Abstract
The role of inflammation in thrombotic complications of primary antiphospholipid syndrome (PAPS) is controversial. The aim of this study was to evaluate levels of inflammation and coagulation markers in patients with thrombotic PAPS (t-PAPS). Patients with t-PAPS and individuals with no history of thrombosis were enrolled. The association of t-PAPS with levels of tumor necrosis factor (TNF)-α, C-reactive protein (hs-CRP), interferon (IFN)-α, interleukins (IL)-6, -8, factor VIII (FVIII), von Willebrand factor (VWF) and tissue factor (TF) was evaluated by regression models. The levels of these markers were also compared between controls and subgroups of t-PAPS patients with triple positivity, recently diagnosed thrombosis, recurrent thrombosis and venous thrombosis. Patients with t-PAPS (n = 101) had a 8.6-fold increased levels of TNF-α, 90% increased levels of hs-CRP, 80% increased levels of IL-6, 30% increased levels of FVIIIAg, 50% increased levels of VWF and 66% increased levels of TF as compared to controls (n = 131), and the differences did not change after adjustments for sex, age and cardiovascular risk factors. Inflammatory markers were elevated in t-PAPS regardless of the aPL profile, number of previous thrombosis or time elapsed since diagnosis. TNF-α and IL-8 levels were higher in t-PAPS patients with venous thrombosis, in comparison with those with arterial thrombosis and controls. Patients with t-PAPS presented with increased levels of inflammatory and coagulation markers, which suggests that t-PAPS is associated not only with hypercoagulability but also with a persistent inflammatory state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Hypercoagulability is present in patients with thrombotic PAPS.
-
An inflammatory state is also detected in thrombotic PAPS.
-
Inflammation is increased in t-PAPS regardless of the severity of the disease.
-
The role of anti-inflammatory and immunomodulatory therapies in t-PAPS are yet to be determined.
Introduction
Primary antiphospholipid syndrome (PAPS) is a chronic immune-mediated disorder in which antibodies directed to phospholipid-binding proteins trigger a procoagulant and inflammatory state that leads to placental vascular complications and thrombotic events of various vascular beds [1, 2]. As opposed to secondary APS, an underlying autoimmune disease is not detected in PAPS.
Thrombotic events in PAPS (t-PAPS) comprise venous thromboembolism (VTE), venous thrombosis in unusual sites, arterial and capillary thrombosis [3] and have high susceptibility to recurrence. The thrombotic risk is primarily conferred by the effect of antiphospholipids (aPL) on monocytes, endothelial cells and platelets, leading to cell membrane expression of tissue factor (TF), adhesion molecules and glycoprotein IIb/IIIa, respectively. Apart from the presence of aPL, additional prothrombotic mechanisms are required to trigger thrombosis, such as transient risk factors for thrombosis or an additional pro-coagulant stimulus [1].
Anticoagulation is the standard of care to prevent thrombosis in PAPS, however the risk for thrombosis recurrence remains high [2, 4,5,6], despite an efficient anticoagulation treatment [2, 5]. This observation raises suspicions that hypercoagulability may not be the only mechanism responsible for thrombosis in PAPS. Besides hypercoagulability, patients with PAPS may also present with a proinflammatory state [7] that contributes to the risk of obstetrical complications [8]. Even though an inflammatory state is also detected in t-PAPS patients [9, 10], the association between inflammation and high-risk APS profile is not established [8].
Therefore, the aim of this study was to investigate the presence of a proinflammatory state in patients with t-PAPS. We also compared the levels of inflammatory markers between patients with high-risk (triple aPL positivity, recurrent thrombosis), non-high-risk t-PAPS and controls.
