Abstract
The crosstalk between immune and coagulation systems plays pivotal roles in host defense, which may involve monocyte-derived dendritic cells (moDCs). Our objectives were to elucidate the role of moDCs in coagulation under inflammatory conditions and the involvement of the complement system. We assessed the effects of lipopolysaccharide (LPS)-stimulated moDCs on coagulation using whole blood thromboelastometry in the presence of complement inhibitors. The sum of clotting time and clot formation time (CT plus CFT) in whole blood thromboelastometry was significantly more reduced in the presence of moDCs than in the absence of monocytes or moDCs and in the presence of monocytes, indicating a more potent coagulability of moDCs. The mRNA expression of coagulation-related proteins in moDCs was analyzed by quantitative PCR, which showed an increase only in the mRNA levels of tissue factor (TF). TF protein expression was assessed by western blot analysis and an activity assay, revealing higher TF expression in moDCs than that in monocytes. The in vitro moDC-associated hypercoagulable state was suppressed by a TF-neutralizing antibody, whereas LPS enhanced the in vitro hypercoagulation further. C1 inhibitor suppressed the in vitro LPS-enhanced whole blood hypercoagulability in the presence of moDCs and the increased TF expression in moDCs. These results suggest a significant role of moDCs and the complement system through TF expression in a hypercoagulable state under inflammatory conditions and demonstrate the suppressive effects of C1 inhibitor on moDC-associated hypercoagulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Whole blood thromboelastometric analysis revealed experimental hypercoagulable states in the presence of monocyte-derived dendritic cells (moDCs)
-
moDCs enhanced the experimental hypercoagulable state in the presence of LPS through tissue factor expression
-
moDCs might be involved in hypercoagulation under inflammatory conditions
-
C1 inhibitor might suppress moDC-induced hypercoagulation by inhibiting tissue factor expression
-
C1 inhibitor might thus be beneficial for suppressing the systemic inflammation-induced hypercoagulable state
Introduction
Innate immunity and inflammatory responses are the front line biological defense mechanism in collaboration with the coagulation system. Coagulation enhanced under inflammatory conditions prevents dissemination of pathogens by hemostatic containment [1]. Monocytes play a key role in the initial step of the hemostatic containment process [2]. When infectious agents activate innate immunity and induce inflammatory responses, monocytes are recruited to the inflammation site and express tissue factor (TF) at the site of pathogen exposure, which initiates the extrinsic coagulation pathway in cooperation with circulating activated factor VII [3]. As monocytes can differentiate into dendritic cells (DCs) under inflammatory conditions, monocyte-derived DCs (moDCs) might play a role in inflammation-induced coagulation similar to monocytes.
DCs occurring at the inflammatory loci are called inflammatory DCs. Primary human monocytes differentiate into inflammatory DCs under inflammatory conditions in vitro and in vivo [4,5,6,7]. Inflammatory DCs are derived from CD14 positive CD16 negative classical monocytes [8]. As existing DCs such as conventional DCs and plasmacytoid DCs are lost because of apoptosis at the inflammatory loci [7], the number of moDCs increases through inflammation-induced differentiation, and their rapid and numerous recruitment to the inflamed site might be significant in inflammation-induced coagulation. In monocytes, TF expression increases under immunological stimulation, which evokes the coagulation system. TF expression in monocytes is hampered by inhibition of the complement system, especially by C1 esterase inhibitor (C1-inh) [9]. However, the role of moDCs in the coagulation under inflammatory conditions remains unclear.
It is critical to consider the cellular fraction as well as the plasma fraction containing coagulation factors for hemostatic assessment, because blood cells, including immune leukocytes, are deeply involved in the coagulation process [10]. Among global coagulation assays that assess the interaction of coagulation factors and blood corpuscles, thromboelastometry measures the viscoelasticity of whole blood during the entire clotting process. To explore whether human moDCs play a role in inflammation-induced coagulation, we investigated the effects of moDCs on global hemostatic function in the presence of lipopolysaccharide (LPS), a pathogen associated molecular pattern that is recognized by innate immune cells, using whole blood thromboelastometry with special reference to TF expression as well as complement system involvement.
