Abstract
The use of intravenous antiplatelet therapy during primary percutaneous coronary intervention (PPCI) is not fully standardized. The aim is to evaluate the effectiveness and safety of periprocedural intravenous administration of cangrelor or tirofiban in a contemporary ST-segment elevation myocardial infarction (STEMI) population undergoing PPCI. This was a multicenter prospective cohort study including consecutive STEMI patients who received cangrelor or tirofiban during PPCI at seven Italian centers. The primary effectiveness measure was the angiographic evidence of thrombolysis in myocardial infarction (TIMI) flow < 3 after PPCI. The primary safety outcome was the in-hospital occurrence of BARC (Bleeding Academic Research Consortium) 2–5 bleedings. The study included 627 patients (median age 63 years, 79% males): 312 received cangrelor, 315 tirofiban. The percentage of history of bleeding, pulmonary edema and cardiogenic shock at admission was comparable between groups. Patients receiving cangrelor had lower ischemia time compared to tirofiban. TIMI flow before PPCI and TIMI thrombus grade were comparable between groups. At propensity score-weighted regression analysis, the risk of TIMI flow < 3 was significantly lower in patients treated with cangrelor compared to tirofiban (adjusted OR: 0.40; 95% CI: 0.30–0.53). The risk of BARC 2–5 bleeding was comparable between groups (adjusted OR:1.35; 95% CI: 0.92–1.98). These results were consistent across multiple prespecified subgroups, including subjects stratified for different total ischemia time, with no statistical interaction. In this real-world multicenter STEMI population, the use of cangrelor was associated with improved myocardial perfusion assessed by coronary angiography after PPCI without increasing clinically-relevant bleedings compared to tirofiban.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immediate platelet inhibition by adjunctive intravenous antiplatelet therapy may mitigate the pro-thrombotic milieu during primary percutaneous coronary intervention (PPCI) and improve clinical outcome in patients with ST-segment elevation myocardial infarction (STEMI) [1].
Third-generation oral P2Y12 inhibitors (ticagrelor or prasugrel) have a more sustained antiplatelet activity than clopidogrel and represent the first-line therapy together with aspirin in the majority of patients with acute coronary syndromes (ACS) [2, 3]. However, oral agents require several hours to induce an effective platelet inhibition, exposing the patient to the risk of early ischemic events [4, 5]. Intravenous antiplatelet agents have the theoretical advantage of a rapid and sustained action in the first few hours and may overcome the drawbacks of oral therapy [6].
Cangrelor is a non-thienopyridine intravenous P2Y12 inhibitor with the unique pharmacokinetic property of a rapid onset/offset of action. In the CHAMPION PHOENIX trial, cangrelor reduced early ischemic events in patients undergoing urgent or elective PCI compared to clopidogrel, without increasing the risk of major bleedings [7].
Glycoprotein IIb/IIIa inhibitors (GPI; i.e., tirofiban and eptifibatide) have shown to reduce major adverse cardiovascular events compared to heparin alone in patients undergoing PCI for ACS, but these ischemic benefits were partially offset by a significant increase in major bleedings [8, 9]. Unfortunately, these studies did not selectively enroll STEMI patients undergoing contemporary pharmacological and PPCI treatments, including the administration of potent P2Y12 inhibitors and the use of radial access as the standard of care [10].
Owing to the lack of evidence from randomized studies, the optimal use of intravenous antiplatelet therapy during PPCI is not fully standardized. In this study, we aimed to evaluate the effectiveness and the safety of periprocedural intravenous administration of cangrelor or tirofiban in a real-world population of STEMI patients undergoing contemporary pharmacological and PPCI treatments.
Methods
Study population
The INVEST-STEMI (Investigating intravenous antiplatelet therapy in patients with ST-Segment elevation myocardial infarction) is an Italian multicenter registry including STEMI patients treated with intravenous antiplatelet therapy during PPCI at seven Italian centers.