Materials and methods
Participant selection
In this case–control study, patients with t-PAPS treated at the Hematology and Hemotherapy Center at the University of Campinas, Brazil, were consecutively enrolled for the study between November 2013 and July 2017. Inclusion criteria comprised a confirmed diagnosis of APS not associated with systemic autoimmune disease and a history of at least one thrombotic event. Patients with active neoplasia, underlying autoimmune disease and pregnant women were excluded. Patients were enrolled for the study on the day of their medical consultation, and clinical information was obtained by reviewing medical records and interviewing patients. Patients with t-PAPS received prolonged anticoagulant treatment; some patients also received antiplatelet agents.
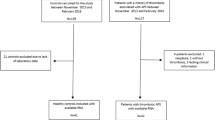
Individuals with no history of antiphospholipid syndrome (APS), arterial or venous thrombosis were enrolled as controls between November 2013 and July 2017. Controls were enrolled among individuals who were accompanying a patient to the medical appointment, employees at the hospital or their relatives or friends. Controls were selected as such that t-PAPS patients and controls had similar distributions of age and sex. Individuals with active neoplasia, underlying autoimmune disease and pregnant women were excluded. Figure 1 illustrates the study enrollment, selection, and reasons for exclusion.
Blood was collected on the day of the enrollment by venipuncture in one serum separating tubes and in two 0.109 M sodium citrate at a proportion of 9:1. The serum separating tubes were always collected before the coagulation tubes and samples were immediately centrifuged. Platelet-poor-plasma was isolated by double centrifugation at 2500×g for 15 min. Serum and platelet-poor plasma obtained were separated and immediately stored at − 80 °C.
Primary APS diagnosis and clinical features
Information on APS diagnosis and classification were assessed in the medical records. APS was diagnosed in patients with persistently positive aPL plus a history of thrombosis (confirmed by imaging examinations) or obstetric complications [3]. Persistently positive aPL was defined as persistently positive lupus anticoagulant (LAC); persistently positive IgG or IgM aCL at moderate to high titers (> 40 GPL or MPL) or persistently positive (> the 99th percentile) IgG/IgM anti-beta2 glycoprotein 1 (aβ2GP1), on two different occasions, with an interval of at least 12 weeks [3].
The detection of aPL was performed at diagnosis following the international guidelines from the International Society of Thrombosis and Haemostasis (ISTH) and Clinical and Laboratory Standard Institute (CLSI). Blood was collected prior to the initiation of any anticoagulant drug regimen or after a sufficient period of drug discontinuation.
To perform LAC testing, low molecular weight heparin and warfarin were withdrawn 24 h and at least five days prior to the blood collection, respectively. Thrombin time and prothrombin time assays were performed in the same sample collected for LAC assay. If thrombin or prothrombin was prolonged, LAC assay was not performed. Two assays based on different principles were applied to determine the presence of LAC in plasma: Dilute Russell's viper venom time (dRVVT) and Silica Clotting Time (SCT). Results of screening tests were potentially suggestive of LAC when the clotting times were longer than the local cut-off value (percentile 99th). In that case, a confirmatory test with excess phospholipid and a mixing test with normal pool plasma were performed. The results were confirmed for LAC if the correction percentage was above the local cut-off value (99th percentile) in the confirmatory test. The aPL with solid phase were tested in patient serum by “in house” ELISA immunological assays, with cardiolipin or β2GP1 as antigen (Sigma-Aldrich, USA), as previously described [11, 12]. A calibration curve and commercial controls were used, positive patient samples were also used as positive controls, and samples were tested in duplicate. The local cut-off value for aβ2GP1 was determined by obtaining the 99th percentile in the population.
APS was classified into primary or secondary depending on the diagnosis of an underlying autoimmune disease, such as SLE. All patients with APS were screened at diagnosis for systemic autoimmune diseases and in the follow-up as necessary. To do that, the following tests were performed: antinuclear antibodies, complement C3 and C4, anti-double-stranded DNA. In the presence of clinical signs and symptoms, such as proteinuria or hematological disorders, further investigations were performed. The diagnosis of SLE was confirmed according to established criteria [13].
