Abstract
The accuracy of non-contrast MRI in diagnosing acute deep vein thrombosis (DVT) of the lower extremities is different. To explore the application of high-resolution non-contrast 3D CUBE T1-weighted MRI in the lower extremities DVT. We recruited 26 patients suspected DVT of the lower extremities from Hebei General Hospital in China. All patients underwent high-resolution non-contrast 3D CUBE T1-weighted MRI. We evaluated the sensitivity, specificity, positive predictive value, and negative predictive value of diagnosing thrombosis. And we divided thrombi into two parts: filling thrombus (FT) and non-filling thrombus (NFT), compared the agreement between MRI and Ultrasound (US) and analysed the locations of thrombi. Compared with US, MRI yielded a sensitivity of 79%, a specificity of 94.2% in mean value, a sensitivity of 85.7%, 97.4%, and 51.7% in iliac, femoral-popliteal, and calf segments respectively, a specificity of 97.6%, 88.3%, and 98.2% in iliac, femoral-popliteal, and in calf segments respectively. The accuracy of MRI in the diagnosis of lower extremity DVT was in very good agreement (κ = 0.711, 95% CI 0.627, 0.795). The FT was the most part in US and CUBE (68/56), CUBE can detect more NFT in femoral vein than US (22/4). 3D CUBE T1-weighted MRI can be used to accurately diagnose acute DVT and detect more NFT. It has the potential of follow-up at the end of treatment to establish a new baseline to stop anticoagulant drug.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Deep vein thrombosis (DVT) is common in clinic and can cause disability.
-
Non-contrast 3D CUBE sequence has been used in cerebral venous thrombosis but not in DVT of the lower limbs.
-
Non-contrast 3D CUBE sequence has a high sensitivity and specificity in showing thrombosis compared with ultrasound and distinguish the type of thrombosis.
-
CUBE has prior to ultrasound in detecting non-filling thrombus.
Introduction
Deep vein thrombosis (DVT) is commonly seen in the clinic and is associated with a high disability rate. The cumulative incidence of post-thrombotic syndrome (PTS) is 22.8–43% in patients after DVT [1, 2]. There is a twofold or higher risk of recurrent DVT after stopping anticoagulant therapy for patients with ongoing non-malignant conditions [3]. However, it is not known if this is due to a lack of efficiency or detection of DVT. The most appropriate management with anticoagulation drugs may eventually result in recurrent DVT or PTS [4]. Misdiagnosis is also associated with an increased risk of recurrent DVT, and the development of pulmonary embolism, and PTS [5].
Ultrasound (US) is the recommended first choice for evaluating DVT. US, has high sensitivity and specificity for patients with symptomatic lower extremity DVT, even though a low sensitivity for the pelvic and calf regions as well as for asymptomatic patients [6]. Meanwhile, depending on the protocol, iliac segment thrombosis is often misdiagnosed [7]. US has difficulty in identifying and interpret recurrent DVT from residual thrombosis, the accuracy of diagnosing is controversial [7]. Computed tomography venography (CTV), and magnetic resonance venography (MRV) or contrast-enhanced MRV (CE-MRV) have been used as secondary methods. The presence of thrombosis was a filling defect in these two techniques, which often fill in the whole lumen. They all had high sensitivity and specificity in diagnosing DVT [8,9,10]. CTV has difficulty in diagnosing femoral segment thrombi since reflux with contrast agent and blood dilution without contrast agent, resulting in uneven density, easy to form false-positive thrombus [11]. And MRV or CE-MRV is for eddy current interference imaging at the bifurcation of blood vessels.
A newly emerging Magnetic resonance imaging (MRI) technique can detect thrombus components and blood coagulation. The use of T1-weighted imaging as an endogenous contrast agent, that is methemoglobin, allows for quantification of the thrombus components and is termed direct thrombus imaging (DTI). DTI does not only demonstrate the lumen thrombus but also shows the wall. Current research clarifies a high sensitivity and specificity of diagnosing thrombosis by DTI. 3D CUBE T1-weighted (T1W) MRI is a high-resolution black blood imaging method based on an ultra-long echo train length and multiple flip angles [12, 13]. This technique also shows an excellent signal-to-noise ratio by advanced suppression of the vessel wall signal and intravascular blood flow artifacts of the femoral vein [14]. It has been widely used in cerebral venous thrombosis[15, 16]. Our study was to evaluate its capability in diagnosing deep venous thrombosis, further we divided thrombi into different types and evaluated the presence by CUBE and US.
