Abstract
Introduction
Cancer-associated thrombosis (CAT) is a significant concern among patients with malignant diseases, leading to increased mortality. While current guidelines recommend primary thromboprophylaxis for venous thromboembolism (VTE) in medium-to-high-risk outpatients, this practice remains controversial. A better understanding of primary thromboprophylaxis is crucial, yet there is a lack of Real-World Evidence (RWE) in Portugal.
Aims
This RWE study aimed to elucidate primary thromboprophylaxis practices among cancer outpatients in Portugal.
Methods
A five-year observational multicentric study in eight Portuguese health institutions enrolled 124 adult cancer outpatients under primary thromboprophylaxis for VTE. The endpoints were CAT, bleeding, cancer progression and death.
Results
High thrombotic risk tumours were prevalent, with 57% (71) of the patients presenting with pancreatic and gastric cancers. Regarding primary thromboprophylaxis, 55% (68) received Low-Molecular-Weight Heparin (LMWH). VTE was presented in 11% (14) of the patients and major bleeding in 2% (2). Vascular compression, elevated D-dimer and previous VTE were significantly associated with VTE occurrence under primary thromboprophylaxis. The Onkotev model was shown to be the best risk assessment model (RAM) in this population (p = 0.007). CAT patients exhibited a lower progression-free survival than non-CAT patients (p = 0.021), while thrombosis did not influence overall survival (p = 0.542).
Conclusion
Primary thromboprophylaxis in medium-to-high-risk cancer outpatients is a safe and effective practice in real-world settings. This study is the first Portuguese RWE on primary thromboprophylaxis, highlighting evidence for improving prophylactic strategies in this population.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The close relationship between malignancy and venous thromboembolism (VTE) is well-established [1]. Up to 20% of cancer patients develop VTE (mainly deep vein thrombosis (DVT) and/or pulmonary embolism (PE)), leading to considerable mortality and a major healthcare burden [2, 3]. Compared with the general population, these patients have a four-to-seven-fold increased risk of VTE events [4]. The condition, known as the Trousseau syndrome, is attributed to factors related to the patient, the tumour and the antineoplastic treatments, setting the pillar for thromboprophylaxis [5, 6].
Over the years, various risk assessment models (RAMs) for cancer-associated thrombosis (CAT) have been proposed. The Khorana score (KS) is the most validated RAM to help select cancer outpatients for primary thromboprophylaxis [7]. The score is directed to those who will start chemotherapy, encompassing clinical and laboratory parameters to categorise patients into three risk categories [8,9,10]. Since KS, several modified RAMs have been created with the addition of new parameters to increase the score sensitivity, mainly for low-risk KS groups. The list includes, among others, the Onkotev model, the Protecht score and the Conko-004 score [11,12,13,14]. These RAMs offer the possibility of better risk stratification [10]. However, they perform differently across different populations. For instance, the Onkotev model seems to be more suitable for the Portuguese population [11, 15].
When deciding on thromboprophylaxis, factors such as VTE risk, bleeding potential (influenced by cancer type, medication and patient’s comorbidities), costs and the impact on the patient's quality of life are pertinent [16, 17]. Unless there is a contraindication, cancer outpatients with a medium-to-high risk of VTE (KS ≥ 2) are candidates for primary thromboprophylaxis [18]. According to randomised trials, prophylactic anticoagulation with Low-Molecular-Weight Heparin (LMWH) can reduce the relative risk of VTE by around 50% [10]. Indeed, it is the safest option in cases involving possible drug interactions and a high anticipated risk of bleeding. Direct oral anticoagulants (DOAC) can also be prescribed if there are no contraindications [19,20,21]. After the recommended six-month period, the decision to continue thromboprophylaxis, whether with DOACs or LMWHs, should be based on the dynamic benefits-to-risk ratio [17, 22,23,24]. Thus, RAMs should be periodically reassessed to accommodate changes in the patient's clinical status [25].