Thrombotic events were characterized as venous or arterial, according to the vascular bed where thrombosis occurred. Venous thrombosis events were further divided into deep vein thrombosis (DVT), pulmonary embolism (PE) or thrombosis of unusual sites. Arterial thrombosis events were divided into stroke and peripheral arterial thrombosis. All thrombotic events were confirmed by image exams, such as ultrasound (US), computerized tomography (CT), magnetic resonance (MR), ventilation/perfusion lung scan, or biopsies, according to the site of thrombosis.
Obesity was defined as body mass index above 30 kg/m2; hypertension was defined as persistent systolic blood pressure above 130 mm Hg, persistent diastolic blood pressure above 90 mm Hg, or use of antihypertensive drugs. Dyslipidemia was defined as high levels of low density cholesterol (LDL ≥ 160 mg/dL or 4.1 mmol/L), high levels of triglycerides (TG ≥ 150 mg/dL or 1.7 mmol/L), or low levels of high density cholesterol (HDL < 40 mg/dL or 1.0 mmol/L for men or HDL < 50 mg/dL or 1.3 mmol/L for women), or use of statins or fibrates. Diabetes was diagnosed according to established criteria [14].
Only patients with PAPS and thrombosis were selected for the study. Blood samples were obtained from all participants (PAPS patients and controls without APS or thrombosis) on the day of their enrollment for the study.
The study was conducted in compliance with the Helsinki Declaration. The local Ethical Committee on Human Research approved this study and written informed consent was obtained from patients or their attending relatives.
Measurements
Serum levels of C-reactive protein (hs-CRP) were measured using the reagent C-Reactive Protein Test System for BN device (ProSpec®, Siemens Healthcare, USA) by nephelometry. Serum levels of interferon -alpha (IFN-α), tumor necrosis factor-alpha (TNF-α), interleukins (IL)-6 and -8 and TF were measured using commercially available ELISA kits:VeriKine Human IFN-alpha ELISA kit (PBL Assay Science, Piscataway Township, NJ, USA), IL-6 Quantikine HS and TNF-αQuantikine HS (R&DSystems, Minneapolis, MN, USA), Human IL -8 ptEIATM (BD Biosciences Pharmingen, San Diego, CA, USA) and IMUBIND® Tissue Factor ELISA (Sekisui Diagnosis, San Diego, CA, USA), respectively. The assays were performed according to the manufacturer’s instructions. Plasma levels of von Willebrand factor (VWF) and factor VIII antigen (FVIIIAg) were measured using HemosIL von Willebrand Factor Activity assay and HemosIL FVIII (Instrumentation Laboratory, Bedford, MA), respectively. ELISA tests were performed in duplicates and if the intra-assay variability was less than 10%, tests were repeated Technicians were not aware whether the samples were from patients or controls.
As TNF-α and hs-CRP have been associated with APS [15, 16] the primary endpoints were defined as the difference in TNF-α and hs-CRP between t-PAPS and controls. The difference in IFN-α, IL -6, IL-8, VWF and TF were considered secondary endpoints. Previous studies reported a mean difference in TNF-α between APS patients and controls of 2.9 pg/mL [15]. We expected to find a powered mean difference of at least 0.26 pg/mL in TNF-α levels between t-PAPS patients and controls, with a 2-sided alpha of 0.05 and 80% power.
Statistical analysis
Clinical characteristics were evaluated as counts and percentage when the variables were categorical. When the variables were continuous, they were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR). Data that were not normally distributed were log-transformed for the regression analysis.
We first determined the between-groups difference in levels of TNF-α, hs-CRP, IFN-α, IL-6, IL-8, FVIII, TF and VWF. This was achieved by calculating the mean difference and 95% confidence intervals (CI) of these markers between t-PAPS patients and controls using linear regression methods. Three regression models were performed: non-adjusted, adjusted for age and sex; and adjusted for age, sex and cardiovascular risk factors (hypertension, diabetes, dyslipidemia and BMI ≥ 30 kg/m2). We chose to adjust for cardiovascular risk factors because they can affect both the risk of thrombosis and the levels of inflammatory markers. Next, we evaluated the correlation between inflammation and coagulation markers in t-PAPS patients using Spearman correlation test.
