Abstract
There is a paucity of data on anticoagulation strategies and clinical outcomes after bleeding events for venous thromboembolism (VTE). In a multicenter Japanese registry enrolling 3027 patients with acute symptomatic VTE, after excluding 430 patients with thrombolysis and 207 patients without anticoagulation therapy, the current study population consisted of 2390 patients, who were divided into patients with major bleeding, clinically relevant non-major (CRNM) bleeding and no bleeding during anticoagulation therapy. All-cause death at 90 days after the bleeding events was evaluated as the primary outcome. There were 189 patients with major bleeding, 147 patients with CRNM bleeding, and 2054 patients without bleeding. Among 189 patients with major bleeding, 142 patients (75%) discontinued anticoagulants, of whom patients with temporary discontinuation and those with permanent discontinuation accounted for 63 patients (44%) and 79 patients (56%), and 58 patients (30.7%) died within 90 days after the bleeding events. The multivariable logistic regression model among patients with bleeding events revealed that active cancer and bleeding events within 90 days after VTE diagnosis were independently associated with 90-day mortality after the bleeding events (active cancer: OR 5.05, 95%CI 2.82–9.05; bleeding events within 90 days after VTE diagnosis: OR 2.23, 95%CI 1.25–3.96). In this practice-based large registry, anticoagulants were frequently discontinued in patients who experienced major bleeding events during anticoagulation therapy and nearly half of them restarted anticoagulants with mortality rate of approximately 30% within 90 days after the bleeding events, and active cancer was the most prevalent cause of death.
Clinical trial registration COMMAND VTE Registry: http://www.umin.ac.jp/ctr/index.htm. Unique identifier: UMIN000021132.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
There is limited data after bleeding during anticoagulation for venous thromboembolism.
-
We investigated anticoagulation strategies and clinical outcomes after bleeding in the real world.
-
Anticoagulants were frequently discontinued and nearly half of them restarted it.
-
The current results could help guiding strategies in the absence of randomized clinical trials.
-
Further studies are warranted to investigate the optimal management strategies after bleeding events.
Introduction
The main goal of treating venous thromboembolism (VTE), including pulmonary embolism (PE) and deep vein thrombosis (DVT), is to prevent recurrent VTE and its complications, which can be achieved by anticoagulation therapy [1, 2]. However, anticoagulation therapy is associated with an increased risk of bleeding events [3, 4]. Previous studies reported that bleeding events during anticoagulation therapy had a considerable impact on mortality in patients with VTE [5,6,7], which should be taken into account when deciding on the optimal duration of anticoagulant therapy for VTE. When a bleeding event occurs during anticoagulation therapy, a decision should be made on whether the anticoagulation therapy should be permanently discontinued, or alternatively, after how long it can be restarted.
Evidence on the optimal anticoagulation strategies after bleeding complications in VTE patients is scarce, and randomized clinical trials focusing on the optimal strategies are currently unavailable. Current VTE guidelines do hardly provide any specific recommendations for the management of anticoagulation therapy after bleeding events in patients with VTE [8,9,10,11], which likely leads to widely varying anticoagulation strategies in daily clinical practice. The aim of the current study therefore was to investigate the anticoagulation strategies and clinical outcomes of anticoagulation-associated bleeding events in VTE patients, using a large practice-based large observational Japanese database.
Methods
Study population
The COMMAND VTE (COntemporary ManageMent AND outcomes in patients with Venous ThromboEmbolism) Registry is a physician-initiated, retrospective, multicenter cohort study in which consecutive patients with acute objectivated symptomatic VTE among 29 centers in Japan were included between January 2010 and August 2014. The design of the registry was previously reported in detail [12, 13]. We searched the hospital databases for clinical diagnosis and imaging examinations, and retrospectively enrolled consecutive patients who met the definition of acute symptomatic VTE diagnosed within 31 days from symptom onset during the study period [14]. The relevant review boards or ethics committees in all 29 participating centers (Online Appendix 1) approved the research protocol. Written informed consent from each patient was waived because we used clinical information obtained in routine clinical practice and none of the patients refused to participate in the study when contacted for follow-up. This method is concordant with the guidelines for epidemiological studies issued by the Ministry of Health, Labor, and Welfare in Japan.