Controversies surround the best use of primary thromboprophylaxis. Few studies have shown that thromboprophylaxis affects overall survival. Furthermore, even under thromboprophylaxis, patients may experience CAT events (6–15%), with some suffering from adverse events, namely bleeding (major bleeding in less than 4% of cases) [4, 19, 20, 26, 27]. A better characterisation of those at increased risk of CAT and bleeding events under primary thromboprophylaxis, and the impact on the patient's quality of life, is crucial to identify those with a better benefits-to-risk ratio. The variability in adherence to guidelines and the lack of data on patient perception contribute to this ongoing debate. The existing evidence mainly stems from clinical trials that often include a selected patient population [7]. Likewise, the few published national data, particularly in Portugal, hampers the ability to comprehend primary thromboprophylaxis practices, adherence rates, and associated outcomes in these populations. Real-world evidence (RWE) is needed to improve clinical decision-making [3, 7, 26]. In this context, an RWE study was designed to investigate the use, effectiveness, and safety of primary thromboprophylaxis among Portuguese cancer outpatients to provide comprehensive insights into the current practices and outcomes of thromboprophylaxis in this population.
Materials and methods
Study cohort description
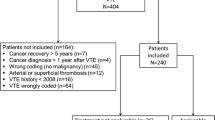
An RWE multicentric cross-sectional study was conducted with cancer outpatients under primary thromboprophylaxis admitted between January 2018 and October 2022 at eight Oncology Departments in Portugal. These health institutions include public and private, peripheral and university tertiary hospitals from the North to the South of Portugal.
The inclusion criteria were: (1) adult patients, (2) histologically confirmed solid tumours, and (3) primary thromboprophylaxis in the ambulatory context. Exclusion criteria were: (1) pregnant or breastfeeding, (2) known haemostatic disorder or (3) anticoagulation for other health reasons. Applying the criteria, a total of 124 patients were enrolled for this study. The decision to start thromboprophylaxis, with which drug and the duration was based on the oncologist’s decision. The patient self-administered the medication after nursing training.
Data concerning the patients' follow-up and demographic and clinicopathological factors at the beginning of systemic treatment (baseline age, sex, performance status (PS), body mass index (BMI), comorbidities, history of previous VTE, use of oral antiplatelet drugs, haemoglobin, leukocytes, platelets and D-dimer levels, primary tumour site, stage, antineoplastic treatments and vascular compression by the tumour) and the systemic (chemotherapy, tyrosine kinase inhibitor (TKI), hormonotherapy, antibody and immunotherapy) and local (radiotherapy and surgery) antineoplastic treatments were collected by reviewing hospital electronic medical records.
Upon enrolling, each patient signed an informed written consent according to the principles of the Helsinki Declaration. The study was approved by the ethics committee of Hospital Center of Trás-os-Montes and Alto Douro (CES. 489/2018, 20th December 2018).
Statistical analysis
Descriptive statistics were used for all objectives. Subgroup descriptive statistics were also tabulated considering distinct groups of patients and treatments.
All categorical variables are presented as numbers and percentages. Given the non-Gaussian distribution of continuous variables, these are presented as the median and interquartile range (IQR). Between-group analysis was conducted using the Mann–Whitney U test for continuous variables and the χ2 test for categorical variables.
Exploratory univariate logistic regression analyses and linear regression analyses were used to find risk factors for events (see Sect. "Study endpoints") in cancer outpatients under thromboprophylaxis. The evaluated variables included the patient’s age, sex, Eastern Cooperative Oncology Group (ECOG) PS, BMI, primary tumour site, vascular/lymphatic compression, previous VTE, level of D-dimer, blood count and platinum and/or gemcitabine-based chemotherapy. These variables were also used to compute the RAMs KS, Onkotev, Protecht and Conko-004. Specifically, the KS score was determined based on the primary tumour site, pre-chemotherapy haemoglobin level, leukocyte and platelet count and BMI [8]. The Onkotev model encompassed previous VTE, metastatic disease, macroscopic vascular/lymphatic compression and a KS > 2 as parameters [11]. The Protecht score was computed by adding information on platinum and/or gemcitabine-based chemotherapy to the KS score [12]. Lastly, the Conko-004 score was determined using the same variables as KS but replacing BMI for PS [13]. The Bonferroni correction was used when necessary. Statistical significance was set at p = 0.05 (two-sided). IBM SPSS Statistics version 23 was the software used for statistical analyses.
Study endpoints
The primary study endpoint was CAT, including confirmed VTE and arterial thromboembolism (ATE), with analysis of its location, conditions of diagnosis (symptomatic or incidental), and date of occurrence. If there was not a clinical suspicion, DVT was not ruled out by duplex compression ultrasonography at the recruitment.
