Abstract
Switching P2Y12 inhibitors is common in clinical practice. However, data on the pharmacodynamic (PD) effects of switching in clinical settings characterized by high platelet reactivity, such as diabetes mellitus (DM), are limited. This is a post-hoc analysis from a prospective, randomized, open-label study conducted in coronary artery disease patients comparing the PD effects of loading dose (LD) and maintenance dose regimens of prasugrel vs ticagrelor according to DM status. A total of 110 patients were enrolled: 42 (38%) with DM and 68 (62%) without DM. All patients were on maintenance dual antiplatelet therapy with aspirin and clopidogrel. PD assessments were performed using whole blood vasodilator-stimulated phosphoprotein (VASP), with results quantified by the platelet reactivity index (PRI), VerifyNow P2Y12 (VN-P2Y12) with results reported as P2Y12 reaction units (PRU), and light transmittance aggregometry (LTA) following 20 and 5 µM adenosine diphosphate stimuli with results reported as maximum platelet aggregation (MPA). PD assessments were performed at baseline (while on clopidogrel), 30 min after LD, 2 h after LD, and 1 week after LD. Overall, platelet reactivity was higher in DM than in non-DM patients while on clopidogrel therapy. After switching to either prasugrel or ticagrelor, platelet reactivity dropped but remained significantly higher among patients with DM at 30 min with all tests (VN-PRU p < 0.01, MPA 20 µM p < 0.01, VASP-PRI p = 0.02) and at 2 h with VN-PRU (p < 0.01) and LTA-MPA 20 µM (p < 0.01) but not with VASP-PRI (p = 0.19). There were no significant differences between prasugrel and ticagrelor both among patients with or without DM, except for lower LTA-MPA 20 at 30 min (p < 0.01) among non-DM patients treated with prasugrel. Patients with DM treated with clopidogrel have higher platelet reactivity compared to patients without DM. Although platelet reactivity markedly reduces to a similar extent after switching to prasugrel or ticagrelor, patients with DM persist with increased platelet reactivity compared to patients without DM.
Study registration: ClinicalTrials.gov identifier: NCT01852175
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
The impact of diabetes (DM) on the comparative pharmacodynamics (PD) effects of prasugrel and ticagrelor after switching from clopidogrel remains poorly explored.
-
In this post-hoc subgroup analysis of a large randomized study including 110 patients undergoing switching from clopidogrel to prasugrel or ticagrelor, we compared PD assessments using three platelet function assays (whole blood vasodilator-stimulated phosphoprotein, VerifyNow P2Y12, and light transmittance aggregometry following 20 μM and 5 μM ADP stimuli) at different time points in DM versus non-DM patients.
-
Patients with DM treated with clopidogrel had higher platelet reactivity compared to patients without DM.
-
Despite switching from clopidogrel to either prasugrel or ticagrelor resulted in a marked reduction in platelet reactivity regardless of DM status, patients with DM had higher platelet reactivity after both loading and maintenance doses of prasugrel or ticagrelor compared to patients without DM. There was no difference between prasugrel and ticagrelor at any time points, irrespective of DM status.
Introduction
Patients with diabetes mellitus (DM) are characterized by platelet hyperreactivity and increased platelet turnover rates leading to impaired pharmacodynamic (PD) response to antiplatelet agents [1,2,3,4,5]. These observations contribute to the higher ischemic event rates observed in DM patients compared with non-DM patients despite the use of antiplatelet therapy [6, 7]. The use of oral P2Y12 inhibiting therapy is key for the prevention of ischemic recurrences, including stent thrombosis, in high risk patients such as those presenting with an acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) [8]. Clopidogrel is the most broadly utilized P2Y12 inhibitor. However, PD studies have consistently shown a broad variability in individual response to clopidogrel with a considerable number of subjects with impaired platelet inhibitory effects [9, 10]. Patients with DM have consistently shown to be associated with impaired clopidogrel response and high on-treatment platelet reactivity (HPR), a marker of thrombotic risk [4, 5, 11, 12].