Additionally, we divided t-PAPS according to the following determinants of high-risk: aPL profile (triple or non-triple positivity), number of thrombotic events (recurrent or non-recurrent thrombosis), time elapsed since the last thrombotic event (less or more than 12 months) and the site of thrombosis (venous or arterial). Patients were classified as having “recent thrombosis” in case they had been enrolled for the study at least 12 months after the thrombotic event occurred and as having “non-recent thrombosis” in case the time interval between thrombosis and enrollment for the study was longer than 12 months. Levels of inflammation and coagulation markers were compared between subgroups and controls using Kruskal–Wallis test.
All statistical analyses were performed with SPSS version 23.0 for Windows (SPSS Inc, Chicago,IL, USA). Graphs were plotted using GraphPad Prism version 6.0 (GraphPad Software Inc. La Jolla, CA, USA).
Results
Clinical characteristics
A total of 231 participants were included in the study; 101 patients with t-PAPS and 131 individuals without APS or history of thrombosis (controls). Figure 1 illustrates the flow chart of participants’ selection and reasons for exclusion. Demographic and clinical characteristics at the date of inclusion in the study are shown in Table 1. Age and sex distribution were similar between t-PAPS patients and controls. The presence of cardiovascular risk factors, such as hypertension, dyslipidemia, obesity and diabetes was more frequent among patients than in controls. Forty-three women with t-PAPS and 53 controls had at least one pregnancy. Among these women, obstetric complications were reported by 60.5% of t-PAPS patients and by 21% of controls. All patients were using an anticoagulant (4 heparin, 95 warfarin and 2 direct oral anticoagulant), however 26 patients on warfarin were using a subtherapeutic dose of the drug (international normalized ratio [INR] below 2.0). Ten patients were also using aspirin.
Table 2 presents the antiphospholipid profile and thrombotic complications in the 101 t-PAPS included in the study. Triple aPL positivity was present in 22% (n = 21) of patients and 45.5% (n = 46) presented with single positivity for LAC. Antinuclear antibody (ANA) test was positive in 27 patients (26.7%). Among all patients, 31% (n = 31) had arterial thrombosis and 35% (n = 35) had recurrent thrombosis. The median time elapsed from the last thrombotic event to enrollment for the study was 53.4 months (IQR = 20.9–108).
Markers of inflammation and coagulation
As shown in Table 3 and Fig. 2, levels of all measured inflammation and coagulation parameters, except for IL-8 and IFN-α, were higher in t-PAPS than in controls. Patients with t-PAPS had an 8.6-fold increased levels of TNF-α, 90% increased levels of hs-CRP, 80% increased levels of IL-6, 30% increased levels of FVIIIAg, 50% increased levels of VWF and 66% increased levels of TF, as compared with controls. The difference in TNF-α, hs-CRP, IL-6, TF and VWF levels between t-PAPS and controls did not change after adjustments for sex, age and cardiovascular risk factors.