The secondary endpoints were bleeding events, cancer progression and death. Bleeding was classified as minor or major (major being defined as bleeding that demands blood transfusion or that lowers the haemoglobin concentration by more than 2 g/dL) [28, 29]. Cancer progression was defined using the Response Evaluation Criteria in Solid Tumours (RECIST) criteria (version 1.1): ≥ 20% increased sum of diameter of target lesions or progression of non-target lesions or new lesions [29].
Results
Among the patients in the cohort, 53% (66) were male. The median (IQR) age of the enrolled patients was 66.0 (14.0) years (9% (11) of the patients were aged 80 years or older). Regarding PS, 75% (93) of the patients had a good functional status (PS ≤ 1). As for BMI, 10% (12) were underweight and 4% (5) presented a BMI ≥ 35 kg/m2. The most common disease group was cardiovascular (68% (85)), but only a minority used antiaggregant (7% (9)). Concerning previous VTE, 20% (25) of the patients had experienced at least one event. Regarding the existence of vascular/lymphatic compression, it was presented in 32% (40) of the patients. In terms of primary tumour site, 68% (85) of the patients had high thrombotic risk cancers, with pancreatic cancer accounting for 41% (51), gastric cancer for 16% (20) and lung cancer for 1% (14) of cases (Fig. 1). Furthermore, 67% (83) of the patients had stage IV cancer (advanced disease). Most patients underwent systemic treatment, with chemotherapy being the most common (72% (89)), preferably in combination schemes (56% (69)) with platinum salts being the most used agents (53% (66)). The full description of the study population is summarized in Table 1.
Across the entire cohort, the strategy for primary thromboprophylaxis strategy included LMWH for 55% (68) of the patients. Regarding specific anticoagulant drugs, the most used were tinzaparin and rivaroxaban, each in 31% (38) of the patients. The LMWH were the preferred drug among patients with tumours associated with a higher risk of bleeding (gastrointestinal and urologic tract). In this subgroup, 66% (61) of the patients were under LMWH compared with 34% (31) under DOAC (p < 0.001). The opposite was seen among those with the remaining studied tumours. The median (IQR) duration of thromboprophylaxis was 3.8 (4.2) months, without a significant difference when comparing LMWH with DOAC (p = 0.263). There were fewer interruptions of thromboprophylaxis in the DOAC group compared to those under LMWH (21% (10) and 43% (29) of the patients respectively, p = 0.016). The reason for drug interruption was not assessed. The high-profile VTE risk of the population was confirmed through all assessed RAMs, but the one with more patients in this risk category was KS (97% (120) of the patients presented a medium–high KS). The full characterization of thromboprophylaxis, baseline RAM and antineoplastic treatment risk factors is summarized in Table 2.
Concerning the endpoints of the study, 81% (124) of the patients did not experience CAT or bleeding events. CAT occurred in 11% (14) of the patients. The median (IQR) time from the start of prophylaxis to a CAT event was 5.8 (6.0) months (Fig. 2). Among those with CAT, most had VTE (71% (10)), in unusual sites (29% (4)) (not PE, DVT nor catheter-related thromboembolism) and most events were symptomatic (79% (11)). On the other hand, ATE occurred in 3% (4) of the patients. There was no association between the incidence of CAT events and the type of thromboprophylaxis drug used (LMWH or DOAC, p = 0.581).
The median (IQR) time between CAT occurrence and cancer progression was 4.1 (5.1) months. The patients that presented a CAT progressed sooner than CAT-free ones (p = 0.021; Fig. 3). CAT occurrence did not influence overall survival (p = 0.542).
In terms of potential predictive biomarkers, significant associations were found between CAT occurrence and the risk factors D-dimer levels, vascular/lymphatic compression and previous VTE. Specifically, patients who experienced CAT had higher baseline D-dimer levels (p = 0.030), and more often presented vascular/lymphatic compression (p = 0.012) and previous VTE (p = 0.036). Among the different RAMs, the only one that showed a significant association with CAT was Onkotev, with the disease associating with a higher ONKOTEV score (p = 0.007).
