Abstract
Emerging evidence suggests the use of peri-procedural bridging during interruptions in warfarin therapy increases bleed risk without reducing thromboembolic events. We implemented a peri-procedural anticoagulant management risk assessment tool in a single, outpatient anticoagulation clinic within an academic teaching institution. In this retrospective, pre-post observational study, we evaluated adults who required an interruption in warfarin therapy for an invasive procedure. The primary outcome was the proportion of patients who received bridging prior to and following implementation of the tool. Secondary outcomes included major bleeding, clinically relevant non-major bleeding, thromboembolic events, and other surgical complications within 30 days of the index procedure. In total, 149 patients were included. Bridging was recommended in 60% of the pre-intervention group and in 39.3% of the post-intervention group (p = 0.012). There were no significant differences in the secondary outcomes between the groups. However, patients who received bridging had numerically more bleeding events than patients who did not (12.3 vs. 3.9%, p = 0.102), and patients who received therapeutic dose bridging had more bleeding events than those who received modified dose bridging (10.9 vs. 1.4%, p = 0.466). Following implementation of the tool, there was a statistically significant decrease in the number of patients who received bridging without an increase in thromboembolic events. There were numerically higher rates of bleeding in those who received bridging. Additional research is needed to evaluate efficacy and safety of prophylactic versus treatment dose bridging and how implementation of peri-procedural antithrombotic tools reflecting the emerging evidence will affect patient outcomes, satisfaction and healthcare costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
It is estimated that approximately 10% of patients on warfarin therapy are evaluated annually for peri-procedural antithrombotic management for an invasive procedure [1]. Peri-procedural bridging strategies have conventionally been employed to prevent thromboembolic complications in patients receiving warfarin who require temporary interruption of their anticoagulant therapy for invasive procedures. Bridging is defined as the implementation of a short-acting parenteral anticoagulant (e.g., low molecular weight heparin) pre-and post-operatively during periods when warfarin is held and/ or the international normalized ratio (INR) is subtherapeutic [2]. This practice, intended to mitigate thromboembolic risk, has largely been based on expert consensus, biologic rationale and pharmacokinetics of anticoagulants rather than robust clinical trial data [3].
Prior to the Fall of 2015, the peri-procedural bridging protocol at the University of New Mexico Hospital (UNMH) mirrored the 3-tiered scheme previously proposed by the American College of Chest Physicians (CHEST) [1] for determining patients’ risk of perioperative thromboembolism. In this guidance statement, patients are categorized into high, moderate, or low risk stratifications, which assists in determining whether bridging should be recommended. In general, bridging is recommended in all patients at high risk and in some patients at moderate risk. However recent studies, including the prospective, randomized, double-blind, placebo-controlled BRIDGE trial, are adding to a growing body of evidence suggesting that bridging significantly increases the incidence of bleeding nearly threefold without providing a reduction in thromboembolic events [3,4,5]. As a result, clinical practice is shifting away from the use of peri-procedural bridging in most patients who are not at high risk for perioperative thromboembolism [2,3,4,5]. UNMH modified its peri-procedural antithrombotic protocol in response to this emerging clinical trial evidence and concomitant paradigm shift in practice.
Following publication of the BRIDGE trial, UNMH instituted a peri-procedural anticoagulant management risk assessment tool (Fig. 1), which utilizes a combination of procedural bleed risk and the patient’s thromboembolic risk to assist in determining which patients should receive bridging therapy. This tool dichotomizes thromboembolic risk into high and low risk stratifications, which differs from previous CHEST guideline recommendations [1]. The impact of this change within our institution has not yet been formally evaluated. Thus, the objective of this study was to evaluate peri-procedural antithrombotic management strategies before and after protocol changes were implemented at our institution. We hypothesized that our modified practices led to a decreased proportion of warfarin patients receiving bridging therapy. Additionally, we hypothesized this would result in a decreased incidence of bleeding without an increase in thromboembolic events in this population.
Methods
Study design
This was a single-center, retrospective, pre-post observational study conducted at UNMH, a 600 + bed academic teaching institution with both inpatient and outpatient pharmacist-driven anti-thrombosis services. Study approval was obtained from the University of New Mexico Health Sciences Center Human Research Review Committee. For this type of study, formal consent is not required.
Patients
Potential patients were identified via the UNMH outpatient Anticoagulation Clinic’s peri-procedural database. This database, maintained by clinic staff, is used to track peri-procedural bridging plans for patients requiring temporary interruption of warfarin therapy for an invasive procedure. Manual review of the electronic health records of all patients meeting eligibility criteria was conducted to abstract study data. Study data were collected and managed using REDCap [6] electronic data capture tools hosted at the University of New Mexico.
Patients were included if they met the following criteria: age ≥ 18 years old, prescribed warfarin therapy for any indication, and underwent an invasive procedure at UNMH requiring temporary warfarin interruption during the pre-intervention period (January 1, 2015–June 30, 2015) or post-intervention period (January 1, 2016–June 30, 2016). A 6-month washout period was chosen to allow for gradual adoption of the tool into clinical practice. Additionally, they must have been followed by the UNMH outpatient Anticoagulation Clinic for ≥ 3 months prior to and 30 days after the index procedure. For patients who underwent more than one invasive procedure in a single intervention period, data was collected surrounding only the first procedure. Patients were excluded if the procedure occurred at a facility outside of UNMH or if they did not undergo the planned procedure.