Prasugrel and ticagrelor have more prompt, potent and predictable antiplatelet effects than clopidogrel, leading to reduced ischemic events in patients with ACS, regardless of DM status [13,14,15,16]. In a recent trial comparing ticagrelor and prasugrel in ACS patients, DM seemed to affect the efficacy of ticagrelor and prasugrel [17, 18]. In particular, in patients with DM, the efficacy of ticagrelor was comparable with that of prasugrel, whereas in non-DM patients, prasugrel was associated with significant reduction of ischemic events [17]. Recently, a randomized controlled trial showed that ticagrelor reduces ischemic events compared to placebo also among aspirin-treated patients with DM and stable CAD who do not have a history of myocardial infarction or stroke, especially in those with prior PCI [19]. Thus, the overall evidence supports the preferential use of potent P2Y12 inhibitors (prasugrel or ticagrelor) among DM patients with CAD, in particular those presenting with an ACS, unless prohibitive bleeding risk is present [20]. This may also entail switching P2Y12 inhibitor from the less potent clopidogrel to the more potent prasugrel or ticagrelor [21]. Nevertheless, the impact of DM on the comparative PD effects of prasugrel and ticagrelor after switching from clopidogrel remains poorly explored.
Methods
Study design and patient population
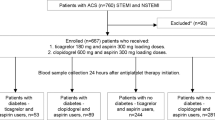
The “A head-to Head Comparison of the Pharmacodynamic Effects of Prasugrel Compared With Ticagrelor in Patients With Coronary Artery Disease” was a prospective, randomized, parallel design, open-label study comparing the PD effects of loading dose (LD) and maintenance dose (MD) regimens of prasugrel vs ticagrelor among patients with stable CAD on maintenance dual antiplatelet therapy (DAPT) with aspirin and clopidogrel (NCT01852175). The key findings of the study were that, when switching from clopidogrel, prasugrel and ticagrelor achieved similarly prompt and potent platelet inhibitory effects following LD administration, which persisted after 1 week of MD therapy [14]. The current study is a post-hoc subgroup analysis aimed at exploring the effects of DM status on the PD effects associated with switching from clopidogrel to prasugrel or ticagrelor. Design, methods and results of the study have been published previously [14]. In brief, 110 patients with CAD taking aspirin (81 mg/daily) and clopidogrel (75 mg/daily) as per standard of care were randomized to stop clopidogrel and switch to either prasugrel (60 mg LD followed by 10 mg/day MD initiated 24 h after the LD) or ticagrelor (180 mg LD followed by 90 mg/b.i.d. MD starting 24 h after LD). PD assessments were performed at five time points by laboratory personnel blinded to treatment assignment: (i) baseline (while on clopidogrel), (ii) 30 min after LD, (iii) 2 h after LD, (iv) 24 h after LD, and (v) 1 week (7 + 2 days) after LD. For the purpose of this analysis, we report PD time points assessment at baseline, 30 min, 2 h and 1 week.
A summary flow diagram of the study and this post-hoc analysis is represented in Fig. 1. PD assessments were conducted using three platelet function assays: whole blood vasodilator-stimulated phosphoprotein (VASP) with results quantified by the platelet reactivity index (PRI), VerifyNow P2Y12 (VN-P2Y12) with results reported as P2Y12 reaction units (PRU), and light transmittance aggregometry (LTA) following 20 µM and 5 µM adenosine diphosphate (ADP) stimuli with results reported as maximum platelet aggregation (MPA) [14]. Results of LTA 5 µM ADP are not shown because were consistent with 20 µM ADP MPA.
Endpoints
The primary aim of this analysis was the comparison of platelet reactivity at baseline (while on clopidogrel) and after switching to LD prasugrel or ticagrelor at 30 min, 2 h and MD at 1 week among patients with DM versus patients without DM. Secondary endpoints were the comparison of platelet reactivity at all time points between (1) DM versus non-DM patients among patients treated with prasugrel; (2) DM versus non-DM patients among patients treated with ticagrelor; (3) prasugrel versus ticagrelor among patients with DM; (4) prasugrel versus ticagrelor among patients without DM. Rates of HPR, defined as PRI > 50% assessed by VASP, PRU > 208 assessed by VN-P2Y12, or MPA > 59% assessed by LTA 20 µM ADP, were also compared between groups [10].