As for bleeding events, 91% (113) of the patients did not have a haemorrhage, and when it occurred, it was mostly minor events (82% (9)). The median (IQR) time from the start of prophylaxis to a bleeding event was 4.2 (15.9) months (Fig. 2). The time from bleeding to death was 0.5 (2.5) months, while no association between the two events (bleeding and death) was detected (p = 0.755). Regarding anticoagulation drugs, there was no association between bleeding occurrence and the use of LMWH or DOAC (p = 0.205). The full characterization of the events is in Table 3.
Discussion
CAT poses a significant concern for patients undergoing antineoplastic treatment, often resulting in increased morbidity and mortality. After cancer itself, VTE is the second death cause among patients with malignant diseases. A personalized approach is crucial for improving patient outcomes and reducing the occurrence of adverse events during primary thromboprophylaxis [5]. While current guidelines recommend primary thromboprophylaxis in medium-to-high-risk cancer outpatients, its implementation is still a subject of controversy. To enhance the quality of care, it is crucial to better understand this prophylactic measure in cancer outpatients. Thus, this RWE study aimed to address this knowledge gap.
This study provides valuable primary thromboprophylaxis RWE among cancer outpatients in Portugal. The main goal was to elucidate thromboprophylaxis's management, effectiveness and safety in this population, which can change clinical practice. Another goal was to evaluate the association of CAT and bleeding events under thromboprophylaxis with known and easily accessible markers and RAMs in clinical settings. By identifying predictive biomarkers, it could be possible to create a new RAM to help tailor intervention.
The study cohort presented mostly an elderly population, with concomitant comorbidities, well balanced on sex, with a good PS and healthy BMI. As expected, most patients had high thrombogenic tumours (with the pancreas followed by gastric and lung cancer being the most common) and presented with advanced-stage diseases. The RAM with most patients in higher categories (97% (120)) was KS, which is probably because it was the most used RAM for the decision of thromboprophylaxis. This shows that KS was the preferred RAM for patient selection. However, Onkotev was shown to be the best RAM in this population. In line with this observation, the Onkotev model was previously deemed superior to KS in guiding individualized thromboprophylaxis for VTE in cancer outpatients [30]. These findings support the importance of a case-by-case evaluation and the discussion of CAT with the patients.
Regarding primary thromboprophylaxis, LMWH were the preferred class. As supported in the guidelines, in tumours with a higher risk of bleeding, LMWH is the recommended option. The same applies to patients with a higher risk of drug interactions and other particularities [18, 22, 25, 31, 32]. Interestingly, in this study, tinzaparin (a LMWH) and rivaroxaban (a DOAC) were equally used. As for the duration, the median period of thromboprophylaxis was 5.4 months, following the current recommendation of 6 months. There was no significant difference in CAT or bleeding event rates or in thromboprophylaxis duration when comparing LMWH with DOAC, which might reveal a good drug-patient decision. The drug selection and the duration of thromboprophylaxis show a tendency towards an individualized choice, considering the VTE and bleeding risks provided by the patient, tumour and treatment characteristics.
The thromboprophylaxis was efficient and safe, with most patients (81% (100)) not experiencing an adverse event. Those with a CAT event (11%) had mostly VTE and symptomatic events, as expected based on earlier trials. In opposition, thrombotic events at unusual sites and ATE had higher rates than expected (3%). This is important as healthcare professionals should be aware of the occurrence of CAT events even under thromboprophylaxis and its occurrence at infrequent sites. In the study, most bleeding events were minor, occurred less than expected and were not significantly associated with the patient’s death. Comparing the two events, CAT occurred more often and two months later than bleeding, around the 6th month of thromboprophylaxis. This highlights the importance of the fulfilment of thromboprophylaxis for at least six months with continuous monitoring until the end.
There was a statistically significant association between CAT and known risk factors: elevated D-dimer levels, vascular compression and previous VTE. This shows the positive relation between tumour aggressiveness and thrombo-inflammation. Whether healthcare professionals should prefer the Onkotev model in Iberian populations or a new RAM should be tailored for this population using these easily accessible risk factors from clinical practice is a matter of discussion. Another question is whether a dose increment could be of benefit in the higher-risk subgroup. In sum, this RWE study supports primary thromboprophylaxis as safe and beneficial for cancer outpatients, while also emphasizing the importance of their continuous surveillance. Also, according to the results, this population demands more tailored thromboprophylaxis strategies.