Materials and methods
Blood sampling
Blood samples were obtained by venipuncture into 3.8% sodium citrate (9:1, v/v) from healthy volunteers comprising three male and three female Asians between 30 and 50 years of age, who had taken no medication for 2 after providing written informed consent. For thromboelastometry analysis, the initial 3 mL of withdrawn blood was discarded.
Isolation of monocytes and generation of moDCs
Peripheral blood mononuclear cells (PBMCs) were separated using Lymphoprep (Axis-Shield, Oslo, Norway). Biomagnetic negative depletion of classical monocytes from PBMCs was performed using a Dynabeads Untouched Human Monocytes kit (Invitrogen, Carlsbad, CA, USA). Flow cytometric analyses showed that CD14 positive CD16 negative classical monocytes constitute at least 94% of the obtained preparations (Supplemental Fig. 1a). Isolated monocytes were differentiated into DCs according to a previous report with slight modification [11]. Briefly, the separated monocytes were cultured in RPMI-1640 containing 10% FBS and 1% penicillin/streptomycin, in the presence of 100 ng/mL of IL-4 and 250 ng/mL of GM-CSF. After 7 days of culture, immature moDCs were obtained and pulsed with 20 ng/mL of recombinant human TNF-α for 3 days to induce maturation. The purity of generated moDCs was confirmed by flow cytometric analysis (Supplemental Fig. 1b). Unless otherwise specified, moDC herein refers to mature cells. To eliminate hemostatic effects of individual differences between subjects, comparisons were made using the same donor-derived monocytes and moDCs. Reagents were purchased from Wako Pure Chemical Industries (Osaka, Japan) unless otherwise noted.
Rotational thromboelastometry (ROTEM)
ROTEM, a modification of traditional thrombelastography, measures the global viscoelastic properties of whole blood clot formation under low shear conditions [12]. Figure 1a (top) shows a typical ROTEM curve. The following numerical parameters are obtained. Clotting time (CT) corresponding to r time in thromboelastography is defined as the time from the start to initial fibrin formation. Clot formation time (CFT) comparable to k time in thrombelastography is a measure of the speed at which a clot forms with a certain viscoelastic strength. Similar to r time plus k time that has long been used as an indicator of whole blood clot formation [13,14,15], CT plus CFT is also used as an index of whole blood clotting in thromboelastometric analysis [16]. Maximum clot firmness (MCF) corresponding to maximum amplitude in thrombelastography is the largest amplitude of the thromboelastometric signal.
Effect of moDCs on whole blood thromboelastometry. Thromboelastometric analysis provides the following parameters: Clotting time (CT), which is the whole blood clot initiation time, and is defined as the time from the start to initial fibrin formation, as noted by a signal amplitude of 2 mm (green line); Clot formation time (CFT) is a measure of the speed at which a clot forms with a certain viscoelastic strength, and is defined as the time from CT until the amplitude of the signal reaches 20 mm (purple portion). Maximum clot firmness (MCF) is the largest amplitude of the thromboelastometric signal and is a measure of clot strength (bidirectional arrow in blue portion). a Representative thromboelastometric traces in the presence or absence of moDCs. b Thromboelastometric variables CT plus CFT and MCF in the presence of monocytes (open circle) and moDCs (closed circle) were plotted against cell concentrations of monocytes or moDCs. The CT plus CFT or MCF of control samples without monocytes or moDCs was set as 100%. The data in the presence of monocytes or moDCs are expressed as a comparative ratio (%) to the control samples. The error bars represent SEM for 5 independent measurements (n = 5). *p < 0.05, ***p < 0.001 whole blood with moDCs vs. whole blood without moDCs (control). #p < 0.05 whole blood with moDCs versus whole blood with monocytes at the corresponding cell concentration
To evaluate the effects of moDCs on ROTEM measurements, 1/10 volume of monocyte or moDC suspension [final concentration (f.c.) 10 and 30 cells/μL] was mixed with normal whole blood and incubated at 37 °C for 2 h. PBS alone was used as a negative control.