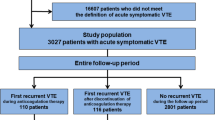
We enrolled 3027 consecutive patients with acute symptomatic VTE after screening of the consecutive 19,634 patients with suspected VTE for eligibility through chart review by the physicians at each institution. After excluding 430 patients who received systemic or local thrombolysis at the index VTE events and 207 patients who did not receive any anticoagulation therapy, the current study population consisted of 2390 patients with acute symptomatic VTE (Fig. 1). In the current study, we evaluated all bleeding events during anticoagulation therapy. According to the occurrence of bleeding events during anticoagulation therapy, the current study population was divided into the following three groups: (1) Major bleeding during anticoagulation therapy; (2) Clinically relevant non-major (CRNM) bleeding during anticoagulation therapy; and (3) No bleeding during anticoagulation therapy. We defined the bleeding event during anticoagulation therapy as the first bleeding event from the index VTE diagnosis to 3 days after first discontinuation of anticoagulants during the follow-up period. Patients who experienced both major and CRNM bleeding were classified according to the first bleeding event. Major bleeding was defined as International Society of Thrombosis and Hemostasis (ISTH) major bleeding, which consisted of a reduction in the hemoglobin level by at least 2 g/dL, transfusion of at least 2 units of blood, symptomatic bleeding in a critical area or organ, or fatal bleeding [15]. CRNM bleeding was defined as overt bleeding that did not meet the criteria for major bleeding, but was associated with medical intervention, unscheduled contact with a physician, or cessation of anticoagulation therapy, based on the previous report [16]. The management strategies of bleeding events were at the discretion of the physicians in charge including temporary/permanent discontinuation of anticoagulation, restarting anticoagulation and use of reversal agents for warfarin.
Data collection and definitions for patient characteristics
Data for the baseline characteristics were collected from the hospital charts or hospital databases according to the pre-specified definitions. The physicians at each institution were responsible for data entry into an electronic case report form in a web-based database system. Data were automatically checked for missing or contradictory input and values out of the expected range. Additional monitoring for the quality of data was performed at the general office of the registry.
Patients with active cancer were defined as those on treatment for cancer such as chemotherapy or radiotherapy, those scheduled to undergo cancer-surgery, those with metastasis to other organs, and/or those with terminal cancer (expected life expectancy of 6 months or less) at the time of the diagnosis. Anemia was defined as hemoglobin level < 13 g/dL for men and < 12 g/dL for women. Thrombocytopenia was defined as platelet count < 100 × 109/L. The detailed definitions of patient characteristics are described in Online Appendix 2.
Clinical follow-up and endpoints
Collection of follow-up information was mainly conducted through review of hospital charts, and additional follow-up information was collected through contact with patients, relatives, and/or referring physicians by phone and/or mail with questions regarding vital status, recurrent VTE, bleeding, stroke, acute myocardial infarction, invasive procedures, and status of anticoagulation therapy. The independent clinical event committee (Online Appendix 3) unaware of the patient characteristics reviewed all the clinical events with the clinical course in Japanese, and checked the accurateness and validity of data entry. Data for international normalized ratio (INR) during follow-up in patients receiving warfarin were collected from the hospital charts of the centers where the index VTE was diagnosed. Time in therapeutic range (TTR) was calculated by the Rosendaal method [17] according to a therapeutic INR range of 1.5–2.5, which is recommended in the Japanese guidelines [18], as well as according to a therapeutic INR range of 2.0–3.0, which is recommended in the Western guidelines [8,9,10]. In the current study, we evaluated the clinical outcomes at 90 days after the bleeding events among patients with bleeding events, and all the clinical outcomes were evaluated irrespective of the status of anticoagulation therapy after the bleeding events.