The strengths of this study include its RWE multicentric design. However, there are limitations to consider. First, the study was underpowered to find small effects given the small cohort size and the small number of patients experiencing CAT and bleeding events. Furthermore, the decisions on thromboprophylaxis beginning and interruption, as well as drug choice, were entirely up to the oncologist without any bias of selection, leading to a more heterogeneous sample. Moreover, the presence of VTE was not actively excluded at the beginning of thromboprophylaxis and the bleeding location was not evaluated. Another constraint is that blood and imaging analyses were performed in different hospitals. At last, drug administration was performed at home without direct observation or another type of control. Taken together, this study must be confirmed in further studies with larger and more homogeneous samples to properly answer the raised questions.
In future studies, the establishment of new and better RAMs for patient selection according to the population group, the knowledge of the best drug duration and dose, and the assessment of patients’ perspectives and impact on the quality of life concerning thromboprophylaxis should be evaluated. These studies should also assess international adherence to guidelines, find potential barriers or challenges to implementation, and guide future actions for improving CAT prevention and management. Research efforts should aim to discover more predictive factors for VTE and bleeding under primary thromboprophylaxis. By doing so, more targeted and effective thromboprophylaxis strategies can be developed, increasing clinicians' confidence in this approach, and putting patients in the focus of the decision. Ultimately, ensuring better VTE prevention could improve the quality of life for cancer patients.
Conclusions
To the best of our knowledge, this is the first Portuguese RWE of primary thromboprophylaxis in cancer outpatients. The findings corroborate a positive risk/benefit ratio of thromboprophylaxis in this population. An association of CAT with vascular compression, earlier VTE, D-Dimer levels and cancer progression was detected. These factors, which are easily assessed in clinical practice, could be used to create a new RAM for the very high-risk Portuguese population. Furthermore, the Onkotev model was shown to be the best RAM in this population. These results are valuable for healthcare professionals involved in the management of cancer patients, aiding their clinical decision-making processes and promoting adherence to guidelines. In summary, these findings support primary thromboprophylaxis in medium-to-high-risk cancer outpatients and emphasize the importance of more tailored strategies to improve cancer patient outcomes.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Soff G (2019) Thrombosis and hemostasis in cancer. Scope of the problem and overview. Cancer Treat Res 179:1–9
Khorana AA et al (2022) Cancer-associated venous thromboembolism. Nat Rev Dis Primers 8(1):1–18
Khorana AA et al (2021) Healthcare costs of patients with cancer stratified by Khorana score risk levels. J Med Econ 24(1):866–873
Karamouzis MV et al (2021) The impact of thromboprophylaxis on the survival of patients with advanced pancreatic cancer. The pancreatic cancer and tinzaparin (PaCT) study. Cancers 13(12):2884
Khorana AA (2010) Venous thromboembolism and prognosis in cancer. Thromb Res 125(6):490–493
Apenteng PN et al (2016) Patients’ perceptions and experiences of the prevention of hospital-acquired thrombosis: a qualitative study. BMJ Open 6(12):e013839
Falanga A, Marchetti M (2023) Cancer-associated thrombosis: enhanced awareness and pathophysiologic complexity. J Thromb Haemost 21(6):1397–1408
Khorana AA et al (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood J Am Soc Hematol 111(10):4902–4907
Yan A-R et al (2021) Risk factors and prediction models for venous thromboembolism in ambulatory patients with lung cancer. Healthcare 9(6):778
Khorana AA et al (2008) Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111(10):4902–4907
Cella CA et al (2017) Preventing venous thromboembolism in ambulatory cancer patients: the ONKOTEV study. Oncologist 22(5):601–608
Verso M et al (2012) A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 7(3):291–292
Pelzer U et al (2015) Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 33(18):2028–2034
Moik F et al (2021) Risk assessment models of cancer-associated thrombosis-potentials and perspectives. Thrombosis Update 5:100075
Godinho J et al (2020) ONKOTEV score as a predictive tool for thromboembolic events in pancreatic cancer-a retrospective analysis. Oncologist 25(2):e284–e290