ROTEM was also performed in the presence of human TF-neutralizing antibody (Goat, American Diagnostica, Stamford, CT, USA). Three microliters of anti-TF antibody (f.c. 10 μg/mL) or PBS were mixed with whole blood (285 μL) and moDC suspension (10 μL, f.c. 30 cells/μL) and incubated at 37 °C for 10 min. The concentration of anti-TF antibody at which the TF activity is neutralized was determined as described previously [17].
To assess the effects of LPS and complement inhibitors on coagulation in the presence of moDCs, 50 μL of LPS (E. coli O11:B4, Sigma-Aldrich, St. Louis, MO, USA) (f.c. 1 μg/mL) was added to the assay mixture [385 μL of whole blood with 15 μL of moDC suspension (f.c. 30 cells/μL)]. Fifty microliters of PBS (vehicle), human C1-inh (Berinert®, CSL Behring, Tokyo, Japan) (f.c. 2.5 mg/mL), or C3-binding cyclic synthetic peptide (C3-inh: Compstatin, Tocris Bioscience, Ellisville, MO, USA) (f.c. 20 μM) was immediately added to the corresponding tubes. The doses of C1-inh and C3-inh were determined according to previous studies [9, 18]. The assay mixtures were incubated at 37 °C for 2 h.
ROTEM measurements were initiated after the addition of 20 μL of 0.2 M CaCl2 in the non-activating thromboelastometry (NATEM) mode.
Gene expression analysis
RNA isolation and quality control were performed as described previously [19, 20]. To select appropriate reference genes for reliable gene expression profiles, we analyzed the stability of expression levels for 7 housekeeping genes by real-time quantitative reverse transcription-PCR (qRT-PCR) using normalization tools and evaluation with the RefFinder software (http://150.216.56.64/referencegene.php) as described previously [21, 22]. Table 1 shows 23 target coagulation-related genes, 7 housekeeping genes, and their TaqMan probes and primer sets (Applied Biosystems, Foster City, CA, USA). Target gene expression in monocytes, immature moDCs, and mature moDCs was analyzed.
To elucidate whether innate immune stimulation affects moDC-associated hypercoagulation, TF expression in mature moDCs treated with LPS (f.c. 1 μg/mL) was examined. Furthermore, to elucidate whether the complement system is involved, vehicle (PBS), C1-inh (f.c. 2.5 mg/mL), or C3-inh (f.c. 20 μM) was added to the corresponding tubes immediately after the addition of LPS to moDCs. Cell mixture samples were incubated at 37 °C for 6 h. Total RNA was extracted from these cell samples, and TF mRNA expression was analyzed by qRT-PCR as described above.
TF protein expression assessed by western blot analysis
Monocytes, immature moDCs, and mature moDCs were lysed using T-PER Tissue Protein Extraction Reagent containing protease and phosphatase inhibitors (Thermo Fisher Scientific, Rockford, IL, USA). Proteins concentrations were determined by the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific) and 20 µg of protein samples were used for western blotting. Goat anti-humanTF antibody was used to detect the TF antigen. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control. Protein band intensity was quantified using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
In a different experiment to examine the effects of LPS, TF protein expression in moDCs incubated with vehicle (PBS) or LPS (f.c. 1 μg/mL) at 37 °C for 6 h was evaluated by western blot analysis as described.
TF protein expression assessed by TF activity assay
moDCs were incubated with vehicle (PBS) or LPS (f.c. 1 μg/mL) at 37 °C for 6 h to investigate the effects of LPS on TF expression in moDCs. Furthermore, to elucidate whether the complement system is involved, vehicle (PBS), C1-inh (f.c. 2.5 mg/mL), or C3-inh (f.c. 20 μM) was added to the corresponding tubes immediately after addition of LPS. After incubation at 37 °C for 6 h, cell samples were washed twice with PBS and dissolved in 15 mM octyl-β-d-glucopyranoside (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C for 15 min. The TF activities of cell lysates were assessed using Tissue Factor Chromogenic Activity Kit (Assaypro, St. Charles, MO, USA). Protein concentrations were determined using BCA assay. The raw data of TF activity were corrected to the amount of total protein, and the results are expressed as a ratio of values obtained from treated cells to those from control cells.
