10.1007/s11240-015-0715-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-015-0715-5