Statistical methods
Continuous variables with normal distribution are expressed as mean ± standard deviation and those with skewed distribution are expressed as median ± interquartile range. Categorical variables are presented as counts and percentages. Means of two continuous variables were compared by independent samples Student’s t-test or Mann–Whitney U test according to the conformity to the normal distribution evaluated with the Kolmogorov–Smirnov test. The frequencies of categorical variables were compared using Chi-square test or Fisher’s exact test as appropriate. An analysis of covariance method with a general linear model, using the baseline value of platelet reactivity as a covariate, was used for prasugrel versus ticagrelor comparisons. A two-tailed P-value of 0.05 was considered to indicate statistically significant differences. Statistical analysis was performed using SPSS version 24.0 software (SPSS Inc., Chicago, IL, USA).
Results
Patient population
A total of 110 patients were analysed: 42 (38%) with DM and 68 (62%) without DM. In particular, among the 55 patients randomized to prasugrel, 20 (48%) were DM and 35 (52%) were non-DM, while among the 55 patients randomized to ticagrelor, 22 (52%) were DM and 33 (48%) were non-DM. Table 1 shows the baseline characteristics of these patients. Overall, the average age was 59 ± 8 years and 59% were men. As compared to non-DM patients, DM patients were older (61.6 ± 7.2 vs 56.9 ± 8.4, p < 0.01), had a higher BMI (34.7 ± 8.5 vs 30.5 ± 5.2, p < 0.01), and more peripheral artery disease (30.9% vs 8.8%, p < 0.01). There were no differences in the other baseline characteristics including concomitant medications, clinical characteristics, and main laboratory tests (Table 1).
Pharmacodynamic findings at baseline and after switching from clopidogrel to prasugrel or ticagrelor according to DM status
At baseline, while patients were on aspirin and clopidogrel, levels of platelet reactivity were significantly higher among patients with DM as compared to patients without DM as assessed by VN-PRU (p < 0.01) and LTA-MPA 20 µM (p < 0.01); the increase did not reach statistical significance with VASP-PRI (p = 0.18) (Fig. 2).
After switching from clopidogrel to either prasugrel or ticagrelor, platelet reactivity significantly dropped at 30 min (VN-PRU p < 0.01, LTA-MPA 20 µM p < 0.01, VASP-PRI p < 0.01) (Fig. 2). However, platelet reactivity remained higher among patients with DM as compared to patients without DM at 30 min with all tests (VN-PRU p < 0.01, MPA 20 µM p < 0.01, VASP-PRI p = 0.02) and at 2 h with VN-PRU (p < 0.01) and LTA-MPA 20 µM (p < 0.01) but not with VASP-PRI (p = 0.19). Higher platelet reactivity was also observed among patients with DM as compared to patients without DM after 1 week of MD with either prasugrel or ticagrelor with VN-PRU (p = 0.01) and LTA-MPA 20 µM p = 0.03), but not with VASP-PRI (p = 0.29) (Fig. 2). When prasugrel and ticagrelor were considered individually, there was a trend towards increased platelet reactivity among patients with DM compared to those without DM at any time point (Fig. 3 and 4). There were no significant differences between prasugrel and ticagrelor both among patients with or without DM, except for lower MPA 20 at 30 min (p < 0.01) among non-DM patients treated with prasugrel (Fig. 5).
Rates of HPR at baseline while patients were on aspirin and clopidogrel varied according to the test used (VN-PRU 34.5%, LTA-MPA 20 µM 31.8%, VASP-PRI 65.5%). Although HPR rates were numerically higher in patients with DM, this did not reach statistical significance (Fig. 6). HPR rates markedly reduced after switching from clopidogrel to either prasugrel or ticagrelor at 30 min (VN-PRU 19.0%, LTA-MPA 20 µM 15.5%, VASP-PRI 34.5%), 2 h (VN-PRU 4.5%, LTA-MPA 20 µM 2.7%, VASP-PRI 6.4%) and 1 week (VN-PRU 0.9%, :TA-MPA 20 µM 5.4%, VASP-PRI 13.6%), with no differences between agents or according to DM status (Fig. 6).