Statistical analysis
Statistical analyses were performed using SPSS software v.19 (IBM SPSS Statistics, Chicago, IL, USA). Data were analyzed using the Student’s t-test. In case of more than two experimental groups, one way ANOVA followed by the Tukey Kramer post-hoc test was performed. Data are expressed as mean ± SEM and p values < 0.05 were considered significant.
Results
moDCs enhance whole blood coagulation
The thromboelastometric traces showed that although addition of monocytes altered neither CT plus CFT nor MCF, addition of moDCs to whole blood dose-dependently reduced the CT plus CFT and increased MCF (Fig. 1a). CT plus CFT was significantly reduced in the presence of moDCs compared with that in the absence of moDCs and in the presence of monocytes at the same cell concentration; however the difference in MCF between monocytes and moDCs was not significant (Fig. 1b). These results indicate that moDCs can enhance whole blood coagulation, whereas monocytes cannot do so in our experimental system. As the figures of the upper measuring limit of MCF are not far from normal values, a significant difference in MCF values between normal and hypercoagulable blood may hardly arise. Therefore, CT plus CFT was adopted as the marker of a hypercoagulable state in subsequent experiments using thromboelastometry.
Upregulation of TF mRNA and protein expression in moDCs
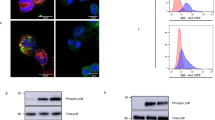
After an analysis of the expression stability of housekeeping genes, HPRT1 was used as the reference gene (Supplemental Fig. 2). Many genes of interest, including fibrinogen β chain, prothrombin, factors VII, IX, X, XI, and XIIIβ subunit, protein C, anti-thrombin, plasminogen, thrombin-activatable fibrinolysis inhibitor, tissue plasminogen activator, hyaluronan binding protein 2 (factor VII activating-protease), protease-activated receptor (PAR) 3, and PAR4 were not expressed during the differentiation of monocytes into mature moDCs. Other genes, including factors V, VIII, and XII, protein S, von Willebrand factor (VWF), PAR1, and PAR2 were downregulated as monocytes differentiated into mature moDCs, except for TF mRNA, which was increased as the differentiation proceeded from monocytes to mature moDCs (Fig. 2a). Western blot analysis confirmed that TF protein synthesis increased during differentiation from monocytes into mature moDCs, consistent with mRNA expression (Fig. 2b).
TF expression. a Gene expression profiling of coagulation-related factors during differentiation from classical monocytes to mature moDCs (n = 5). The data are normalized to the expression of the reference gene HPRT1 and are expressed as a comparative ratio to the monocyte samples. mono: monocytes, iDC: immature moDCs, moDC: mature moDCs. All values are presented as mean ± SEM. Only TF mRNA expression was increased with differentiation. b TF synthesis during differentiation from monocytes into mature moDCs (n = 5). Upper: Western blots demonstrating the relative levels of TF and GAPDH, which was used as a loading control. Lower: Analysis of band intensities quantified with ImageJ software. Band intensities of TF in immature moDCs and mature moDCs relative to that of monocytes are shown. Note that TF protein expression was increased during differentiation. mono: monocytes, iDC: immature moDCs, moDC: mature moDCs. The error bars represent the SEM of triplicate experiments. *p < 0.05, ***p < 0.001. c Effect of TF-neutralizing antibody on moDC-induced whole blood hypercoagulation (n = 5). Reduced CT plus CFT in the presence of moDCs was restored to control levels by the addition of TF-neutralizing antibody. CT plus CFT in the presence of TF antibody was not prolonged to more than that of the control levels. These results suggest that whole blood hypercoagulability in the presence of moDCs was primarily attributed to TF activity. The error bars represent the SEM of triplicate experiments. *p < 0.05
TF-inhibition suppresses moDC-associated hypercoagulability of whole blood
Reduced CT plus CFT in the presence of moDCs was restored to control levels by the addition of a TF-neutralizing antibody (Fig. 2c).