Methods
Study populations
We enrolled twenty-six patients with suspected acute DVT in hospital at Hebei General Hospital in China between April 2018 and October 2019. The study is a prospective, single-center study. Inclusion criteria for this study are: (1) age ≥ 18 years, (2) ability of participant to understand the character and individual consequences of this study, (3) all patients have taken US and MRI examinations, and the interval time is less than 5 days. Exclusion criteria are: (1) general contraindications for MRI, (2) survived acute conditions, such as acute pulmonary embolism, acute symptomatic DVT, coma or hemodynamic instability, et al. This study was approved by the institute’s ethics committee and all patients provided written informed consent.
MRI examination
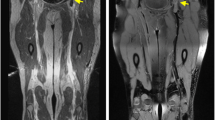
Patients underwent MRI using a 3.0 T GE scanner (Discovery MR750w, GE Healthcare, USA) with a 12-channel phased-array body coil and a 24-channel phased-array spine coil. Patients were examined feet-first in a supine position with the body array coils covering from the navel to the thigh area (Fig. 1a), the middle femoris to the calf. First, coronal T2-weighted IDEAL imaging was used to locate the height of the inferior vena cava opening followed by coronal 3D CUBE T1W fat-suppressed imaging (Fig. 1b). The positioning of 3D CUBE T1W sequence included the inferior vena cava opening and the pubic symphysis was included in the sagittal frame (Fig. 1c). The scanning parameters were as follows: 40 × 40 cm FOV, 640 ms TR, TE minimum, 2.0 NEX, 62.50 bandwidth, 256 × 192 matrix, 248 layers, and 6′55″ duration.
Compression US examination
US examinations were performed using a PHILIPS EPIQ 7 machine. The standardized protocol involved machine setting using optimal contrast, adaptive image processing (AIP), and broadband harmonics. A line array transducer, L12-3 (frequency range 3–7 MHZ), was used when studying the venous system from the vena cava down towards the venous tibial confluence below the knee, with the patient in a supine position. Consequently, the patient was examined in an upright standing position. Throughout the examination, all color modalities available on the machine were applied to determine flow, in particular directional E-flow, in both transverse and longitudinal planes. Venous compression in a transverse plane was used to determine the level of obstruction in each segment. Inspiration and expiration were used to enhance flow as well as to displace bowel gases when necessary. The examination was performed with the same equipment and by 5-year experienced physicians.
Diagnostic evaluation
The CUBE imagines were evaluated by two independent readers with 5-year experience in image reading, who were blinded to the final clinical diagnosis. Observing the following vessel segments of both sides: the common and external iliac vein, the common, superficial, and deep femoral vein, as the first part scan; the popliteal vein, the anterior and posteriors tibial vein, the peroneal vein, as well as intramuscular, as the second part scan. MRI used the signal from muscle tissue as the reference standard to classify the thrombus signal according to the characteristics of different thrombus components [17]. Thrombosis in the acute phase consists mostly of high-intensity than adjacent muscles, chronic phase thrombosis exhibits low or iso-signal near muscles accompanied by thickened and irregular blood vessel walls.
According to the shape and signal of CUBE, thrombi were divided into the following two patterns.
-
1.
Filling thrombus (FT) was demonstrated diffuse or strip-like high signal in the cavity, lumen expanded or not (Fig. 2).
-
2.
Non-filling thrombus (NFT) was demonstrated with high signal than muscles in the wall, high signal noted as node or ring in the wall, the vessel’s cavity was seen (Fig. 3).
Blocked or strip-like low or iso signals were ignored, which was describes as chronic thrombus or residual material. Arc low or iso signals were considered as artifacts in three-dimension construction. (Fig. 3).
Based on the ultrasound guidelines for blood vessels [18], thrombi were divided into the following two patterns [19].
-
1.
FT was demonstrated as a diffuse or strip-like echoic clot within the venous lumen. The color flow was not detected or just line flow and the vein was noncompressible. Below the obstruction site, vein distention was found in limbs (Fig. 2).
-
2.