The following demographic and baseline information was collected for all patients: age, gender, race, weight, start date of anticoagulation therapy, indication for anticoagulation, CHADS2 (congestive heart failure, hypertension, age > 75 years, diabetes mellitus, history of stroke/transient ischemic attack) and CHA2DS2VASc scores (congestive heart failure, hypertension, age > 75 years, diabetes mellitus, history of stroke/transient ischemic attack, vascular disease, age 65–74 years, sex category) for patients with atrial fibrillation, diagnosis of hypertension, smoking status, history of (or current) ethanol abuse, documented diagnosis of liver or renal disease, estimated renal function (based on Cockcroft-Gault equation), malignancy (active or treated within last 6 months), history of stroke, history of bleeding event, and concomitant antiplatelet therapy. Information was also collected on: type of procedure, procedural setting, duration of procedure, procedural bleed risk stratification, thrombotic risk stratification as suggested by national guidelines and expert opinion [1, 5], time in therapeutic range (TTR), use of bridging versus no bridging, and the use of prophylactic versus therapeutic doses of the parenteral anticoagulant.
For this study, bridging was defined as the receipt of pre- and post-operative parenteral anticoagulation. Patients who received bridging at prophylactic doses were categorized into the modified dose group, while those who received therapeutic doses were categorized into the treatment dose group.
Study outcomes
The primary outcome was the proportion of patients who received bridging therapy in the pre- and post-intervention groups. Secondary outcomes included the incidence of major and clinically relevant non-major bleeding as defined by the International Society of Thrombosis and Hemostasis (ISTH) [7, 8], as well as the incidence of thromboembolic complications. All thromboembolic complications, defined as stroke, systemic arterial thromboembolism, transient ischemic attack, deep vein thrombosis, or pulmonary embolism [4], were identified via objective diagnostic confirmation. Additionally, secondary outcomes included the proportion of patients with other complications, such as delayed procedures or need for warfarin reversal prior to procedure. Secondary outcomes were assessed up to 30 days following the index procedure.
Statistical analyses
Baseline characteristics and outcomes were described using descriptive statistics. The independent t-test was used to compare results between groups for continuous variables with normal distributions. For non-normally distributed variables, the Mann–Whitney U test was used. Categorical variables were compared using the Chi square test, except for variables with expected values < 5, for which the Fisher’s exact test was used. REDCap and IBM SPSS Statistical software, version 19, were used for analyses.
Results
Patients
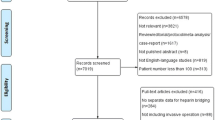
There were 282 patients in the UNMH peri-procedural database with procedures scheduled during the specified intervention periods (Fig. 2). Of these 282 patients, 149 met inclusion criteria. There were 65 patients in the pre-intervention period and 84 patients in the post-intervention period. Patient characteristics of the two intervention periods were similar at baseline (Table 1). Patients had a mean age of 61.52 years, 56.4% were female, and 79.9% were white. On average, patients had been receiving anticoagulation therapy for a mean of 5.45 years with the most common indication being non-valvular atrial fibrillation/flutter. Notably, 26.2% of patients had more than one indication for anticoagulation. Of patients with atrial fibrillation, the mean CHADS2 score was 2.32, and the mean CHA2DS2VASc score was 3.6, with a trend toward significance for higher CHA2DS2VASc scores in the post-intervention group. Concomitant antiplatelet use was seen in 29.5% of patients, with aspirin as the most common agent used. History of bleed, either major or clinically relevant non-major, was found in 9.4% of patients. History of stroke or TIA was found in 14.8% of patients. The post-intervention group had a significantly higher prevalence of patients who were former ethanol abusers as well as patients who were former smokers (p = 0.048 and p = 0.035, respectively).
Procedures
Procedural characteristics were similar in both intervention groups (Table 1). The most common procedures were colonoscopy at 36.9%, endoscopy at 22.8%, and biopsy at 14.8%. Notably, 27.5% of patients had more than one procedure at a time. This is a common practice, intended to minimize the number of interruptions in a patient’s anticoagulation therapy. Outpatient procedures accounted for 84.6% of the total, with 73.8% lasting less than 45 min. On average, 6.0% of patients had a high procedural bleed risk, and 22.1% of patients had a high risk for thromboembolism. There were no significant differences in the pre- and post-intervention groups regarding the prevalence of procedures with high bleed risk or patients with high risk of thromboembolism.