High platelet reactivity according to diabetes status and treatment arms. There were no significant differences in the rate of HPR were observed at baseline or after switching to prasugrel and/or ticagrelor between patients with or without diabetes. There were also no differences in the rate of HPR with prasugrel versus ticagrelor irrespective of diabetes status, DM diabetes mellitus, HPR high platelet reactivity, ADP adenosine diphosphate
Discussion
The results of the present analysis can be summarized as follows: (1) clopidogrel-mediated platelet P2Y12 receptor blockade is impaired in patients with DM compared with non-DM patients; (2) switching from clopidogrel to either prasugrel or ticagrelor is associated with, marked reduction of platelet reactivity regardless DM status; (3) patients with DM still exhibit higher platelet reactivity than non-DM following LD and during MD treatment of prasugrel and ticagrelor; (4) prasugrel and ticagrelor achieve comparable P2Y12 inhibitory effects, irrespective of DM status.
The prevalence of DM in patients with CAD reaches up to 50% and represents a strong independent predictor of ischemic events, including mortality [1, 22]. The increased atherothrombotic risk of DM patients has been largely attributed to abnormalities in platelet function and increased platelet turnover rates resulting in increased platelet reactivity and impaired PD response to antiplatelet agents used for prevention of ischemic recurrences [2, 6]. Since PD studies have consistently shown reduced clopidogrel-mediated platelet P2Y12 receptor blockade [4, 5, 11, 12, 23], several clinical studies have assessed whether the use of potent P2Y12 inhibitors (prasugrel and ticagrelor) could improve clinical outcomes. Indeed, the benefit of both prasugrel and ticagrelor over clopidogrel in ACS was confirmed in patients with DM [24, 25]. The benefit of ticagrelor was also confirmed in DM patients with CCS, both with and without prior MI [19, 26]. There is limited data to the latter extent with prasugrel [27]. Nevertheless, the use of potent P2Y12 inhibitors was associated with an increased risk of bleeding in the overall population, underscoring the importance of patient selection for the use of these agents [26, 28]. Clinical and PD data suggest that DM status may affect the comparative efficacy of ticagrelor and prasugrel [4, 17, 23]. In particular, a pre-specified analysis of the ISAR-REACT (Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment) 5 trial found prasugrel and ticagrelor show similar efficacy in patients with DM, while prasugrel was superior to ticagrelor in patients without DM [17]. The results of the present analysis support that while DM status also affects the antiplatelet effects of potent P2Y12 inhibitors, their PD profile is comparable in both DM and non-DM patients [4, 23]. However, the results of our PD investigation is too limited in sample size to explain the clinical findings in patients with and without DM in the ISAR-REACT 5 trial.
Switching P2Y12 inhibitor is common in clinical practice [20, 21]. Data from large-scale clinical investigations to guide the optimal approach towards switching P2Y12 inhibitors are limited, and most data are derived from PD studies [21]. The prospective, randomized, parallel design trial from which this sub-analysis was performed was one of the largest PD studies comparing prasugrel vs ticagrelor after switching from clopidogrel [14]. Nevertheless, the impact of DM on the PD effects of prasugrel and ticagrelor after switching from clopidogrel have not been previously investigated. A better understanding of the PD effects of switching from clopidogrel to either prasugrel or ticagrelor among specific settings characterized by high platelet reactivity (e.g., ACS, elderly, chronic kidney disease, obesity, DM) may help optimize antithrombotic regimens in these challenging and common clinical scenarios [29]. This study provides new evidence in the field, showing that a trend towards increased platelet reactivity is present at all time points with the use of potent P2Y12 inhibitors among DM as compared to non-DM patients. This occurs both after LD administration and during MD treatment and underscores the need for further optimization of antithrombotic therapy also in high-risk CCS patients. This may include the use of intravenous antiplatelet therapies at the time of PCI as well as the implementation of tools allowing for a guided selection of antiplatelet therapy when the thrombotic risk is high, and novel non-platelet mediated strategies to reduce long term ischemic risk (i.e. proprotein convertase subtilisin/kexin type 9 inhibitors, Sodium-Glucose Cotransporter-2 inhibitors) [30,31,32,33,34].