Box plot graphs illustrate the levels (median, interquartile range, minimum and maximum) of a TNF-α, IFN-α, hs-CRP, IL-8, IL-6 and b FVIII, VWF and TF in individuals without a history of thrombosis (n = 131) and PAPS patients (n = 101). *values were significantly different from controls (P value < 0.05) by Mann–Whitney test. TNF alpha tumor necrosis factor-alpha, CRP C-reactive protein, IFN-alpha interferon-alpha, IL-6 interleukin-6, IL-8 interleukin-8, FVIII factor VIII, VWF von Willebrand factor, TF tissue factor
In patients with t-PAPS, there was no correlation between levels of TNF-α and TF (r = 0.185; P = 0.13), VWF (r = 0.074; P = 0.61) or FVIII (r = -0.167; P = 0.22). Also, there was no correlation between levels of hs-CRP and TF (r = -0.054; P = 0.66), VWF (r = 0.065; P = 0.64) or FVIII (r = 0.227; P = 0.08). Levels of IL-6 were weakly correlated with levels of TF (r = 0.262; P = 0.03) but not with VWF (r = 0.100; P = 0.48) or FVIII (r = -0.069; P = 0.617). We observed that time since the last thrombotic event was slightly correlated with levels of TNF- α (r = 0.299, P = 0.01), IL-6 (r = 0.369, P = 0.002) and IL-8 (r = 0.297, P = 0.04). Although, these correlations were statistically significant, the correlation coefficients were low and there was no correlation between time since the last thrombotic event and other inflammatory or coagulation marker. Altogether, these findings suggest that inflammatory markers did not change substantially with time.
Table 4 demonstrates the analysis of the subgroups. When compared to controls, levels of TNF-α, hs-CRP, IL-6, TF and VWF were higher in triple (n = 21) and non-triple positivity patients (n = 80), between patients with (n = 17) and without recent thrombosis (n = 84), and between patients with incident (n = 66) or recurrent thrombosis (n = 35). TF levels were higher in triple aPL positive patients in comparison with patients without triple positivity and controls. Also, triple aPL positive patients had higher IL-8 levels. PAPS patients using anticoagulant plus aspirin (n = 10) had levels of TNF-α, hs-CRP, FVIII and TF similar to controls.
Patients with a history of venous thrombosis had higher levels of TNF-α (mean 4.6 pg/mL [SD 14.3]) in comparison with patients with arterial thrombosis (mean 1.95 pg/mL [SD 2.7]) and controls (mean 0.34 [SD 0.60], P = 0.002). Levels of IL-8 were also higher in patients with venous thrombosis (mean 53.6 pg/mL [SD 65.7]) than in those with arterial thrombosis (mean 22.2 pg/mL [SD 9.3]) and controls (mean 29.9 [SD 62.0], P = 0.03).
Discussion
In the present study, we first evaluated whether t-PAPS patients presented with a proinflammatory state. t-PAPS was associated with increased levels of TNF-α, hs-CRP and IL-6 when compared with controls (individuals without APS or thrombosis). Levels of IL-8 and IFN-α did not differ substantially between patients and controls. These associations were not changed after adjustments for potential confounding.
TNF-α and IL-6 are cytokines released by macrophages and involved in local and systemic inflammation [17]. IL-6 also stimulates hepatocytes to release acute phase proteins, such as CRP [18]. In vitro studies demonstrated that antiphospholipid antibodies, in particular anti-beta2 glycoprotein 1, are capable of binding to monocytes and inducing the release of both TNF-α and TF [19]. From a clinical perspective, increased levels of TNF-α and IL-6 have been consistently reported in patients with SLE and APS [15, 16, 20,21,22] and an enhanced type I IFN gene signature has been demonstrated in PAPS patients with thrombosis [23, 24]. Therefore, our findings provide additional evidence for an association between t-PAPS and a proinflammatory state.
In addition to inflammation, hypercoagulability was observed as the levels of VWF, FVIII and TF were higher in t-PAPS than in controls. Factor VIII, VWF and TF are coagulation-related proteins that may be released after an inflammatory stimulus [25, 26] and have been related to APS before [27, 28]. In vitro studies demonstrated that anti-beta2 glycoprotein 1 antibodies are capable of inducing the release of VWF from endothelial cells [29] and the expression of TF in monocytes [19]. These antibody-mediated mechanisms may be at the basis of the observed association of APS with increased TF, FVIII and VWF levels.