All consecutive STEMI patients undergoing PPCI between January 2020 and January 2022 and receiving either cangrelor or tirofiban according to the physician's decision were prospectively enrolled in the study. STEMI diagnosis was performed according to current guidelines and systematically confirmed by coronary angiography [11, 12].
Patients treated with both cangrelor and tirofiban, if any, were excluded. In all patients, demographic, clinical, laboratory, echocardiographic, angiographic and PCI data were systematically collected and reported in an electronic dataset. The study population was divided into two groups according to the intravenous antiplatelet agent used (cangrelor or tirofiban) during PPCI.
The local Ethical Committees approved the study. The investigation conforms to the principles outlined in the Declaration of Helsinki. All patients were informed of the nature and aims of the study and asked to sign an informed consent for the anonymous management of their data.
Patient and public involvement statement
Eligible patients were asked to participate in the INVEST-STEMI registry after the confirmation of STEMI diagnosis. Participants were not involved in determining the research question or outcome measures, nor were they involved in recruitment, design or implementation of the study. Participants were not asked for advice on the interpretation of results.
Angiographic and procedural features
All the coronary angiograms were systematically reassessed offline by two experienced operators at each center. The extension and the severity of coronary artery disease was expressed as type and number of coronary vessels involved, and by using the SYNTAX (Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac surgery) score (SS) as previously described [13].
For each patient, the infarct-related artery (IRA) was reported. Data on thrombolysis in myocardial infarction (TIMI) flow grade of the IRA at baseline and after PPCI were systematically collected (Online Table 1) [14].
Angiographic coronary thrombus burden was visually evaluated through the TIMI thrombus grade, which was scored from 0 to 5 based on the initial diagnostic angiogram, as previously described (Online Table 2) [15].
PPCI was performed according to the standard techniques. Data on minimum and maximum stent diameter and on the total length of stents implanted in the IRA were collected.
Myocardial blush grade (MBG) at the end of the procedure was assessed using the semiquantitative densitometric method as previously described (Online Table 3) [16, 17]. Successful myocardial reperfusion was defined as MBG grades 2 or 3.
Medications
Intravenous antiplatelet therapy was administered in all cases once the coronary anatomy was defined.
Tirofiban was administered at a 25 μg/kg bolus + 0.15 μg/kg per minute infusion for 2 h (or infusion at 0.075 μg/kg per minute if creatinine clearance was < 30 mL/min). Cangrelor was administered at a 30 μg/kg bolus + 4 μg/kg per minute infusion for 2 h.
Study outcome measures
The primary outcome measure of effectiveness was the angiographic evidence of TIMI flow < 3 after PPCI. The primary safety outcome was the occurrence of clinically-relevant bleedings during the hospitalization, defined as BARC (Bleeding Academic Research Consortium) ranging from 2 to 5 [18].
The secondary outcome measures were TIMI flow < 2 and MBG < 2 after PPCI, the occurrence of BARC 3–5 bleeding during the hospitalization, and in-hospital mortality.
The relative change in left ventricular ejection fraction (LVEF) assessed by transthoracic echocardiography from admission to discharge was considered as an exploratory outcome measure.
Statistical analysis
The normal distribution of continuous parameters was tested with the Kolmogorov-Smirnov test. Normally distributed variables were expressed as mean ± standard deviation (SD), whereas non-normally distributed ones as median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. Continuous normally distributed variables were compared using the Student t-test; continuous non-normally-distributed variables were compared with the Mann-Whitney U test. Categorical variables were compared with the chi-squared test, or Fisher exact test, as appropriate. Ordinal variables were compared with the Kendall’s Tau-c test.
The association between treatments and the study outcome measures was assessed using logistic regression analysis and reported as unadjusted and adjusted odds ratios (OR) with their 95% confidence intervals (CI). We used the propensity score weighting technique to account for potential selection bias in treatment assignment between patients receiving cangrelor or tirofiban (average effect weights). The propensity score model was developed using a non-parsimonious approach and by incorporating a pre-procedural covariates potentially related to the treatment decision and/or outcome regardless of their statistical significance or collinearity with other covariates included in the model [19, 20]. The list of variables included in the propensity score model are reported in Online Table 4. After weighting, a standardized mean difference (SMD) below 0.10, which reflects an optimal balance for all covariates included in the propensity score model, was achieved (Online Fig. 1).