The primary outcome measure in the current study was all-cause death at 90 days after the bleeding events. The independent clinical event committee (Online Appendix 3) unaware of the patient characteristics reviewed all the death events, and classified the causes of deaths as due to bleeding events, due to cancers, due to PE, due to other non-cardiac causes, due to cardiac causes, or due to unknown causes [19]. Death was judged to be bleeding related if it followed an intracranial hemorrhage or a bleeding episode leading to hemodynamic deterioration. Death in patients with the end-stage cancer without a specific cause of death was regarded as cancer in origin. Death was judged to be due to PE (fatal PE) if it was confirmed by autopsy or if death followed a clinically severe PE, either initially or after recurrent pulmonary embolic events. Final classifications for the causes of deaths were made on the basis of the full consensus of the independent clinical event committee.
The secondary outcome measure in the current study were recurrent VTE, bleeding, acute myocardial infarction, and ischemic stroke at 90 days after the bleeding events. Recurrent VTE was defined as PE and/or DVT with symptoms accompanied by confirmation of new thrombus or exacerbation of the thrombus by objective imaging examinations or autopsy [20, 21]. Acute myocardial infarction was defined in accordance with the universal myocardial infarction guidelines [22]. Ischemic stroke was defined stroke either requiring or prolonging hospitalization with symptoms lasting more than 24 h accompanied by confirmation by objective imaging examinations.
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables are presented as the mean and standard deviation or the median and interquartile range (IQR) based on their distributions. Categorical variables were compared using the chi-squared test when appropriate; otherwise, Fisher’s exact test was used. Continuous variables were compared using one-way analysis of variance or Kruskal–Wallis test based on their distributions. The 90-day clinical outcomes after the bleeding events are presented as numbers of events and percentages with 95% confidence intervals (CI). Furthermore, to explore the potential risk factors for 90-day all-cause death after the all bleeding events, we constructed a multivariable logistic regression models to estimate the odds ratio (OR) and the 95% CI of the potential risk factors. Based on the previous reports [8,9,10, 23] and consideration of clinical relevance, we selected those potential risk factors such as age, sex, location of thrombus at diagnosis, chronic kidney disease, active cancer, anemia, thrombocytopenia, bleeding type (major bleeding or CRNM bleeding), and timing of bleeding events after diagnosis of the index VTE (bleeding events within 90 days after VTE diagnosis or beyond 90 days). All statistical analyses were conducted using JMP version 14.0.0 (SAS Institute Inc., Cary, NC, USA). All reported P-values were 2-tailed, and P-values < 0.05 were considered statistically significant.
Results
Patient characteristics
The mean age of the study population was 68 years, 38% were men, and mean body weight and body mass index were 57.4 kg and 23.1 kg/m2, respectively, and 2273 patients (95%) received warfarin as anticoagulation therapy. According to the first bleeding event, there were 189 patients (7.9%) with major bleeding, 147 patients (6.2%) with CRNM bleeding, and 2054 patients (86%) without bleeding during anticoagulation therapy (Fig. 1). The patient characteristics were different in several aspects across the three groups (Table 1). Patients in the two groups with bleeding during anticoagulation therapy had a lower mean body weight and body mass index, and more often had chronic kidney disease, active cancer, anemia, and thrombocytopenia than those without bleeding. Patients with major bleeding showed the highest-bleeding risk profile based on VTE BLEED score (Table 1). The median TTR for warfarin users according to a therapeutic INR range of 1.5–2.5 in the Japanese guidelines were 61.3 in patients with major bleeding, 57.1 in those with CRNM bleeding and 72.6 in those without bleeding events (P < 0.001) (Table 1).