NFT was usually demonstrated as a laminar echogenic clot adhering to the venous intima, the intima was rough and the vessel wall was thick. The color doppler signal could be detected with filling defect, and the vein was not completely compressible, the lumen became stenotic and velocity increased (Fig. 3).
Statistical analysis
All experimental data were statistically analysed using SPSS 24.0. We calculated Cohen’s kappa coefficient and intra-class correlation coefficient to determine the sensitivity, specificity, and positive and negative predictive value (Fig. 4).
Results
Baseline characteristics
Among the 26 suspected acute DVT patients, based on Chinese guidelines for the prevention and treatment of thrombosis [20], nine cases were highly possibility, 17 cases were low possibility, among them, two cases were excluded for negative D-dimer value (< 500 ng/ml). All remaining 24 patients underwent US and 3D CUBE MRI examinations (Table 1). The average age was 60 years (range 26–86 years) and seven cases were male. Twenty-four patients were diagnosed with acute DVT by US with a total of 480 venous vascular segments and 81 lesions, of which 14 were in the iliac vein, 38 were in the femoral-popliteal vein, and 29 were in the calf vein. The average interval between MRI and US examination was 1.3 days.
Evaluation of the thrombus
The interobserver agreement was very good in the evaluation of the iliac thrombosis (κ = 0.838; 95% CI 0.685, 0.991), good in the evaluation of the femoral-popliteal thrombosis (κ = 0.733; 95% CI 0.623, 0.843), moderate in the calf thrombosis (κ = 0.591; 95% CI 0.419, 0.763). The result was highly consistent with US, and had a sensitivity of 79%, a specificity of 94.2%, a positive predictive value of 73.5%, a negative predictive value of 95.7%, and Youden Index of 0.73. A sensitivity of 85.7%, 97.4%, and 51.7%, a specificity of 97.6%, 88.3%, and 98.2%, a positive predictive value of 85.7%, 67.3%, and 83.3%, a negative predictive value of 97.6%, 99.3%, and 93.1% in iliac, femoral-popliteal, and calf segments, respectively. (Table 2).
Thrombi were detected in 480 vessel segments by US and CUBE, FT was 68 and 56, NFT was 13 and 31. Table 3 shows the locations and patterns of thrombi detected by US and CUBE.
Discussion
We demonstrated 3D CUBE T1W sequence had high sensitivity and specificity in diagnosing thrombus and had a good consistent with US. Importantly we first classified the patterns of thrombi by MRI. The filling type was the most common in our series. CUBE can detect more non-filling type thrombus than US. To date, no study has reported the comparison of the different types of thrombus between US and MRI.
Non-contrast DTI sequence achieved a high sensitivity (SE) (77.78–98%) and specificity (SP) (88–99%) in diagnosing DVT [21,22,23]. In our study, we had a consistency of SE and SP with literature in iliac, femoral-popliteal segment thrombosis (85.7% and 97.2%, 97.4% and 88.3%). But it was far below in calf segment to the literature, we thought it was because we used body oil, which was larger than the bilateral calf, ambient noise increased the signal of muscle as well as decreased the differences between thrombosis and muscle, leading to misdiagnosing strikingly exalted. The agreement between CE-MRI and US was excellent (κ = 0.857) [24]. Our results were very good (κ = 0.711), coincident with non-contrast MRI [25], despite lower than CE-MRI. In femoral-popliteal thrombosis, the consistency was not better. In one case, MRI examination was taken 4 days later than US. US diagnosed thrombosis in left common and external iliac vein, femoral vein, popliteal vein, but only left femoral and posterior tibial thrombi by CUBE. During the interval, the patient was administrated low molecular weight heparin at 100 unit per kilogram body weight hypodermic, and oral warfarin once diagnosed thrombosis by US, most may be dissolved when MRI was taken.
Accurate diagnosis of DVT is important for patients not to be treated with overdoing anticoagulation, extra risk and cost, meanwhile decrease potential risks of DVT extension and embolization [26]. CUBE, a high-spatial-resolution 3D T1W TSE sequence, has emerged as one of the leading non-invasive imaging modalities allowing for directly visualizing diseased vessel–wall [27], and it allows for a better distinction between arterial wall and lumen due to the black blood effect [27]. Incomplete resolution of thrombus occurs in up to 30–50% of patients after DVT [28], thus separate FT and NFT is important. In our study, we first classified the types of thrombi into two parts: FT and NFT. CUBE can detect more non-filling thrombi than US in femoral vein (22/4), while equal in iliac (4/4), popliteal (4/4), and calf vein (4/4). The FT number of CUBE was close to US in all segments. Either CUBE can be a good substitute tool in diagnosing DVT or has an advantage in detecting small thrombus.