The rate of missing baseline values, if any, is shown in Online Table 5. Missing data were handled using multiple imputations with the method of chained equations. Twenty imputed data sets were generated and combined using Rubin’s rules [21].
A sensitivity analysis was conducted to verify the consistency of the main results for the primary outcomes of the study was investigated in prespecified subgroups of clinical interest: males or females, age ≥ 65 or < 65 years, presence or not of diabetes, presence or not of chronic kidney disease, clinical presentation with or without cardiogenic shock, use of radial or femoral access for PCI, and total ischemia time ranges.
A further sensitivity analysis was conducted to test the association between study treatments and BARC 2–5 bleedings within 48 h.
Heterogeneity between centers of the treatment effect for both primary outcome measures was assessed using the Q-statistic and I2 tests. Significant heterogeneity was considered present for p values < 0.10 or I2 > 50%. For all the other tests, a p-value < 0.05 was considered statistically significant. Analysis was performed using SPSS software version 28.0 (SPSS Inc., Chicago, Illinois) and R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Of 3,556 STEMI patients undergoing PPCI at the participating centers, 646 (18.2%) received intravenous antiplatelet therapy during procedure; 19 patients (2.9%) who received both cangrelor and tirofiban as a bailout strategy were excluded. The final study population included 627 patients (median age 63 years, 79.1% males): 312 (49.8%) received cangrelor and 315 (50.2%) tirofiban. The clinical characteristics of the study population are summarized in Table 1.
Patients treated with tirofiban had a higher prevalence of prior myocardial infarction (MI; 6.8% vs. 15.9%, p < 0.001) and prior PCI (8.5% vs. 16.5%, p = 0.002) compared to patients treated with cangrelor. There was no difference between groups in terms of history of bleeding or atrial fibrillation. The estimated glomerular filtration rate was significantly higher in patients treated with cangrelor compared to those treated with tirofiban (p < 0.001). The percentage of pulmonary edema or cardiogenic shock at clinical presentation, and the need for inotropic agents or mechanical circulatory support during the hospitalization, were not statistically different between groups.
The angiographic and procedural characteristics of the study population are summarized in Table 2. Patients receiving cangrelor had lower ischemia time than patients treated with tirofiban (p = 0.016); however, the proportion of patients with total ischemia time lower than 6 h was comparable between groups. The proportion of P2Y12 receptor blockers preloading was comparable between groups. No difference was reported in terms of vascular access for PPCI between groups. SS score was higher in the tirofiban than in the cangrelor group (p < 0.001). TIMI flow before PPCI and TIMI thrombus grade were comparable between groups. Moreover, there was no difference in terms of minimum stent diameter, total stent length, and number of stents implanted per procedure. The type of lesions treated at the index PPCI was not significantly different between groups.
Study outcomes
TIMI flow < 3 after PPCI was reported in 140 patients (22.3%) and was less frequent in patients treated with cangrelor compared to those treated with tirofiban (14.1 vs. 30.5%, p < 0.001; Table 3). Furthermore, TIMI flow < 2 and MBG < 2 after PPCI were less frequently observed in patients treated with cangrelor.
The percentage of BARC 2–5 bleedings during the hospitalization was comparable between groups. No difference in terms of periprocedural MI, stent thrombosis and in-hospital death was observed between groups.
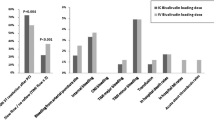
The unadjusted and adjusted OR for the study outcomes are shown in Fig. 1. At propensity score-weighted adjusted regression analysis, there was a lower risk of TIMI flow < 3 in patients treated with cangrelor compared to those treated with tirofiban (adjusted OR:0.40; 95% CI:0.30–0.53). No difference was detected for the risk of BARC 2–5 bleeding between groups (adjusted OR:1.35; 95% CI:0.92–1.98).