Bleeding events and management strategies of anticoagulants
The detailed timing of bleeding events after VTE diagnosis is described in Supplementary Fig. 1, and 59% (111 patients) of major bleeding and 42% (62 patients) of CRNM bleeding during anticoagulation therapy occurred within 90 days after the VTE diagnosis (Table 1). The median INR values at bleeding events were 2.6 in major bleeding and 2.1 in CRNM bleeding. Among 189 patients with major bleeding during anticoagulation therapy, 142 patients (75%) discontinued anticoagulants, of whom 63 patients (44%) restarted anticoagulants with a median interval of 14 days from bleeding to restart. Among 147 patients with CRNM bleeding during anticoagulation therapy, 95 patients (65%) discontinued anticoagulants, of whom 43 patients (45%) restarted anticoagulants with a median interval of 3 days from bleeding to restart.
Clinical outcomes after bleeding events
Among the patients with bleeding during anticoagulation therapy, 58 patients (30.7%) in the major bleeding group, and 30 patients (20.4%) in the CRNM bleeding group died within 90 days after the bleeding events (Table 2). Of the 58 deaths after major bleeding, deaths due to bleeding events accounted for 22 (38%), and deaths due to cancer accounted for 23 (40%). The incidence of bleeding-related death after major bleeding was 11.6%. Among 22 bleeding-related death, 10 deaths (45%) and 8 deaths (36%) were due to intracranial bleeding and gastrointestinal bleeding, and the median day from bleeding to death was 1 day. Among the 30 deaths in patients with CRNM bleeding during anticoagulation therapy, no patients died due to bleeding events, and deaths due to cancer accounted for 18 (60%).
Patients with major bleeding during anticoagulation therapy experienced recurrent VTE in 8 patients (4.2%), recurrent bleeding in 9 patients (4.8%) and ischemic stroke in 2 patients (1.1%), and no patient experienced acute myocardial infarction at 90 days after the bleeding events (Table 2). Patients with CRNM bleeding during anticoagulation therapy experienced recurrent VTE in 8 patients (5.4%), recurrent bleeding in 6 patients (4.1%) and ischemic stroke in 2 patients (1.4%), and no patient experienced acute myocardial infarction at 90 days after the bleeding events.
Among 142 patients who discontinued anticoagulants, patients with temporary discontinuation accounted for 63 patients (44%) and those with permanent discontinuation accounted for 79 patients (56%). Among 95 patients who discontinued anticoagulants, patients with temporary discontinuation accounted for 43 patients (45%) and those with permanent discontinuation accounted for 52 patients (55%). Patient characteristics according to the status of anticoagulation therapy after the bleeding events are shown in Online Table 1. Patients who discontinued anticoagulation therapy permanently showed a higher prevalence of bleeding within 90 days after VTE diagnosis compared with patients who continued anticoagulation therapy and patients who restarted anticoagulation therapy. As for clinical outcomes according to the status of anticoagulation therapy, recurrent VTE and recurrent bleeding occurred in 2 patients (2.0%) and in 1 patients (1.0%) who continued anticoagulation therapy, in 8 patients (7.5%) and in 8 patients (7.5%) who restarted anticoagulation therapy (temporary discontinuation) and in 6 patients (4.6%) and in 6 patients (4.6%) who discontinued anticoagulation therapy permanently (permanent discontinuation) (Online Table 2).
Independent risk factors for all-cause death within 90 days after the all bleeding events
The multivariable logistic regression model revealed that active cancer and bleeding events within 90 days after VTE diagnosis were independently associated with all-cause death within 90 days after the all bleeding events (active cancer: OR 5.05, 95% CI 2.82–9.05; bleeding events within 90 days after VTE diagnosis: OR 2.23, 95% CI 1.25–3.96) (Online Table 3). The absolute 90-day incidence rate of all-cause death after bleeding events in patients with active cancer and that in those without were 46.4% (64/138) and 12.1% (24/198). Major bleeding was associated with a trend toward higher risk for all-cause death within 90 days after the bleeding events as compared with CRNM bleeding, although it was not statistically significant (OR 1.59, 95% CI 0.90–2.82).