Study limitations
Some limitations have to be considered in the present analysis. This was a post-hoc subgroup analysis of a prospective study randomizing patients according to treatment group (prasugrel or ticagrelor) rather than to the presence of DM, potentially leading to some imbalances between number of patients and baseline characteristics between DM versus non-DM cohorts. Nevertheless, the cohort of DM patients in this study was highly represented, allowing a balanced comparison between DM and non-DM patients in both prasugrel and ticagrelor groups (20 vs 35 and 22 vs 33, respectively). Moreover, except for a slight but significant difference in average age (62 vs 58 years), the remaining differences in baseline characteristics between DM vs non-DM patients were found only for BMI and concomitant peripheral artery disease, all conditions that are known to be associated with DM status. Given the post-hoc nature of the study, the sample size, despite the largest evidence available, was not powered to assess PD findings according to DM status, leading to non-significant p values for some of the PD assays, although a directional trend was consistently shown. Nevertheless, this limitation was at least partially overcome using multiple PD assays allowing for a comprehensive assessment of P2Y12 signalling. Ultimately, our study did not include pharmacokinetic assessments and the sample size did not allow for identifying predictors of HPR among DM vs non-DM patients. This study was a post-hoc exploratory analysis not pre-specified at the time of study design and as such results should be considered as hypothesis-generating. However, the endpoints of this analysis had indeed been prespecified before inspecting the data for post hoc analysis. Finally, we did not adjust for multiple comparisons as this would have potentially masked meaningful functional differences between groups within the scope of this retrospective analysis. This approach is in line with previously published studies [4].
Conclusion
Patients with DM treated with clopidogrel have higher platelet reactivity compared to patients without DM. Despite switching from clopidogrel to either prasugrel or ticagrelor results in a marked reduction in platelet reactivity regardless of DM status, patients with DM continue to have higher platelet reactivity after both loading and maintenance doses of prasugrel or ticagrelor compared to patients without DM. However, there was no difference between prasugrel and ticagrelor at any time points, irrespective of DM status.
References
Capodanno D, Angiolillo DJ (2020) Antithrombotic therapy for atherosclerotic cardiovascular disease risk mitigation in patients with coronary artery disease and diabetes mellitus. Circulation 142(22):2172–2188
Patti G, Cavallari I, Andreotti F, Calabrò P, Cirillo P, Denas G et al (2019) Prevention of atherothrombotic events in patients with diabetes mellitus: from antithrombotic therapies to new-generation glucose-lowering drugs. Nat Rev Cardiol 16(2):113–130
Rollini F, Franchi F, Muñiz-Lozano A, Angiolillo DJ (2013) Platelet function profiles in patients with diabetes mellitus. J Cardiovasc Transl Res 6(3):329–345
Franchi F, Rollini F, Aggarwal N, Hu J, Kureti M, Durairaj A et al (2016) Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (optimizing antiplatelet therapy in diabetes mellitus)-4 study. Circulation 134(11):780–792
Angiolillo DJ, Jakubowski JA, Ferreiro JL, Tello-Montoliu A, Rollini F, Franchi F et al (2014) Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J Am Coll Cardiol 64(10):1005–1014
Ferreiro JL, Angiolillo DJ (2011) Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 123(7):798–813
Zhang R, Mamza JB, Morris T, Godfrey G, Asselbergs FW, Denaxas S et al (2022) Lifetime risk of cardiovascular-renal disease in type 2 diabetes: a population-based study in 473,399 individuals. BMC Med 20(1):63
Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donghue M (2022) Antiplatelet therapy after percutaneous coronary intervention. EuroIntervention 17(17):e1371–e1396
Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K et al (2015) Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 36(27):1762–1771
Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L et al (2019) Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 12(16):1521–1537
Angiolillo DJ, Bernardo E, Sabaté M, Jimenez-Quevedo P, Costa MA, Palazuelos J et al (2007) Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol 50(16):1541–1547
Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Sabaté M, Jimenez-Quevedo P et al (2006) Clopidogrel withdrawal is associated with proinflammatory and prothrombotic effects in patients with diabetes and coronary artery disease. Diabetes 55(3):780–784
Navarese EP, Khan SU, Kołodziejczak M, Kubica J, Buccheri S, Cannon CP et al (2020) Comparative efficacy and safety of oral P2Y(12) inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation 142(2):150–160