A low degree of heterogeneity between centers of the treatment effect was observed for both primary outcomes of effectiveness (I2 = 31.3%; p = 0.188) and safety (I2 = 0%; p = 0.427).
The results for the primary outcomes were consistent across multiple subgroups, with no significant interactions in the propensity score-weighted regression analyses (Online Table 6 and 7). Patients treated with cangrelor also showed a lower risk of TIMI flow < 2 (adjusted OR:0.41; 95% CI:0.24–0.71) and of MBG < 2 (adjusted OR:0.39; 95% CI:0.29–0.52) compared to patients treated with tirofiban.
There was no difference between study treatments for the risk of BARC 2–5 bleedings within 48 h both at unadjusted (OR:1.01; 95% CI:0.55–1.84) and adjusted (adjusted OR:0.98; 95% CI:0.63–1.55) regression analyses.
Patients treated with cangrelor showed a statistically significant improvement in LVEF at discharge compared to patients treated with tirofiban (p = 0.008; Online Fig. 2).
Discussion
The in-hospital outcome of STEMI patients is closely time-dependent and the gap in the onset of action of oral P2Y12 inhibitors may influence the effectiveness of myocardial revascularization during PPCI, particularly in patients at higher thrombotic risk or with compromised gastrointestinal absorption due to hemodynamic impairment [1, 6].
This is the first multicenter study evaluating the effectiveness and safety of cangrelor and tirofiban in a real-world STEMI population. The main findings of the study are as follows:
-
i)
patients treated with cangrelor had a higher chance of successful myocardial reperfusion assessed by coronary angiography indices after PPCI compared to those treated with tirofiban;
-
ii)
the use of cangrelor or tirofiban did not influence the risk of clinically-relevant bleedings during the hospitalization;
-
iii)
the use of tirofiban was preferred over cangrelor in patients with prior MI and prior PCI; cangrelor was preferred in patients with early presentation within 3 h from symptoms onset;
-
iv)
the thrombus burden and the type of coronary lesion did not influence the choice of the intravenous antiplatelet agent during PPCI;
-
v)
patients treated with cangrelor showed a slightly greater improvement of LVEF at discharge.
Current guidelines recommend the use of GPI as a bailout strategy and in the setting of high-risk PCI in patients who have not been pre-treated with P2Y12 inhibitors. On the other hand, cangrelor may be considered in P2Y12 inhibitor-naïve patients [11, 12]. Therefore, the use of GPIs should be preferred in STEMI patients with high thrombotic burden, who have higher chance of no-reflow or thrombotic complications during PPCI. Cangrelor should be used before PPCI, particularly in whom oral therapy with P2Y12 inhibitors is not feasible or desirable [6].
In this study including a contemporary cohort of STEMI patients, preloading with P2Y12 inhibitors was performed in about one fifth of patients, with no difference between cangrelor and tirofiban groups. Clinicians considered feasible the co-administration of cangrelor with P2Y12 receptor blockers in patients with STEMI, as well as the combination with GPI. This result was consistent with an observational study by Grimfjӓrd et al. including 899 STEMI patients from the Swedish Coronary Angiography and Angioplasty Registry, which reported ticagrelor preloading in 35% of cases [22]. The use of cangrelor in patients pre-treated with P2Y12 inhibitors is supported by the CANTIC trial, a pharmacodynamic study on 50 STEMI patients randomized to treatment with either cangrelor or matching placebo co-administered with ticagrelor 180 mg loading [23]. The use of cangrelor was associated with an early reduction of P2Y12 reaction units, which persisted during the entire duration of drug infusion, without drug-drug interaction. This pharmacokinetic and pharmacodynamic profile of cangrelor in patients pretreated with a 180 mg ticagrelor loading dose was confirmed by the SWAP-5 study, a recent randomized trial including 20 patients with coronary artery disease [24].