Discussion
The main findings of the current study were as follows: (1) Anticoagulants were discontinued in 65–75% of patients who experienced bleeding events during anticoagulation therapy, and restarted in 44–45% of patients among those who had discontinued anticoagulants after a median interval of 14 days from major bleeding to restart and 3 days from CRNM bleeding to restart; (2) Approximately 30% of patients died within 90 days after the major bleeding, and 38% of deaths were due to bleeding events; (3) Active cancer and bleeding events within 90 days after VTE diagnosis were independent risk factors for all-cause death within 90 days after the bleeding events.
When patients with VTE experience clinically relevant bleeding events during anticoagulation therapy, anticoagulant therapy usually is discontinued or even reversed, aiming at achieving hemostasis. The important question for clinicians is whether anticoagulants should be restarted or discontinued permanently. A previous study reported that restart of anticoagulants after intracranial hemorrhage in patients with atrial fibrillation was associated with a lower rate of thromboembolic events and a higher rate of recurrent intracranial hemorrhage, compared with persistent discontinuation of anticoagulants [24]. Another study reported that restart of anticoagulants after upper gastrointestinal bleeding in patients with anticoagulants was associated with a reduced risk of thromboembolism and all-cause death, but with an increased risk of recurrent gastrointestinal bleeding [25]. However, there has been limited data in patients with VTE. The current VTE guidelines state only a few non-specific recommendations for this issue [8,9,10]. The American Society of Hematology 2018 guidelines weakly recommends restart of anticoagulants within 90 days rather than persistent discontinuation for patients with VTE who survive an episode of major bleeding based on very low certainty in the evidence [11]. The current study showed more than half of patients with bleeding events during anticoagulation therapy discontinued anticoagulants at least temporally after a diagnosis of bleeding events, suggesting that control of bleeding and avoidance of recurrent bleeding are often the priority of clinicians in the real world practice. The current study showed that patients diagnosed with major or CRNM bleeding faced a high incidence of relevant complications. Because the incidence rates of recurrent VTE and recurrent bleeding were numerically higher in patients who restarted anticoagulant therapy than in those patients who discontinued anticoagulant therapy permanently, it may be assumed that the assumed risk of recurrent VTE plays a major role in anticoagulation management decisions after bleeding complications. Interestingly, the incidence rate of recurrent bleeding in patients who discontinued anticoagulant therapy permanently was non-neglectable and in the same order of magnitude than the incidence rate of recurrent VTE.
Previous studies performed mostly in patients with atrial fibrillation reported that mortality rates after major bleeding during anticoagulation therapy ranged from 10 to 20% and up to more than 30% for intracranial hemorrhage [5, 26, 27]. Consistent with previous reports, the current study showed a relatively high mortality rate within 90 days after major bleeding (30.7%), which could be explained by the large proportion of patients with active cancer. Notably, deaths due to bleeding events accounted for more than one third of all mortalities, and bleeding-related deaths occurred soon after bleeding events, which suggested the clinical importance of anticoagulation-related bleeding events. The previous studies reported the case-fatality rate of long-term major bleeding events was 8–10% [5, 6], which was line with the current study (bleeding-related death rate of 11.6%). Even so, the most common cause of death was due to cancer, which should be taken into consideration when evaluating the clinical impact of major bleeding on mortality in patients with VTE. Patients with advanced cancer could be at a high risk of bleeding [28] as well as at a high risk of mortality after bleeding events. In daily clinical practice, patient preference of anticoagulation therapy also could have a certain influence on management strategies of anticoagulation therapy.
The risk factors for mortality after bleeding events found in the current study inform VTE caretakers on the prognosis of patients with bleeding complications and may help determining the decision to continue or discontinue anticoagulation therapy. From the current study, we cannot conclude that either restarting or permanently discontinuing anticoagulation therapy is the optimal strategy for all patients. If anything, the current study confirms the major impact of a bleeding complication on the prognosis of a VTE patient, and shows that management decisions are mostly based on the specific risk profile of the patients. Considering the low incidence of fatal VTE and fatal bleeding in patients with a first-anticoagulant associated VTE, the chosen anticoagulation strategy may not be the most important determinant of survival.