Rollini F, Franchi F, Cho JR, DeGroat C, Bhatti M, Muniz-Lozano A et al (2016) A head-to-head pharmacodynamic comparison of prasugrel vs ticagrelor after switching from clopidogrel in patients with coronary artery disease results of a prospective randomized study. Eur Heart J 37(35):2722–2730
Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G et al (2022) Comparative effects of guided vs potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J 43(10):959–967
Franchi F, Angiolillo DJ (2015) Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 12(1):30–47
Ndrepepa G, Kastrati A, Menichelli M, Neumann FJ, Wöhrle J, Bernlochner I et al (2020) Ticagrelor or prasugrel in patients with acute coronary syndromes and diabetes mellitus. JACC Cardiovasc Interv 13(19):2238–2247
Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J et al (2019) Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med 381(16):1524–1534
Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA et al (2019) Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med 381(14):1309–1320
Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL et al (2021) 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 42(14):1289–1367
Angiolillo DJ, Rollini F, Storey RF, Bhatt DL, James S, Schneider DJ et al (2017) International expert consensus on switching platelet P2Y(12) receptor-inhibiting therapies. Circulation 136(20):1955–1975
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141(9):e139–e596
Erlinge D, Varenhorst C, Braun OO, James S, Winters KJ, Jakubowski JA et al (2008) Patients with poor responsiveness to thienopyridine treatment or with diabetes have lower levels of circulating active metabolite, but their platelets respond normally to active metabolite added ex vivo. J Am Coll Cardiol 52(24):1968–1977
Franchi F, James SK, Ghukasyan Lakic T, Budaj AJ, Cornel JH, Katus HA et al (2019) Impact of diabetes mellitus and chronic kidney disease on cardiovascular outcomes and platelet P2Y(12) receptor antagonist effects in patients with acute coronary syndromes: insights from the PLATO trial. J Am Heart Assoc 8(6):e011139
Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW et al (2008) Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Circulation 118(16):1626–1636
Bhatt DL, Bonaca MP, Bansilal S, Angiolillo DJ, Cohen M, Storey RF et al (2016) Reduction in ischemic events with ticagrelor in diabetic patients with prior myocardial infarction in PEGASUS-TIMI 54. J Am Coll Cardiol 67(23):2732–2740
Meredith IT, Tanguay JF, Kereiakes DJ, Cutlip DE, Yeh RW, Garratt KN et al (2016) Diabetes mellitus and prevention of late myocardial infarction after coronary stenting in the randomized dual antiplatelet therapy study. Circulation 133(18):1772–1782
Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA et al (2019) Ticagrelor in patients with stable coronary disease and diabetes. New Engl J Med 381(14):1309–1320
Angiolillo DJ, Capodanno D, Danchin N, Simon T, Bergmeijer TO, Ten Berg JM et al (2020) Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: the ABCD-GENE score. JACC Cardiovasc Interv 13(5):606–617
Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW et al (2013) Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med 368(14):1303–1313
Cosentino F, Bhatt DL, Marx N, Verma S (2022) The year in cardiovascular medicine 2021: diabetes and metabolic disorders. Eur Heart J 43(4):263–270
Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D et al (2021) Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 397(10283):1470–1483
Galli M, Franchi F, Rollini F, Angiolillo DJ (2021) Role of platelet function and genetic testing in patients undergoing percutaneous coronary intervention. Trends Cardiovasc Med S1050–1738(21):00157–00162
Ferreiro JL, Ueno M, Tello-Montoliu A, Tomasello SD, Capodanno D, Capranzano P et al (2013) Effects of cangrelor in coronary artery disease patients with and without diabetes mellitus: an in vitro pharmacodynamic investigation. J Thromb Thrombolysis 35(2):155–164
Acknowledgements
The present study was funded by Institutional Grant of the University of Florida College of Medicine-Jacksonville.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.G. declares that he has received honoraria from Terumo. F.F. declares that he has received payment as an individual for consulting fee or honorarium from AstraZeneca, Bayer and Sanofi. Institutional payments for grants from PLx Pharma, and The Scott R. MacKenzie Foundation. F.R. declares that he has received honoraria from Chiesi. D.J.A. declares that he has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi,. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and Scott R. MacKenzie Foundation. The remaining authors report no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Galli, M., Rollini, F., Been, L. et al. Impact of diabetes mellitus on the pharmacodynamic effects of prasugrel and ticagrelor after switching from clopidogrel in patients with coronary artery disease. J Thromb Thrombolysis 54, 461–469 (2022). https://doi.org/10.1007/s11239-022-02696-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02696-4