In our study, the use of tirofiban was higher in patients with history of prior MI or PCI compared to cangrelor. This different use of intravenous antiplatelet agents was not influenced by preloading with P2Y12 inhibitors, which was comparable between groups. Moreover, the thrombus burden, the TIMI flow before PPCI and the type of coronary lesion did not orient toward the use of either drug during procedure. Thus, we hypothesized that these differences reflect a different behavior of the interventional cardiologists in the use of these agents in real-world PPCI practice.
Cangrelor was preferred over tirofiban in patients undergoing PPCI in the first three hours from symptoms onset. This result is consistent with the study by Grimfjӓrd et al., who reported a preferential use of cangrelor in patients undergoing PPCI within 3 h from the first ECG, reflecting the pharmacodynamic profile of cangrelor and the theoretical benefit in patients with very early presentation [22].
There are limited data about the use of cangrelor in the clinical scenario of STEMI patients with hemodynamic instability. In this real-world study, intravenous antiplatelet therapy was widely used in patients with cardiogenic shock who required infusion of inotropic agents or mechanical circulatory support, reflecting the intention to achieve more potent and rapid antiplatelet activity. In these very high-risk patients, we found a numerically higher use of cangrelor than tirofiban, albeit in absence of statistical differences. This suggests that the use of cangrelor is preferred in these patients who have a higher likelihood of ischaemic complications in the first hours after PPCI [25, 26].
The main finding of this real-world study was the association of cangrelor with better myocardial reperfusion assessed by coronary angiography indices after PPCI compared to tirofiban. Of note, this result was consistent among prespecified patients’ subgroups, including patients with different ischemia time and patients with or without chronic kidney disease.
The angiographic assessment of reperfusion in the IRA at the end of the procedure has a major prognostic significance in STEMI and has been routine in most centers for decades [14, 27, 28]. However, there is no study assessing the angiographic indices of myocardial reperfusion as measures of effectiveness in STEMI patients undergoing either cangrelor or tirofiban treatment during PPCI.
The FABOLUS-FASTER trial was a multicenter, randomized, pharmacodynamic study including 122 P2Y12-naïve patients with STEMI randomized to cangrelor, tirofiban, or 60-mg loading dose of prasugrel [29]. At 30 min from administration, tirofiban yielded a higher inhibition of platelet aggregation than cangrelor, and both were superior to prasugrel.
It is reasonable to assume that a drug with the highest inhibition of platelet aggregation would result in a better coronary flow after PPCI. However, these divergent results need to be interpreted against some limitations of the FABOLUS-FASTER study. Indeed, the rates of high platelet reactivity on cangrelor assessed with light transmittance aggregometry in the FABOLUS-FASTER were several-fold higher than shown with other assays requiring minimal sample processing (eg. VerifyNow, Multiplate impedance aggregometry) [30]. Furthermore, the level of inhibition of platelet aggregation assessed in the FABOLUS FASTER was extremely low for a highly selective and potent P2Y12 receptor blocker such as cangrelor. This pharmacodynamic evidence also diverges from an exploratory analysis by Vaduganathan et al. on 2042 patients enrolled in the CHAMPION trials, showing that cangrelor was at least as effective as GPI in reducing ischemic complications within 48 h after PCI, which is consistent with our results [31]. However, compared with our study, the analysis by Vaduganathan et al. included only 12% patients with STEMI, the preferred GPI were eptifibatide (78%) and abciximab (15%), and cangrelor was tested against clopidogrel as P2Y12 inhibitor.
The angiographic evidence of an improved myocardial reperfusion in the IRA at the end of the procedure in patients receiving cangrelor suggests a potentially higher clinical benefit from PPCI in these patients compared to those receiving tirofiban [32]. Although our study was not powered to evaluate clinical events, treatment with cangrelor was associated with a significantly higher improvement of LVEF at discharge compared to tirofiban. Although this analysis was merely exploratory and other mechanisms may have influenced the LVEF changes during hospitalization, it could provide a functional correlate of the more effective myocardial reperfusion observed in cangrelor-treated patients compared to tirofiban.