Despite the results having demonstrated that both inflammation and hypercoagulability are associated with t-PAPS, there was no correlation between the levels of inflammatory and coagulation markers. In addition, inflammation was not associated with the severity of t-PAPS, since inflammatory markers were elevated in t-PAPS regardless of the aPL profile, number of previous thrombosis or time elapsed since the acute thrombotic event. TNF-α and IL-8 levels were higher in t-PAPS patients with venous thrombosis, in comparison with those with arterial thrombosis and controls. Levels of TNF-α and IL-8 were higher in patients with venous thrombosis than in those with arterial thrombosis and in controls, suggesting that these markers may be associated with venous events; however, the numbers were too small in these subgroups to allow any conclusion. Altogether, our results pointed towards the presence of an inflammatory state in t-PAPS that seems to be related with both high and low-risk disease profile.
There are some limitations in this study that are addressed in the following paragraph. First, this is a retrospective observational study and we cannot rule out that data may have been misreported in the medical records. Second, although we demonstrated that patients with t-PAPS have increased levels of inflammation markers, the direction of this association was not possible to determine as all parameters were measured after t-PAPS diagnosis. Third, inflammatory and coagulation markers were measured a relevant time after the acute thrombosis, which may have affected the results. However, the levels of inflammatory and coagulation markers were similar between patients with recent (less than 1 year) and non-recent (more than 1 year) thrombosis, which suggests that the time interval between the thrombotic event and the inclusion in the study may not have substantially affected the results. The diagnosis of APS is often confirmed long time after the acute thrombosis, when anticoagulation is withdrawal. Forth, subgroup analysis revealed that the levels of inflammatory markers were similar between patients with triple positivity and non-triple positivity for aPL, incident and recurrent thrombosis. Additionally, subgroup analysis revealed that inflammatory and coagulation markers in PAPS patients taking anticoagulants plus aspirin were lower than in those taking only anticoagulants. Although these findings may suggest that inflammation is detected in t-PAPS regardless of the disease severity and that aspirin use may revert this scenario, subgroup analysis must be interpreted with caution due to the sample size in these subgroups and issues with multiple testing, which could yield type I errors. Finally, circulating TF can be measured by either ELISA, flow cytometry or functional assays and the choice of the assay may affect the clinical interpretation of the results [30, 31]. Here, we chose to measure TF antigen by using a commercially available ELISA as we wanted to use an assay that could be easily reproduced.
In conclusion, patients with t-PAPS have an increase in levels of inflammatory and coagulation markers even long time after diagnosis, which suggests that the disease is associated not only with hypercoagulability but also with a chronic proinflammatory profile. The role of inflammation in t-PAPS and the need for anti-inflammatory or immunomodulatory therapies are yet to be determined.
References
Ruiz-Irastorza G, Crowther M, Branch W, Khamashta MA (2010) Antiphospholipid syndrome. Lancet 376(9751):1498–1509. https://doi.org/10.1016/s0140-6736(10)60709-x
Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Boffa MC, Hughes GR, Ingelmo M (2002) Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum 46(4):1019–1027
Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera RH, Derksen RH, de Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4(2):295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x