LPS-enhanced hypercoagulability in whole blood with moDCs
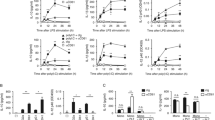
Thromboelastometric analysis showed that LPS-treatment of whole blood with moDCs further shortened the CT plus CFT. However, the CT plus CFT reduced by LPS was restored to baseline levels in the presence of C1-inh, whereas C3-inh showed little effect (Fig. 3a).
Hypercoagulable effect of LPS and its inhibition by C1-inh. a Thromboelastometric analysis (n = 5). CT plus CFT in the presence of LPS, LPS and C1-inh, and LPS and C3-inh is expressed as a comparative ratio (%) to the control samples (whole blood supplemented with moDCs). CT plus CFT was reduced in the presence of LPS, indicating that LPS challenge further enhanced the moDC-associated hypercoagulable state. C1-inh restored the LPS-induced reduction of CT plus CFT, whereas C3-inh did not. b TF mRNA expression (n = 3). The data obtained in the presence of LPS, LPS and C1-inh, and LPS and C3-inh are expressed as comparative ratios to those for moDCs alone. C1-inh suppressed the LPS-enhanced TF expression. c Western blot analysis for TF protein synthesis (n = 3). The data in the presence of LPS are expressed as a comparative ratio to those for moDCs alone. d TF activity (n = 5). The data in the presence of LPS, LPS and C1-inh, and LPS and C3-inh are expressed as comparative ratios (%) to those of moDCs alone. Significant upregulation of TF expression was confirmed at the mRNA and protein levels, both of which reflected a similar trend. An activity assay was employed for TF protein expression in this experiment, because coagulability depends more on TF activity than upon TF antigen levels. *p < 0.05. Error bars represent SEM of 3 or 5 independent experiments
LPS-enhanced upregulation of TF mRNA and protein expression in moDCs
TF mRNA expression was further enhanced in moDCs after LPS treatment (Fig. 3b). Consistently, western blot analysis and TF activity assay also demonstrated increased TF protein expression in LPS-treated moDCs (Fig. 3c, d).
Enhanced upregulation of both TF mRNA expression and TF activity by LPS was significantly suppressed in the presence of C1-inh, whereas C3-inh had no effect (Fig. 3b, d).
Discussion
In the present study, we showed that moDCs may be involved in the hypercoagulable state induced under inflammatory conditions through their endogenous TF expression.
First, thromboelastometric analysis demonstrated that moDCs had a significantly more potent coagulability compared to monocytes. Gene expression analysis of coagulation-related genes showed that only TF expression was upregulated in moDCs. Increased TF protein synthesis was confirmed by western blot analysis and activity assay. As TF inactivation using a TF-neutralizing antibody suppressed the in vitro hypercoagulable state of the whole blood with moDCs to a control state in the absence of moDCs, the moDC-associated hypercoagulability likely depends on TF upregulation. In the current cell-based model of coagulation, the initiation phase occurs on the TF-bearing cell surface [10]. When TF bearing cells transmit a procoagulant signal to platelets and the stimulus is strong enough, thrombin and activated factors IX and X are formed to initiate intrinsic coagulation leading to a thrombin burst [23]. Therefore, marked upregulation of TF in moDCs under inflammatory conditions might result in hypercoagulation in vivo. TF upregulation was significantly more pronounced in moDCs than that in monocytes, which may explain the difference of the hypercoagulable effects between monocytes and moDCs shown in our thromboelastometric analysis.
On the other hand, as PARs serve as a link between inflammation and coagulation [24], expression of PARs on moDCs might contribute to the experimental hypercoagulable state. However, we failed to show the expression of any PAR in mature moDCs, which is consistent with a previous observation that only PAR2—which is not involved in coagulation—is expressed in immature moDCs and decreases as maturation progresses [25].