In our study, the use of either cangrelor or tirofiban was not associated with a different risk of clinically-relevant bleedings. Yerasi et al., in a recent study including 2072 all-comers patients undergoing PCI and adjunctive therapy with either cangrelor or GPI (eptifibatide in 99%), reported a lower incidence of major bleedings in patients receiving cangrelor [32]. This result, partially diverging from our study, may be explained by the different clinical scenario of STEMI and by the selective use of tirofiban as a GPI in our study. Moreover, our results were consistent with the propensity-matched analysis by Vaduganathan et al., reporting at least comparable risk of moderate-to-severe bleedings in patients receiving cangrelor vs. GPI [31].
This study has some limitations that should be acknowledged. Owing to the observational nature of the study, our results should be considered as hypothesis-generating and interpreted with caution. To account for potential selection bias in treatment assignment, we used the propensity score weighting technique to adjust for several baseline patient-related characteristics. Although we included several variables in the model (non-parsimonious approach), we cannot exclude a residual selection bias related to other concealed confounders [33]. We also conducted a sensitivity analysis showing the consistency of the primary results among multiple prespecified subgroups.
The INVEST-STEMI registry protocol does not include the re-evaluation of coronary angiography indices by a central core laboratory. The study does not provide information on the timing of administration of intravenous antiplatelet therapy (before PCI or during PCI as bailout treatment). However, we provided information on TIMI thrombus grade and TIMI flow before PCI, which do not seem to have influenced the use of either cangrelor or tirofiban.
In this real-world study, we did not perform a formal calculation of the sample size, but we included all consecutive patients meeting the study inclusion criteria at each participating center throughout the study period.
This study evaluated the safety and effectiveness of tirofiban and the results cannot be generalized to other GPIs. Another limitation is the lack of follow-up information.
Conclusions
In this real-world multicenter study including patients with STEMI, the use of cangrelor was associated with improved myocardial perfusion assessed by coronary angiography after PPCI, without increasing clinically-relevant bleedings compared to tirofiban.
Balancing individual haemorrhagic risk against the expected benefit of intravenous antiplatelet therapy is critical in patients with STEMI. Whether cangrelor is the best choice in this high-risk clinical scenario needs confirmation by randomized studies.
Data Availability
The datasets generated and/or analyzed during the current study may be available from the first author upon reasonable request.
References
Franchi F, Rollini F, Angiolillo DJ (2017) Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol 14(6):361–379. https://doi.org/10.1038/nrcardio.2017.18
(1997) EUROASPIRE. A European Society of Cardiology survey of secondary prevention of coronary heart disease: principal results. EUROASPIRE Study Group. European Action on Secondary Prevention through Intervention to Reduce Events. Eur Heart J 18(10):1569–82. https://doi.org/10.1093/oxfordjournals.eurheartj.a015136
Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ (2018) ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol 72(23 Pt A):2915–31. https://doi.org/10.1016/j.jacc.2018.09.057
Scudiero F, Canonico ME, Sanna GD et al (2023) Dual antiplatelet therapy with 3(rd) generation P2Y(12) inhibitors in STEMI patients: impact of body mass index on loading dose-response. Cardiovasc Drugs Ther 37(4):695–703. https://doi.org/10.1007/s10557-022-07322-2
Parodi G, Valenti R, Bellandi B et al (2013) Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol 61(15):1601–1606. https://doi.org/10.1016/j.jacc.2013.01.024
De Luca L, Steg PG, Bhatt DL, Capodanno D, Angiolillo DJ (2021) Cangrelor: clinical data, contemporary use, and future perspectives. J Am Heart Assoc 10(13):e022125. https://doi.org/10.1161/jaha.121.022125