Bertero MT, Bazzan M, Carignola R, Montaruli B, Silvestro E, Sciascia S, Vaccarino A, Baldovino S, Roccatello D (2012) Antiphospholipid syndrome in northwest Italy (APS Piedmont Cohort): demographic features, risk factors, clinical and laboratory profile. Lupus 21(7):806–809. https://doi.org/10.1177/0961203312446974
Cervera R, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Kiss E, Zeher MM, Tincani A, Kontopoulou-Griva I, Galeazzi M, Bellisai F, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Piette JC, Espinosa G, Bucciarelli S, Pisoni CN, Bertolaccini ML, Boffa MC, Hughes GR (2009) Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis 68(9):1428–1432. https://doi.org/10.1136/ard.2008.093179
Saraiva Sda S, Custodio IF, Mazetto Bde M, Collela MP, de Paula EV, Appenzeller S, Annichino-Bizzachi J, Orsi FA (2015) Recurrent thrombosis in antiphospholipid syndrome may be associated with cardiovascular risk factors and inflammatory response. Thromb Res 136(6):1174–1178. https://doi.org/10.1016/j.thromres.2015.10.029
Ames PR, Antinolfi I, Ciampa A, Batuca J, Scenna G, Lopez LR, Delgado Alves J, Iannaccone L, Matsuura E (2008) Primary antiphospholipid syndrome: a low-grade auto-inflammatory disease? Rheumatology (Oxford) 47(12):1832–1837. https://doi.org/10.1093/rheumatology/ken382
Meroni PL, Borghi MO, Grossi C, Chighizola CB, Durigutto P, Tedesco F (2018) Obstetric and vascular antiphospholipid syndrome: same antibodies but different diseases? Nat Rev Rheumatol 14(7):433–440. https://doi.org/10.1038/s41584-018-0032-6
Salmon JE, Girardi G, Lockshin MD (2007) The antiphospholipid syndrome as a disorder initiated by inflammation: implications for the therapy of pregnant patients. Nat Clin Pract Rheumatol 3 (3):140–147; quiz 141 p following 187. https://doi.org/10.1038/ncprheum0432
de Groot PG, Urbanus RT (2015) Antiphospholipid syndrome-not a noninflammatory disease. Semin Thromb Hemost 41(6):607–614. https://doi.org/10.1055/s-0035-1556725
Averina M, Johannesen S, Brox J (2016) Diagnostic accuracy of silica clotting time method for lupus anticoagulant in a clinical population with various symptoms of antiphospholipid syndrome. Lupus 25(4):418–422. https://doi.org/10.1177/0961203315617540
Freire PV, Watanabe E, dos Santos NR, Bueno C, Bonfa E, de Carvalho JF (2014) Distinct antibody profile: a clue to primary antiphospholipid syndrome evolving into systemic lupus erythematosus? Clin Rheumatol 33(3):349–353. https://doi.org/10.1007/s10067-013-2472-3
Font J, Cervera R (1993) 1982 revised criteria for classification of systemic lupus erythematosus–ten years later. Lupus 2(5):339–341. https://doi.org/10.1177/096120339300200512
American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. https://doi.org/10.2337/dc14-S081
Forastiero RR, Martinuzzo ME, de Larranaga GF (2005) Circulating levels of tissue factor and proinflammatory cytokines in patients with primary antiphospholipid syndrome or leprosy related antiphospholipid antibodies. Lupus 14(2):129–136. https://doi.org/10.1191/0961203305lu2048oa
Swadzba J, Iwaniec T, Musial J (2011) Increased level of tumor necrosis factor-alpha in patients with antiphospholipid syndrome: marker not only of inflammation but also of the prothrombotic state. Rheumatol Int 31(3):307–313. https://doi.org/10.1007/s00296-009-1314-8
Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449(7164):819–826. https://doi.org/10.1038/nature06246
Rauch J, Dieude M, Subang R, Levine JS (2010) The dual role of innate immunity in the antiphospholipid syndrome. Lupus 19(4):347–353. https://doi.org/10.1177/0961203310361492
Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Rigano R, Ortona E, Valesini G (2007) Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum 56(8):2687–2697. https://doi.org/10.1002/art.22802