Study limitations
The current study has several limitations. First, the current study was an observational study, which can be subject to various biases inherent to observational study design. The therapeutic decision-making was left to the discretion of the attending physicians, which could have influences on clinical outcomes. In addition, the current study was conducted based on the search through hospital databases for clinical diagnosis and imaging examinations, and collection of follow-up information was mainly conducted through review of hospital charts. Second, detailed management strategies of medical intervention for hemostasis such as surgical treatment, plasma transfusion, use of prothrombin complex concentrate were not evaluated in the current study, which should be interpreted with caution. Third, demographics, practice patterns as well as clinical outcomes in patients with VTE in Japan may be different from those outside Japan. Fourth, the current study evaluated long-term bleeding events, and clinical features of bleeding events could be changed during the follow-up period. Fifth, the clinical outcomes according to the status of anticoagulation therapy after the bleeding events could be influenced by immortal time bias. Finally and most importantly, the current study was conducted before introduction of direct oral anticoagulants for VTE in Japan. Thus, it should be interpreted with caution whether the present results could be extrapolated to patients treated with direct oral anticoagulants.
Conclusions
In this practice-based large registry, anticoagulants were frequently discontinued in patients who experienced major bleeding events during anticoagulation therapy and nearly half of them restarted anticoagulants with mortality rate of approximately 30% within 90 days after the bleeding events, and active cancer was the most prevalent cause of death, which could help guiding anticoagulation strategies in the absence of randomized clinical trials.
References
Huisman MV, Barco S, Cannegieter SC, Le Gal G, Konstantinides SV, Reitsma PH, Rodger M, Vonk Noordegraaf A, Klok FA (2018) Pulmonary embolism. Nat Rev Dis Primers 4:18028. https://doi.org/10.1038/nrdp.2018.28
Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N (2015) Venous thrombosis. Nat Rev Dis Primers 1:15006. https://doi.org/10.1038/nrdp.2015.6
Landefeld CS, Beyth RJ (1993) Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 95(3):315–328. https://doi.org/10.1016/0002-9343(93)90285-w
Klok FA, Huisman MV (2020) How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood 135(10):724–734. https://doi.org/10.1182/blood.2019001605
Linkins LA, Choi PT, Douketis JD (2003) Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 139(11):893–900. https://doi.org/10.7326/0003-4819-139-11-200312020-00007
Khan F, Tritschler T, Kimpton M, Wells PS, Kearon C, Weitz JI, Buller HR, Raskob GE, Ageno W, Couturaud F, Prandoni P, Palareti G, Legnani C, Kyrle PA, Eichinger S, Eischer L, Becattini C, Agnelli G, Vedovati MC, Geersing GJ, Takada T, Cosmi B, Aujesky D, Marconi L, Palla A, Siragusa S, Bradbury CA, Parpia S, Mallick R, Lensing AWA, Gebel M, Grosso MA, Thavorn K, Hutton B, Le Gal G, Fergusson DA, Rodger MA, Collaborators M (2021) Long-term risk for major bleeding during extended oral anticoagulant therapy for first unprovoked venous thromboembolism: a systematic review and meta-analysis. Ann Intern Med 174(10):1420–1429. https://doi.org/10.7326/M21-1094
Carrier M, Le Gal G, Wells PS, Rodger MA (2010) Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med 152(9):578–589. https://doi.org/10.7326/0003-4819-152-9-201005040-00008
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jimenez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ni Ainle F, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL, Group ESCSD (2020) 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, Vedantham S, White RJ, Zierler BK, American Heart Association Council on Cardiopulmonary CCP, Resuscitation, American Heart Association Council on Peripheral Vascular D, American Heart Association Council on Arteriosclerosis T, Vascular B (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123(16):1788–1830. https://doi.org/10.1161/CIR.0b013e318214914f