Bhatt DL, Stone GW, Mahaffey KW et al (2013) Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 368(14):1303–1313. https://doi.org/10.1056/NEJMoa1300815
Stone GW, Bertrand ME, Moses JW et al (2007) Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: the ACUITY timing trial. JAMA 297(6):591–602. https://doi.org/10.1001/jama.297.6.591
Giugliano RP, White JA, Bode C et al (2009) Early versus delayed, provisional eptifibatide in acute coronary syndromes. N Engl J Med 360(21):2176–2190. https://doi.org/10.1056/NEJMoa0901316
Valgimigli M, Bueno H, Byrne RA et al (2018) 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 39(3):213–260. https://doi.org/10.1093/eurheartj/ehx419
Byrne RA, Rossello X, Coughlan JJ et al (2023) 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J Acute Cardiovasc Care. https://doi.org/10.1093/ehjacc/zuad107
Ibanez B, James S, Agewall S et al (2018) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39(2):119–177. https://doi.org/10.1093/eurheartj/ehx393
Farooq V, van Klaveren D, Steyerberg EW et al (2013) Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet (London, England) 381(9867):639–650. https://doi.org/10.1016/s0140-6736(13)60108-7
(1985) The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N Engl J Med 312(14):932–6. https://doi.org/10.1056/nejm198504043121437
Gibson CM, de Lemos JA, Murphy SA et al (2001) Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation 103(21):2550–2554. https://doi.org/10.1161/01.cir.103.21.2550
Poli A, Fetiveau R, Vandoni P et al (2002) Integrated analysis of myocardial blush and ST-segment elevation recovery after successful primary angioplasty: real-time grading of microvascular reperfusion and prediction of early and late recovery of left ventricular function. Circulation 106(3):313–318. https://doi.org/10.1161/01.cir.0000022691.71708.94
Baldi C, Polito MV, Citro R et al (2017) Prognostic value of clinical, echocardiographic and angiographic indicators in patients with large anterior ST-segment elevation myocardial infarction as a first acute coronary event. J Cardiovasc Med (Hagerstown) 18(12):946–953. https://doi.org/10.2459/jcm.0000000000000528
Mehran R, Rao SV, Bhatt DL et al (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123(23):2736–2747. https://doi.org/10.1161/circulationaha.110.009449
Glynn RJ, Schneeweiss S, Stürmer T (2006) Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 98(3):253–259. https://doi.org/10.1111/j.1742-7843.2006.pto_293.x
Patrick AR, Schneeweiss S, Brookhart MA et al (2011) The implications of propensity score variable selection strategies in pharmacoepidemiology: an empirical illustration. Pharmacoepidemiol Drug Saf 20(6):551–559. https://doi.org/10.1002/pds.2098
Sterne JA, White IR, Carlin JB et al (2009) Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 338:b2393. https://doi.org/10.1136/bmj.b2393
Grimfjärd P, Lagerqvist B, Erlinge D, Varenhorst C, James S (2019) Clinical use of cangrelor: nationwide experience from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J Cardiovasc Pharmacother 5(3):151–157. https://doi.org/10.1093/ehjcvp/pvz002
Franchi F, Rollini F, Rivas A et al (2019) Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation 139(14):1661–1670. https://doi.org/10.1161/circulationaha.118.038317
Franchi F, Ortega-Paz L, Rollini F et al (2023) Cangrelor in patients with coronary artery disease pretreated with ticagrelor: the switching antiplatelet (SWAP)-5 study. JACC Cardiovasc Interv 16(1):36–46. https://doi.org/10.1016/j.jcin.2022.10.034
Zeymer U, Lober C, Richter S et al (2023) Cangrelor in patients with percutaneous coronary intervention for acute myocardial infarction after cardiac arrest and/or with cardiogenic shock. Eur Heart J Acute Cardiovasc Care 12(7):462–463. https://doi.org/10.1093/ehjacc/zuad041
Kordis P, Bozic Mijovski M, Berden J, Steblovnik K, Blinc A, Noc M (2023) Cangrelor for comatose survivors of out-of-hospital cardiac arrest undergoing percutaneous coronary intervention: the CANGRELOR-OHCA study. EuroIntervention 18(15):1269–1271. https://doi.org/10.4244/EIJ-D-22-00675