TF mRNA and protein upregulation in moDCs was found to be even more enhanced in the presence of LPS. Under in vivo inflammatory conditions, LPS activates monocytes and macrophages as well as moDCs to induce proinflammatory cytokine production, which may cause endothelial damage and thrombotic tendency [26].
TF is not only a primary initiator of blood coagulation, but also has signaling activity and promotes pleiotropic inflammatory responses via PARs expressed on various types of cells in concert with other coagulation factors. Therefore, when an intense inflammatory response allows differentiation into moDCs and extends systemically beyond the inflammatory site, systemic, pathogenic immune-mediated thrombosis—potentially leading to disseminated intravascular coagulation—may develop. However, C1-inh might suppress a series of events caused by the inflammation-coagulation axis associated with moDCs and may be beneficial in systemic immune-mediated thrombosis. In earlier studies, pentraxin 3 (PTX3) has been shown to upregulate TF expression and is involved in LPS-enhanced TF expression in monocytes [9, 27, 28]. Furthermore, the inhibitory activity of C1-inh on LPS-induced TF expression in monocytes is partly ascribed to the suppression of PTX3 generation [9]. We therefore speculate that PTX3 also participates in the mechanism underlying TF upregulation in moDCs, which might involve C1. Further studies are warranted to address these mechanisms.
The limitations of this study were that it was performed in laboratory conditions and that the blood samples used were all from Asians. Considering the ethnic differences in coagulation [29], further experiments using samples of non-Asian origin are warranted.
In conclusion, human moDCs, which are induced from classical monocytes under inflammatory conditions and are accumulated at inflammatory sites, may contribute to hemostatic containment and immune-mediated thrombosis through TF expression. Our findings elucidate the role of moDCs in the inflammation-induced hypercoagulable state.
References
Alcock J, Brainard AH (2008) Hemostatic containment—an evolutionary hypothesis of injury by innate immune cells. Med Hypotheses 71:960–968
Ito T (2014) PAMPs and DAMPs as triggers for DIC. J Intensive Care 2:67
Engelmann B, Massberg S (2013) Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 13:34–45
Segura E, Amigorena S (2013) Inflammatory dendritic cells in mice and humans. Trends Immunol 34:440–445
Qu C, Brinck-Jensen NS, Zang M, Chen K (2014) Monocyte-derived dendritic cells: targets as potent antigen-presenting cells for the design of vaccines against infectious diseases. Int J Infect Dis 19:1–5
Puck A, Aigner R, Modak M, Cejka P, Blaas D, Stockl J (2015) Expression and regulation of Schlafen (SLFN) family members in primary human monocytes, monocyte-derived dendritic cells and T cells. Results Immunol 5:23–32
Fan X, Liu Z, Jin H, Yan J, Liang HP (2015) Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int 2015:903720
Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM (2017) Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 12:e0176460
Landsem A, Nielsen EW, Fure H, Christiansen D, Ludviksen JK, Lambris JD, Osterud B, Mollnes TE, Brekke OL (2013) C1-inhibitor efficiently inhibits Escherichia coli-induced tissue factor mRNA up-regulation, monocyte tissue factor expression and coagulation activation in human whole blood. Clin Exp Immunol 173:217–229
Hoffman M, Monroe 3rd DM (2001) A cell-based model of hemostasis. Thromb Haemost 85:958–965
Posch W, Lass-Florl C, Wilflingseder D (2016) Generation of human monocyte-derived dendritic cells from whole blood. J Vis Exp 118:1–7
Kozek-Langenecker S (2007) Monitoring of hemostasis in emergency medicine. In: Vincent JL (ed) Intensive Care Medicine. Springer, Berlin, pp 847–860
Sheehy TW, Eichelberger JW (1958) Alimentary lipemia and the coagulability of blood: analysis by thrombelastography and silicone clotting time. Circulation 17:927–935