Stashenko G et al (2011) Prophylaxis for venous thromboembolism: guidelines translated for the clinician. J Thromb Thrombolysis 31(1):122–132
Munoz Martin AJ et al (2020) SEOM clinical guideline of venous thromboembolism (VTE) and cancer (2019). Clin Transl Oncol 22(2):171–186
Key NS et al (2020) Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 38(5):496–520
Carrier M et al (2019) Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med 380(8):711–719
Khorana AA et al (2019) Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med 380(8):720–728
Agnelli G (2019) Direct oral anticoagulants for thromboprophylaxis in ambulatory patients with cancer. N Engl J Med 380(8):781–783
Mandalà M et al (2011) Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 22 Suppl 6:vi85-92
Xynogalos S et al (2022) Can thromboprophylaxis build a link for cancer patients undergoing surgical and/or chemotherapy treatment? The MeTHOS cohort study. Support Care Cancer 30(8):6973–6984
Moik F, Pabinger I, Ay C (2020) How I treat cancer-associated thrombosis. ESMO Open 5(1):e000610
Streiff MB et al (2021) Cancer-associated venous thromboembolic disease, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19(10):1181–1201
Christopoulou A et al (2022) Prophylaxis of cancer‑associated venous thromboembolism with low‑molecular‑weight heparin‑tinzaparin: real world evidence. Oncol Lett 23(4):115
Maraveyas A et al (2012) Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer 48(9):1283–1292
Schulman S, Kearon C (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Cella CA et al (2023) Comparison of Khorana vs. Onkotev predictive score to individualize anticoagulant prophylaxis in ambulatory patients with cancer. Blood 142:661
Lyman GH, Kuderer NM (2020) Clinical practice guidelines for the treatment and prevention of cancer-associated thrombosis. Thromb Res 191:S79–S84
Pachón V et al (2018) Cancer-associated thrombosis: beyond clinical practice guidelines—A multidisciplinary (SEMI–SEOM–SETH) expert consensus. TH Open 02(04):e373–e386
Acknowledgements
The authors would like to thank GESCAT, Hospital Center of Trás-os-Montes e Alto Douro, Health Local Unit of Nordeste, Hospital Center of Barreiro Montijo, Hospital Center of Lisboa Ocidental, Hospital of Luz Setúbal, Hospital of Espírito Santo de Évora, Hospital Center of Vila Nova de Gaia / Espinho, University Hospital Center of São João and Fundação para Ciência e Tecnologia (FCT).
Funding
Scholarship program “Trombose & Cancro 2018: Advancing the knowledge of Cancer Associated Thrombosis” supported by GESCAT – Study Group of Cancer Associated Thrombosis, the fund holder being J.-L.P. V.T. is a PhD scholarship holder (2020.08969.BD; https://doi.org/10.54499/2020.08969.BD) supported by Fundação para a Ciência e Tecnologia (FCT), co-financed by European Social Funds (FSE) and national funds of MCTES.
Author information
Authors and Affiliations
Contributions
Conceptualization, J.-L.P.; Methodology: J.L.P., J.G., M.B., J.R., M.M., H.G. and J.R. Writing—original draft preparation: J.-L.P. and V.T. Writing—review and editing: J.-L.P., V.T., C.G., R.G., M.S. and A.A.K; Funding acquisition: J.L.P. Supervision: M.B., A.A.K and R.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Research involving human participants and/or animals
The study was approved by the ethics committee of Hospital Center of Trás-os-Montes and Alto Douro (CES. 489/2018, 20th December 2018).
Informed consent
Patients signed an informed written consent according to the principles of the Helsinki Declaration.
Conflicts of interest
J.-L.P. received funding from LEO Pharma to conduct this investigation. The funder had no role in the collection, analysis, and interpretation of data, writing of the manuscript, or in the decision to submit the paper for publication. The remaining authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bullet Points
1. Thrombosis contributes to elevated morbidity and mortality rates among cancer patients;.
2. Despite the importance of primary thromboprophylaxis, there is a lack of Real-World Evidence;.
3. This five-year observational multicentric study elucidated the practices of primary thromboprophylaxis in Portugal;.
4. Vascular compression, elevated D-dimer and a history of previous VTE were significantly associated with VTE occurrence under primary thromboprophylaxis;.
5. New insights could help refine the guidelines for primary thromboprophylaxis, improving the clinical outcomes of cancer patients.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liz-Pimenta, J., Tavares, V., Gramaça, J. et al. Primary thromboprophylaxis in cancer outpatients – real-world evidence. J Thromb Thrombolysis 57, 805–814 (2024). https://doi.org/10.1007/s11239-024-02984-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-024-02984-1