Outcomes
For the primary outcome, 60% of the patients in the pre-intervention group received bridging compared to 39.3% of the post-intervention group (p = 0.012) (Fig. 3). When comparing intervention periods, the incidence of secondary outcomes was similar (Table 2). In the pre-intervention group, all bleeding events occurred in patients who received bridging (1.5% vs. 0 for major bleeding, p = 0.6; 7.7% vs. 0 for clinically relevant non-major bleeding, p = 0.08). There was no difference in thromboembolic events between intervention periods (0 pre-intervention vs. 1.2% post-intervention, p = 0.607). A single surgical complication was noted in the post-intervention group in a patient who did not receive bridging. This patient’s surgery was delayed by 1 day due to an elevated pre-procedure INR. Rather than holding warfarin 5 days prior to the procedure, as is most commonly recommended by the Anticoagulation Clinic, this patient was instructed to hold warfarin for 2 days prior to the procedure per the surgeon’s request.
In comparing patients who received bridging to those who did not (Table 3), major bleeding events occurred only in patients who received therapeutic dose bridging (p = 0.238). Clinically relevant non-major bleeding events occurred in patients who received bridging as well as in those who did not (p = 0.147). Although not statistically significant, bleeding events were more common in patients who received bridging (12.3 vs. 3.9%, p = 0.102) and especially in patients who received therapeutic dosing rather than modified dosing (10.9 vs. 1.4%, p = 0.466). The difference in thromboembolic events was not significant between patients who were bridged and those who were not (p = 0.510).
Discussion and conclusions
Emerging evidence suggesting peri-procedural bridging increases bleed risk without reducing thromboembolic events have prompted a paradigm shift in clinical practice. Implementing a modified peri-procedural anticoagulant management risk assessment tool within a large academic teaching institution resulted in a statistically significant decrease in the number of patients receiving peri-procedural bridging without an increase in thromboembolic events. Although we found no statistically significant differences in bleeding, there were numerically higher rates of bleeding in patients who received bridging, which is consistent with recent evidence [2,3,4]. Overall low rates of bleeding across both groups may explain why no significant differences were identified. Of those who received bridging, the majority of the major and clinically relevant non-major bleeding occurred in patients who received therapeutic dose bridging. In a retrospective cohort study, conducted at Kaiser Permanente Colorado, evaluating bleeding rates and recurrent venous thromboembolism (VTE) in warfarin patients with prior history of VTE, they found no differences in recurrent thromboembolic events regardless of receiving therapeutic or prophylactic dose bridging [2]. Additional research into the use of prophylactic dose bridging in patients at high peri-operative thromboembolic risk may provide clarity regarding its broader utility to further mitigate bleed risk.
This study has several limitations owing to its retrospective study design. First, although manual review of all electronic health records (EHR) were completed to ensure data were collected and categorized as accurately as possible, it cannot be ruled out that some procedures or outcomes were documented incorrectly in the EHR or some patients were misclassified in bleeding and thromboembolic risk stratifications. Second, despite collecting confounders for both bleeding and thromboembolic risk, we cannot confirm all confounders were adjusted for in our analyses. However, we had broad inclusion criteria including all indications for anticoagulation and collected numerous relevant patient characteristics. Finally, due to our small sample size, overall low event rates, and inclusion of only a single site within a single academic teaching institution, there may be difficulty in detecting small, statistically significant differences which may limit generalizability. However, this study does provide real-world evidence that implementing a peri-procedural antithrombotic protocol recommending bridging for those only at the highest peri-procedural thromboembolic risk can significantly reduce the number of patients receiving bridging therapy, thereby perhaps reducing overall bleed risk. This, in turn, contributes to the growing body of evidence supporting change in clinical practice and may lead to additional research evaluating patient outcomes, patient satisfaction and overall reduced cost to the patient and healthcare system.
References
Douketis JD, Spyropoulos AC, Spencer FA et al (2012) Perioperative management of antithrombotic therapy. Chest 141(2 suppl):e326S–e350S. https://doi.org/10.1378/chest.11-2298
Clark NP, Witt DM, Davies LE et al (2015) Bleeding, recurrent venous thromboembolism, and mortality risks during warfarin interruption for invasive procedures. J Am Med Assoc Intern Med 175(7):1163–1168. https://doi.org/10.1001/jamainternmed.2015.1843
Rose AJ, Allen AL, Minichello T (2016) A call to reduce the use of bridging anticoagulation. Circulation 9(1):64–67. https://doi.org/10.1161/CIRCOUTCOMES.115.002430
Douketis JD, Spyropoulos AC, Kaatz S et al (2015) Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med 373(9):823–833. https://doi.org/10.1056/NEJMoa1501035
Spyropoulos AC, Al-Badri A, Sherwood MW et al (2016) Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost 14:875–885. https://doi.org/10.1111/jth.13305
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, on behalf of the subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis (2010) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 8(1):202–204. https://doi.org/10.1111/j.1538-7836.2009.03678.x
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, for the Subcommittee on Control of Anticoagulation (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13:2119–2126. https://doi.org/10.1111/jth.13140
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Marquez, J., Togami, J.C., Dant, C.R. et al. Peri-procedural antithrombotic management: time to burn the bridge?. J Thromb Thrombolysis 45, 337–344 (2018). https://doi.org/10.1007/s11239-018-1616-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1616-3