Baldi C, Silverio A, Esposito L et al (2021) Clinical outcome of patients with ST-elevation myocardial infarction and angiographic evidence of coronary artery ectasia. Catheter Cardiovasc Interv 99(2):340–347. https://doi.org/10.1002/ccd.29738
De Luca G, Silverio A, Verdoia M et al (2022) Angiographic and clinical outcome of SARS-CoV-2 positive patients with ST-segment elevation myocardial infarction undergoing primary angioplasty: a collaborative, individual patient data meta-analysis of six registry-based studies. Eur J Intern Med 105:69–76. https://doi.org/10.1016/j.ejim.2022.08.021
Gargiulo G, Esposito G, Avvedimento M et al (2020) Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS-FASTER trial. Circulation 142(5):441–454. https://doi.org/10.1161/circulationaha.120.046928
Angiolillo DJ, Bhatt DL, Stone GW (2021) Letter by Angiolillo et al regarding article, “Cangrelor, tirofiban, and chewed or standard prasugrel regimens in patients with ST-segment-elevation myocardial infarction: primary results of the FABOLUS FASTER trial.” Circulation 143(13):e795–e796. https://doi.org/10.1161/circulationaha.120.050205
Vaduganathan M, Harrington RA, Stone GW et al (2017) Evaluation of ischemic and bleeding risks associated with 2 parenteral antiplatelet strategies comparing cangrelor with glycoprotein IIb/IIIa inhibitors: an exploratory analysis from the CHAMPION Trials. JAMA Cardiol 2(2):127–135. https://doi.org/10.1001/jamacardio.2016.4556
Yerasi C, Case BC, Chezar-Azerrad C et al (2021) Cangrelor vs. glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention. Am Heart J 238:59–65. https://doi.org/10.1016/j.ahj.2021.04.013
Silverio A, Buccheri S, Venetsanos D et al (2020) Percutaneous treatment and outcomes of small coronary vessels: a SCAAR report. JACC Cardiovasc Interv 13(7):793–804. https://doi.org/10.1016/j.jcin.2019.10.062
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the manuscript.
- Conception and design or analysis and interpretation of data: A. Silverio, M. Bellino, F. Scudiero, M. Di Maio, M. Centore.
-Drafting of the manuscript: A. Silverio, M. Bellino, L. Esposito.
- Revising critically the manuscript: T. Attisano, C. Baldi, A. Catalano, A. Cesaro, G. Granata, F. Maiellaro, I. Muraca, G. Musumeci, G. Parodi, D. Personeni, R. Valenti.
- Final approval of the manuscript: Angelo Silverio, Michele Bellino, Fernando Scudiero, Tiziana Attisano, Cesare Baldi, Angelo Catalano, Mario Centore, Arturo Cesaro, Marco Di Maio, Luca Esposito, Giovanni Granata, Francesco Maiellaro, Iacopo Muraca, Giuseppe Musumeci, Guido Parodi, Davide Personeni, Renato Valenti, Carmine Vecchione, Paolo Calabrò, Gennaro Galasso.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional ethics committee of the University Hospital of Salerno and conforms to the Declaration of Helsinki.
Consent to participate
All patients were informed of the nature and aims of the study and asked to sign an informed consent for the anonymous management of their data.
Competing interests
Dr. Luca Esposito received a research grant by the CardioPath program from the Federico II University of Naples, Italy. The other authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The use of intravenous antiplatelet therapy during primary percutaneous coronary intervention (PPCI) is not fully standardized.
• This is the first multicentre study comparing the effectiveness and safety of cangrelor and tirofiban in a real-world population of patients with ST-segment elevation myocardial infarction (STEMI).
• Compared to tirofiban, the use of cangrelor was associated with a higher chance of successful myocardial reperfusion after PPCI, with no difference in terms of clinically-relevant bleedings during the hospitalization.
• Our study reinforces the role of cangrelor for periprocedural platelet inhibition during PPCI in patients with STEMI. These findings should be confirmed by future randomized clinical trials.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Silverio, A., Bellino, M., Scudiero, F. et al. Intravenous antiplatelet therapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Thromb Thrombolysis 57, 757–766 (2024). https://doi.org/10.1007/s11239-024-02970-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-024-02970-7