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, Huisman M, King CS, Morris TA, Sood N, Stevens SM, Vintch JRE, Wells P, Woller SC, Moores L (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149(2):315–352. https://doi.org/10.1016/j.chest.2015.11.026
Witt DM, Nieuwlaat R, Clark NP, Ansell J, Holbrook A, Skov J, Shehab N, Mock J, Myers T, Dentali F, Crowther MA, Agarwal A, Bhatt M, Khatib R, Riva JJ, Zhang Y, Guyatt G (2018) American Society of Hematology 2018 guidelines for management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv 2(22):3257–3291. https://doi.org/10.1182/bloodadvances.2018024893
Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Konishi T, Akao M, Kobayashi Y, Inoue T, Oi M, Izumi T, Takahashi K, Tada T, Chen PM, Murata K, Tsuyuki Y, Sakai H, Saga S, Sasa T, Sakamoto J, Yamada C, Kinoshita M, Togi K, Ikeda T, Ishii K, Kaneda K, Mabuchi H, Otani H, Takabayashi K, Takahashi M, Shiomi H, Makiyama T, Ono K, Kimura T, Investigators CVR (2018) Anticoagulation therapy for venous thromboembolism in the real world—from the COMMAND VTE Registry. Circ J 82(5):1262–1270. https://doi.org/10.1253/circj.CJ-17-1128
Yamashita Y, Morimoto T, Amano H, Takase T, Hiramori S, Kim K, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Nishimoto Y, Saga S, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Yoshikawa Y, Shiomi H, Kato T, Makiyama T, Ono K, Kimura T, Investigators CVR (2020) Usefulness of simplified pulmonary embolism severity index score for identification of patients with low-risk pulmonary embolism and active cancer: from the COMMAND VTE Registry. Chest 157(3):636–644. https://doi.org/10.1016/j.chest.2019.08.2206
Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353(9162):1386–1389. https://doi.org/10.1016/s0140-6736(98)07534-5
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4):692–694. https://doi.org/10.1111/j.1538-7836.2005.01204.x
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of A (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13(11):2119–2126. https://doi.org/10.1111/jth.13140
Rosendaal FR, Cannegieter SC, van der Meer FJ, Briet E (1993) A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost 69(3):236–239
Group JCSJW (2011) Guidelines for the diagnosis, treatment and prevention of pulmonary thromboembolism and deep vein thrombosis (JCS 2009). Circ J 75(5):1258–1281. https://doi.org/10.1253/circj.cj-88-0010
Faller N, Limacher A, Mean M, Righini M, Aschwanden M, Beer JH, Frauchiger B, Osterwalder J, Kucher N, Lammle B, Cornuz J, Angelillo-Scherrer A, Matter CM, Husmann M, Banyai M, Staub D, Mazzolai L, Hugli O, Rodondi N, Aujesky D (2017) Predictors and causes of long-term mortality in elderly patients with acute venous thromboembolism: a prospective cohort study. Am J Med 130(2):198–206. https://doi.org/10.1016/j.amjmed.2016.09.008
Barco S, Konstantinides S, Huisman MV, Klok FA (2018) Diagnosis of recurrent venous thromboembolism. Thromb Res 163:229–235. https://doi.org/10.1016/j.thromres.2017.05.026
Huisman MV, Klok FA (2013) Diagnostic management of acute deep vein thrombosis and pulmonary embolism. J Thromb Haemost 11(3):412–422. https://doi.org/10.1111/jth.12124
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S (2012) Third universal definition of myocardial infarction. Circulation 126(16):2020–2035. https://doi.org/10.1161/CIR.0b013e31826e1058
Kim K, Yamashita Y, Morimoto T, Kitai T, Yamane T, Ehara N, Kinoshita M, Kaji S, Amano H, Takase T, Hiramori S, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Saga S, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Shiomi H, Kato T, Makiyama T, Ono K, Furukawa Y, Kimura T, Investigators CVR (2019) Risk factors for major bleeding during prolonged anticoagulation therapy in patients with venous thromboembolism: from the COMMAND VTE Registry. Thromb Haemost 119(9):1498–1507. https://doi.org/10.1055/s-0039-1692425