The NFT may resolve completely or scar. Thrombus becomes infiltrated with fibroblasts over several months, fibrosis producing scaring, wall thickening [7]. In the Ultrasound guideline, repeat ultrasound at or near the end of anticoagulation is recommended to establish a new baseline and to determine if scaring is present [7]. DVT can cause thickening of the vein wall, which in turn may lead to thrombosis [29]. The formation of scar tissues during thrombolysis is accompanied by thrombus adhesion, lumen dilation, and subsequent fibrotic changes [29]. Thrombosis in the chronic phase is manifested as a scar on the blood vessel wall composed of collagen [30]. In our study, non-filling thrombosis presents node, ring-like, the wall was thick in different level, some cavity became stenosis. NFT often was too small to be noticed in suspected patients, let alone in asymptomatic patients. Thrombus is not considered by US if the diameter increases by less than 2 mm [31]. In an animal study, the establishment of a nonocclusive thrombus in the femoral vein was observed at the site of vessel trauma within two or three minutes [32]. For the detection of thrombi in proximal veins in asymptomatic patients by US, specificities were maintained, but sensitivity was lower, up to 47–62%, and in calf was around 50% [33], far away from the sensitivity of 89–96% in symptomatic patients [33]. DVT in iliac-femoral has a high risk to occur pulmonary embolism with reported incidence rates of up to 33.7% [34]. US has limited diagnostic capacity above the inguinal canal and below the knee joint [35] while 20% of DVT occurs in the pelvic iliac femoral vein [36]. CUBE can detect more non-filling thrombosis than US (18/0). Four NFT all were diagnosed by US and CUBE. Three cases were diagnosed NFT by CUBE and FT by US, which may be associated with the interval time or the several stenoses.
Either FT or NFT, acute DVT burden increased the risk of residual venous obstruction (RVO), following ROV was a significant risk factor for DVT recurrence or propagation (rDVT) (OR, 3.90) [37]. A parallel randomized trial demonstrated tailoring the duration of anticoagulation based on residual thrombosis findings reduces the rate of recurrent VTE in adults with proximal DVT [38]. Thrombus above popliteal produces pulmonary thrombosis easily [39]. A popliteal vein rarely develops into a distal thrombus pe, but if not treated, 15% develops into a proximal thrombus [40]. CUBE can directly observe the size and location of thrombosis. Therefore, MRI may be superior for the detection of small thrombus, allowing clinicians to adjust anticoagulation treatments. In our study, the capability of CUBE in detecting non-filling thrombosis was over than US, we suggested CUBE was recommended or assisted to establish the new baseline at the ending the treatment.
Limitations
There are two shortcomings in this study. First, the positive predictive value was low. This is because once patients were diagnosed DVT by US, they were required to lie in bed, don’t moving. Second, we just classified two types of thrombosis. There were three types: FT, local thrombus, mural thrombus [19], but this was the first and only time to classify thrombosis, however, this is not mentioned in the guideline of Ultrasound in Chinese [18] or American [7], we combined the latter two for easy evaluation. Further, we should classify more types to ensure the risk in different types and follow-up is needed.
Conclusion
In short, 3D CUBE T1 sequence can become the first-line replacement when US cannot be used as a detection method. CUBE can test more NFT than US in the femoral vein, maybe it can be the baseline of stop anticoagulant therapy. Improving the accuracy of the algorithms in different types of DVT is also the challenge of MRI in the future.