Manganelli V, Capozzi A (2017) Elevated serum level of HMGB1 in patients with the antiphospholipid syndrome. J Immunol Res 2017:4570715. https://doi.org/10.1155/2017/4570715
Sacre K, Criswell LA, McCune JM (2012) Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 14(3):R155. https://doi.org/10.1186/ar3895
Willis R, Seif AM, McGwin G Jr, Martinez-Martinez LA, Gonzalez EB, Dang N, Papalardo E, Liu J, Vila LM, Reveille JD, Alarcon GS, Pierangeli SS (2012) Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 21(8):830–835. https://doi.org/10.1177/0961203312437270
Ugolini-Lopes MR, Torrezan GT, Gandara APR, Olivieri EHR, Nascimento IS, Okazaki E, Bonfa E, Carraro DM, de Andrade DCO (2019) Enhanced type I interferon gene signature in primary antiphospholipid syndrome: association with earlier disease onset and preeclampsia. Autoimmun Rev 18(4):393–398. https://doi.org/10.1016/j.autrev.2018.11.004
Palli E, Kravvariti E, Tektonidou MG (2019) Type I interferon signature in primary antiphospholipid syndrome: clinical and laboratory associations. Front Immunol 10:487. https://doi.org/10.3389/fimmu.2019.00487
Kawecki C, Lenting PJ, Denis CV (2017) von Willebrand factor and inflammation. J Thromb Haemost 15(7):1285–1294. https://doi.org/10.1111/jth.13696
Levi M, van der Poll T, ten Cate H (2006) Tissue factor in infection and severe inflammation. Semin Thromb Hemost 32(1):33–39. https://doi.org/10.1055/s-2006-933338
Habe K, Wada H, Matsumoto T, Ohishi K, Ikejiri M, Matsubara K, Morioka T, Kamimoto Y, Ikeda T, Katayama N, Mizutani H (2016) Presence of antiphospholipid antibodies as a risk factor for thrombotic events in patients with connective tissue diseases and idiopathic thrombocytopenic purpura. Intern Med 55(6):589–595. https://doi.org/10.1177/1076029616643820
Tobaldini LQ, Arantes FT, Saraiva SDS, Mazetto BM, Colella MP, de Paula EV, Annichino-Bizzachi J, Orsi FA (2018) Circulating levels of tissue factor and the risk of thrombosis associated with antiphospholipid syndrome. Thromb Res 171:114–120. https://doi.org/10.1016/j.thromres.2018.09.058
Ng CJ, McCrae KR, Ashworth K, Sosa LJ, Betapudi V, Manco-Johnson MJ, Liu A, Dong JF, Chung D, White-Adams TC, Lopez JA, Di Paola J (2018) Effects of anti-beta2GPI antibodies on VWF release from human umbilical vein endothelial cells and ADAMTS13 activity. Res Pract Thromb Haemost 2(2):380–389. https://doi.org/10.1002/rth2.12090
Hernandez C, Orbe J, Roncal C, Alvarez-Hernandez M, Martinez de Lizarrondo S, Alves MT, Garcia Mata J, Paramo JA (2013) Tissue factor expressed by microparticles is associated with mortality but not with thrombosis in cancer patients. Thromb Haemost 110(3):598–608. https://doi.org/10.1160/TH13-02-0122
Brambilla M, Rossetti L, Zara C, Canzano P, Giesen PLA, Tremoli E, Camera M (2018) Do methodological differences account for the current controversy on tissue factor expression in platelets? Platelets 29(4):406–414. https://doi.org/10.1080/09537104.2017.1327653
Acknowledgments
This work was supported by the São Paulo Research Foundation, FAPESP [Grant Number 2016/14172-6] and by Brazilian Federal Agency for the Support and Evaluation of Graduate Education, CAPES [to Fernanda T. Arantes and Laís Q. Tobaldini].
Author information
Authors and Affiliations
Contributions
FTA performed the laboratory and statistical analyses and drafted the manuscript; BMM was responsible for the laboratory analyses and revised the manuscript; LQT and APRdS performed the laboratory analyses and revised the manuscript; SSS and JA-B revised the manuscript and FAO designed the study, the analyses and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests:
The author(s) declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arantes, F.T., Mazetto, B.M., Saraiva, S.S. et al. Inflammatory markers in thrombosis associated with primary antiphospholipid syndrome. J Thromb Thrombolysis 50, 772–781 (2020). https://doi.org/10.1007/s11239-020-02155-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02155-y