Nielsen PB, Larsen TB, Skjoth F, Lip GY (2017) Outcomes associated with resuming warfarin treatment after hemorrhagic stroke or traumatic intracranial hemorrhage in patients with atrial fibrillation. JAMA Intern Med 177(4):563–570. https://doi.org/10.1001/jamainternmed.2016.9369
Majeed A, Wallvik N, Eriksson J, Hoijer J, Bottai M, Holmstrom M, Schulman S (2017) Optimal timing of vitamin K antagonist resumption after upper gastrointestinal bleeding. A risk modelling analysis. Thromb Haemost 117(3):491–499. https://doi.org/10.1160/TH16-07-0498
Halbritter K, Beyer-Westendorf J, Nowotny J, Pannach S, Kuhlisch E, Schellong SM (2013) Hospitalization for vitamin-K-antagonist-related bleeding: treatment patterns and outcome. J Thromb Haemost 11(4):651–659. https://doi.org/10.1111/jth.12148
Beyer-Westendorf J, Forster K, Pannach S, Ebertz F, Gelbricht V, Thieme C, Michalski F, Kohler C, Werth S, Sahin K, Tittl L, Hansel U, Weiss N (2014) Rates, management, and outcome of rivaroxaban bleeding in daily care: results from the Dresden NOAC registry. Blood 124(6):955–962. https://doi.org/10.1182/blood-2014-03-563577
Nishimoto Y, Yamashita Y, Kim K, Morimoto T, Saga S, Amano H, Takase T, Hiramori S, Oi M, Akao M, Kobayashi Y, Toyofuku M, Izumi T, Tada T, Chen PM, Murata K, Tsuyuki Y, Sasa T, Sakamoto J, Kinoshita M, Togi K, Mabuchi H, Takabayashi K, Yoshikawa Y, Shiomi H, Kato T, Makiyama T, Ono K, Sato Y, Kimura T, Investigators CVR (2020) Risk factors for major bleeding during anticoagulation therapy in cancer-associated venous thromboembolism—from the COMMAND VTE Registry. Circ J 84(11):2006–2014. https://doi.org/10.1253/circj.CJ-20-0223
Acknowledgements
We appreciate the support and collaboration of the co-investigators participating in the COMMAND VTE Registry. We are indebted to the independent clinical research organization (Research Institute for Production Development, Kyoto, Japan) for technical support.
Funding
The COMMAND VTE Registry is supported by the independent clinical research organization (Research Institute for Production Development, Kyoto, Japan) and research funding from Mitsubishi Tanabe Pharma Corporation. The research funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
YY, FK, SB contributed to the design of the study, the analysis and interpretation of data, and drafted the manuscript. TM and TK contributed to the analysis and interpretation of data, and drafted the manuscript. All other authors contributed to acquisition and interpretation of data, and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Yamashita received lecture fees from Daiichi-Sankyo, Bristol-Myers Squibb, Pfizer, and Bayer Healthcare. Dr Morimoto reports lecturer's fees from Bristol-Myers Squibb, Daiichi Sankyo, Japan Lifeline, Kowa, Kyocera, Novartis, and Toray; manuscript fees from Bristol-Myers Squibb and Kowa; advisory board for Sanofi. Dr. Klok reports research grants from Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, MSD, Daiichi-Sankyo, Actelion, the Dutch thrombosis association, The Netherlands Organization for Health Research and Development and the Dutch Heart foundation, all outside the current work. Dr. Nishimoto received lecture fees from Daiichi-Sankyo, Bristol-Myers Squibb, Pfizer, and Bayer Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For the COMMAND VTE Registry of retrospective study formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamashita, Y., Morimoto, T., Klok, F.A. et al. Anticoagulation strategies and clinical outcomes after bleeding events during anticoagulation therapy for venous thromboembolism in the practice-based Japanese registry. J Thromb Thrombolysis 54, 524–534 (2022). https://doi.org/10.1007/s11239-022-02665-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-022-02665-x