References
Prandoni PL, Cogo AWA, Cuppini A, Villalta S, Prins S (1996) The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 125:1–7
Kahn SS, Julian I, Ginsberg JA (2008) Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 149(10):698–707
Tran HHG, Merriman E, Curnow JL, Young L, Bennett A, Tan CW, Chunilal SD, Ward CM, Baker R, Nandurkar H (2019) New guidelines from the thrombosis and haemostasis society of Australia and New Zealand for the diagnosis and management of venous thromboembolism. Med J Aust 210(5):227–235
Kahn SS, Julian I, Ducruet JA, Arsenault TLM, Roussin MJ, Ginsberg A (2008) Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med 149:698–707
Patel P et al (2020) Systematic review and meta-analysis of outcomes in patients with suspected deep vein thrombosis. Blood Adv 4(12):2779–2788
Michel Dauzat M, Jean-Pierre Laroche MD, Ghislaine Deklunder MD, Jean Ayoub MD, Isabelle Que´re´ MD, Franc¸ois-Michel Lopez MD, Charles Janbon MD (1997) Diagnosis of acute lower limb deep venous thrombosis with ultrasound: trends and controversies. J Clin Ultrasound. 25(7):343–358
Needleman LC, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, DeJong MR, Streiff MB, Meissner MH (2018) Ultrasound for lower extremity deep venous thrombosis: multidisciplinary recommendations from the society of radiologists in ultrasound consensus conference. Circulation 137(14):1505–1515
Arnoldussen CS, Lambregts RHW, Lahaye MJ, Graaf RD, Wittens CHA (2014) Feasibility of identifying deep vein thrombosis characteristics with contrast enhanced MR-Venography. Phlebology 29(1S):119–124
Mendichovszky IH, Priest AN, Graves MJ, Bowden DJ, Baglin T, Hunter S, Lomas DJ, Joubert I (2017) Combined MR direct thrombus imaging and non-contrast magnetic resonance venography reveal the evolution of deep vein thrombosis: a feasibility study. Eur Radiol 27(6):2326–2332
Wang SZ, Gu JP, Feng M, Wu G, Lu LQ, Yin XD, Wu QZ (2008) Evaluation of the clinical values of MRA and MSCTA in detection of deep vein thrombosis in the low legs-comparison of DSA. Chin J Med Imaging Technol 24(3):370–373
Kang JL, Yuan YT, Quan GM, Geng ZJ (2017) Evaluation of lower extremity venous thrombosis by direct CT angiography at low dose低剂量直接法静脉CT造影评估下肢静脉血栓. Hebei Med J 39(21):3244–3247
Liang JJG, Liu D, Shi C, Luo L (2019) Application of high-resolution CUBE sequence in exploring stroke mechanisms of atherosclerotic stenosis of middle cerebral artery. J Stroke Cerebrovasc Dis 28(1):156–162
Gold GB, Beehler RFC, Han E, Brau AC, Beatty PJ, Beaulieu CF (2007) Isotropic MRI of the knee with 3D fast spin-echo extended echo-train acquisition (XETA): initial experience. AJR Am J Roentgenol 188(5):1287–1293
Xie G et al (2017) Black-blood thrombus imaging (BTI): a contrast-free cardiovascular magnetic resonance approach for the diagnosis of non-acute deep vein thrombosis. J Cardiovasc Magn Reson 19(1):4
Li ML et al (2016) High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. Eur J Radiol 85(4):803–807
Liang J et al (2019) Application of high-resolution CUBE sequence in exploring stroke mechanisms of atherosclerotic stenosis of middle cerebral artery. J Stroke Cerebrovasc Dis 28(1):156–162
Wang SF, Gu MJP, Wu G, Sun J, Mao CN, Lu LS, Yin XD (2008) Clinical application of magnetic resonance direct thrombus imaging in detection of deep vein thrombosis in the low legs. J Pract Radiol 24(5):624–627
Association CMAUM (2009) Guidelines for blood vessel ultrasound血管超声检查指南. Chin J Ultrasonog 18(11):993–1012
Zhang GA, Duan I, He ZS (1996) The accuracy of color doppler flow imaging for the detection of symptomatic deep venous thrombosis in chinese patients. Surg Today 26:683–687
Expert Panel on Vascular, Imaging (2018) Chinese guidelines for the prevention and treatment of Thrombosis中国血栓性疾病防治指南. Natl Med J China 98(36):2861–2888
Huang YK, Lin CH, Tsai YH, Hsu YC, Wang SC, Chen CW (2019) Evaluation of venous pathology of the lower extremities with triggered angiography non-contrast-enhanced magnetic resonance imaging. BMC Med Imaging 9(1):96