Serradimigni A, Audier BM (1960) The variations in the ratio (am/r plus k) of the thromboelastogram during treatment with anticoagulants. Arch Mal Coeur Vaiss 53:796–801
Senzolo M, Agarwal S, Zappoli P, Vibhakorn S, Mallett S, Burroughs AK (2009) Heparin-like effect contributes to the coagulopathy in patients with acute liver failure undergoing liver transplantation. Liver Int 29:754–759
Spiel AO, Mayr FB, Firbas C, Quehenberger P, Jilma B (2006) Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost 4:411–416
Watanabe T, Yasuda M, Yamamoto T (1999) Angiogenesis induced by tissue factor in vitro and in vivo. Thromb Res 96:183–189
Landsem A, Fure H, Mollnes TE, Nielsen EW, Brekke OL (2016) C1-inhibitor efficiently delays clot development in normal human whole blood and inhibits Escherichia coli-induced coagulation measured by thromboelastometry. Thromb Res 143:63–70
Kasuda S, Tatsumi K, Sakurai Y, Kato J, Taminishi S, Takeda T, Ohashi K, Okano T, Hatake K, Shima M (2011) Expression of coagulation factors from murine induced pluripotent stem cell-derived liver cells. Blood Coagul Fibrinolysis 22:271–279
Kasuda S, Sakurai Y, Tatsumi K, Kato J, Takeda T, Shima M, K H (2014) Sequential gene expression analysis of coagulation factors and protease activated receptors in hematopoietic lineage development. Curr Angiogenesis 3:139–143
Tatsumi K, Ohashi K, Taminishi S, Okano T, Yoshioka A, Shima M (2008) Reference gene selection for real-time RT-PCR in regenerating mouse livers. Biochem Biophys Res Commun 374:106–110
Rydbirk R, Folke J, Winge K, Aznar S, Pakkenberg B, Brudek T (2016) Assessment of brain reference genes for RT-qPCR studies in neurodegenerative diseases. Sci Rep 6:37116
Monroe DM, Hoffman M, Roberts HR (1996) Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul Fibrinolysis 7:459–464
Ma L, Dorling A (2012) The roles of thrombin and protease-activated receptors in inflammation. Semin Immunopathol 34:63–72
Johansson U, Lawson C, Dabare M, Syndercombe-Court D, Newland AC, Howells GL, Macey MG (2005) Human peripheral blood monocytes express protease receptor-2 and respond to receptor activation by production of IL-6, IL-8, and IL-1b. J Leukoc Biol 78:967–975
Roumenina LT, Rayes J, Frimat M, Fremeaux-Bacchi V (2016) Endothelial cells: source, barrier, and target of defensive mediators. Immunol Rev 274:307–329
Napoleone E, Di Santo A, Bastone A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R (2002) Long pentraxin PTX3 upregulates tissue factor expression in human endothelial cells: a novel link between vascular inflammation and clotting activation. Arterioscler Thromb Vasc Biol 22:782–787
Napoleone E, di Santo A, Peri G, Mantovani A, de Gaetano G, Donati MB, Lorenzet R (2004) The long pentraxin PTX3 up-regulates tissue factor in activated monocytes: another link between inflammation and clotting activation. J Leukoc Biol 76:203–209
Gurbel PA, Bliden KP, Cohen E, Navickas IA, Singla A, Antonino MJ, Fissha M, Kreutz RP, Bassi AK, Tantry US (2008) Race and sex differences in thrombogenicity: risk of ischemic events following coronary stenting. Blood Coagul Fibrinol 19:268–275
Acknowledgements
The authors thank Emeritus Professor Yoshihiro Fujimura of Nara Medical University for critical reading of the manuscript. The authors also thank Ms. Junko Kato for providing technical assistance.
Funding
This work was supported by grants-in-aid from The Cardiovascular Research Fund, Tokyo, Japan (S.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Nara Medical University Ethical Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kasuda, S., Sakurai, Y., Tatsumi, K. et al. Experimental hypercoagulable state induced by tissue factor expression in monocyte-derived dendritic cells and its modulation by C1 inhibitor. J Thromb Thrombolysis 46, 219–226 (2018). https://doi.org/10.1007/s11239-018-1688-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1688-0