Ye Y, et al (2020) Comparison between the diagnostic performance of 1.5 T and 3.0 T field strengths for detecting deep vein thrombosis using magnetic resonance black-blood thrombus imaging. Clin Appl Thromb Hemost 26:1–8
Treitl KT, Kooijman-Kurfuerst H, Kammer NM, Coppenrath E, Suderland E, Czihal M, Hoffmann U, Reiser MF, Saam T (2015) Three-dimensional black-blood T1-weighted turbo spin-echo techniques for the diagnosis of deep vein thrombosis in comparison with contrast-enhanced magnetic resonance imaging. Invest Radiol 50(6):401–408
Kaya F (2019) Diagnostic performance of contrast-enhanced and unenhanced combined pulmonary artery MRI and magnetic resonance venography techniques in the diagnosis of venous thromboembolism. BJR 92(1095):20180695
Dronkers CK, Langevelde KV, Sramek A, Haren GRV, Huisman MV, Roos AD, Kroft LJM (2019) Diagnosing recurrent DVT of the leg by two different non-contrast-enhanced magnetic resonance direct thrombus imaging techniques: a pilot study. TH Open 3(1):e37–e44
Bhatt M et al (2020) Diagnosis of deep vein thrombosis of the lower extremity: a systematic review and meta-analysis of test accuracy. Blood Adv 4(7):1250–1264
Bapst BA, Vignaud JL, Kauv A, Maraval P, Kalsoum A, Tuilier E, Benaissa T, Brugieres A, Leclerc P, Hodel J (2020) Post-contrast 3D T1-weighted TSE MR sequences (SPACE, CUBE, VISTA/BRAINVIEW, isoFSE, 3D MVOX): technical aspects and clinical applications. J Neuroradiol 47(5):358–368
Carrier MR, Wells PS, Righini M, Legal G (2011) Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta-analysis. J Thromb Haemost 9:1119–1125
Deatrick KB et al (2011) Postthrombotic vein wall remodeling: preliminary observations. J Vasc Surg 53(1):139–146
Comerota AJ et al (2015) A histological and functional description of the tissue causing chronic postthrombotic venous obstruction. Thromb Res 135(5):882–887
Maufus M et al (2018) Diagnosis of deep vein thrombosis recurrence: ultrasound criteria. Thromb Res 161:78–83
Stockmans FS, Vermylen J, Nyström A (1997) A technique to investigate mural thrombus formation in small arteries and veins: I. Comparative morphometric and histological analysis. Ann Plast Surg 38(1):56–62
Segal JE, Tamariz LJ, Bass EB (2007) Review of the evidence on diagnosis of deep venous thrombosis and pulmonary embolism. Ann Fam Med 5(1):63–73
Luo XZ, Zhang CM, Hu L, Feng YP, Liang GZ, Niu LY, Zhang H, Cheng L, Qi HS (2015) Risk factors associated with the severity of pulmonary embolism in patients with acute deep venous thrombosis of lower extremities. Chin J Surg 53(8):580–583
Hanley MS, Ahmed O, Azene EM, Bennett SJ, Chandra A, Desjardins B, Gage KL, Ginsburg M, Mauro DM, Oliva IS, Ptak T, Strax MR, Verma N, Dill KE (2018) ACR appropriateness criteria suspected lower extremity deep vein thrombosis. J Am Coll Radiol 15:S413–S417
Spritzer CEA, Freed KS (2001) Isolated pelvic deep venous thrombosis: relative frequency as detected with MR imaging1. Radiology 219:521–525
Yoo TA, Wang TF, Satiani B, Haurani MJ (2018) Presence and degree of residual venous obstruction on serial duplex imaging is associated with increased risk of recurrence and progression of infrainguinal lower extremity deep venous thrombosis. J Vasc Surg: Venous and Lym Disorders 6(5):575.e1–583.e1
Prandoni PP, Lensing MH, AWAESOPUS Investigators (2009) Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis a randomized trial. Ann Intern Med 150(9):577–585
Roberts SHL (2017) Venous thromboembolism: updated management guidelines. Am J Nurs 117(5):38–47
Masuda EK, Musikasinthorn C, Liquido S, Geling O, He Q (2011) The controversy of managing calf vein thrombosis. J Vasc Surg 55(2):550–561
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, S., Lan, Y., He, L. et al. Non-contrast-enhanced magnetic resonance imaging technique diagnoses DVT and classifies thrombus. J Thromb Thrombolysis 53, 663–670 (2022). https://doi.org/10.1007/s11239-021-02538-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02538-9