Abstract
In this feasibility study, a novel catheter prototype for laser thrombolysis under the guidance of optical coherence tomography (OCT) was designed and evaluated in a preclinical model. Human arteries and veins were integrated into a physiological flow model and occluded with thrombi made from the Chandler Loop. There were four experimental groups: placebo, 20 mg alteplase, laser, 20 mg alteplase + laser. The extent of thrombolysis was analyzed by weighing, OCT imaging and relative thrombus size. In the alteplase group, thrombus size decreased to 0.250 ± 0.036 g (p < 0.0001) and 14.495 ± 0.526 mm2 (p < 0.0001) at 60 min. The relative thrombus size decreased to 73.6 ± 4.1% at 60 min (p < 0.0001). In the laser group, thrombus size decreased significantly to 0.145 ± 0.028 g (p < 0.0001) and 11.559 ± 1.034 mm2 (p < 0.0001). In the alteplase + laser group, thrombus size decreased significantly (0.051 ± 0.026 g; p < 0.0001; 9.622 ± 0.582 mm2; p < 0.0001; 47.4 ± 6.1%; p < 0.0001) in contrast to sole alteplase and laser application. The reproducibility and accuracy of the OCT imaging was high (SD <10%). Histological examination showed no relevant destruction of the vascular layers after laser ablation (arteries: 745.8 ± 5.5 μm; p = 0.69; veins: 448.3 ± 4.5 μm; p = 0.27). Thus, laser ablation and OCT imaging are feasible with the novel catheter and thrombolysis combining alteplase with laser irradiation appears highly efficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Reperfusion strategies in thrombotic vessel occlusion are mainly focused on rapid and complete restoration of blood flow in order to reduce hypoxia and potential tissue injury [1, 2]. Despite major advances in surgical techniques, operative thrombectomy is still related to the general risks of open surgery, whereas pharmacological thrombolysis often only limits thrombus growth and progress but without complete restoration of circulation [2]. Due to these limitations, new interventional procedures have been intensively studied in the endovascular era. Laser-recanalization has become a further treatment option in vascular therapy during the last two decades [3]. However, former studies have mainly focused on atherosclerotic plaque extraction in femoro-popliteal lesions and in-stent stenosis in cardiovascular therapy [3, 4]. Today, only a few studies have yet investigated the potential treatment of thrombosis with laser irradiation but mostly without evaluating safety and efficiency for clinical use [3, 4].
While optical coherence tomography (OCT) has become a valuable tool in interventional cardiology, it’s use in other fields of endovascular therapy is still limited and less frequent [5, 6]. Along with the rapid development of endovascular therapies being introduced for peripheral vascular diseases, there is an increasing need for dynamic and safe intravascular imaging [7]. Besides reducing the amount of contrast agent, additional measurements of the vascular wall, the thrombus and atherosclerotic plaque formation may provide relevant diagnostic and therapeutic informations [7, 8]. Frequent comparisons between OCT and intravascular ultrasound have shown major advantages for the use of OCT. Especially higher field depth and resolution of the OCT allows live image processing during endovascular treatment [9, 10].
On the assumption that OCT guidance in peripheral vessels could lead to an effective and safe use of laser ablation, our research group has developed a new catheter prototype for endovascular laser ablation. Aim of this preclinical study was to evaluate the safety, efficiency and accuracy of the new catheter prototype combining laser ablation and OCT imaging for the treatment of thrombosis.
Materials and methods
Experimental protocol
As there is currently no viable device combining laser surgery and OCT imaging, a catheter prototype was designed and constructed in collaboration with the Institute of Medical Technology at Hamburg University of Technology (Fig. 1). Efficacy and safety of the catheter prototype was evaluated in a flow model providing physiologically conditions for the arterial and venous system. Human vessels were occluded with thrombi and integrated into a circulation model in order to investigate the precision of OCT imaging and putative damage due to laser irradiation.
Illustration of different design strategies for the catheter prototype. a First sketch of the inner scaffold of the catheter prototype, b the final design comprised an inner Y-shaped scaffold with an inner tip diameter of 8.1 Fr and two working channels, c glass fibers for laser ablation were arranged in an outer sheath (red arrow) and carbon-coated imaging fibers (red star) were located in the center, d the first feasibility model based originally on the SLS II 16 F laser catheter (Spectranectics Inc., Colorado Springs, USA), e the final design had a maximal outer diameter of 12.3 Fr and a length of 55 cm (with kindly permission of Spectranectics)
There were four experimental groups: the first group (n = 9) was the placebo group representing spontaneous degeneration of the thrombi within the flow model. Thrombi from the second group (n = 9) were only treated with alteplase following a high-dose protocol. The third group (n = 9) was treated with sole laser ablation and thrombi from the fourth group (n = 9) were treated with a combination of laser ablation and alteplase. All experiments were performed by two high-trained senior interventionists. Venous blood collection was taken from 19 voluntary, healthy male persons underwent full coagulation diagnostics prior to the study. Haematologic investigations were realized by the Center for Coagulation Disease, University Medical Center Schleswig–Holstein, Kiel and included testing for Von Willebrand disease, haemophilia, the factor-V Leiden mutation and thrombophilia diagnosis. Arteries and veins were taken from 18 voluntary donors underwent cardiovascular surgery. Prior surgery, every donor was clinically examined and all donors underwent mandatory examination of the vessels of the lower and upper extremities by Doppler sonography in order to exclude macroangiopathy. Microangiopathy was further excluded by histological analysis. All experiments were designed in consensual with the university internal ethic commission.
Flow model
A validated circulatory system with physiological flow and pressure characteristics was used to simulate the human vessel system. A pneumatically driven pulsatile pump generated a flow rate of 0.35 L min at 15 beats per minute and with a peak flow at 1.1 L min. As reported before, these flow conditions correspond with the parameters in a healthy, normal human [11, 12]. Superficial femoral arteries and human saphenous veins from voluntary donors were carefully prepared and transferred into the flow model. The working fluid (1000 mL) was a suspension of water and whole blood at a volume ratio of 1:3. The working fluid was constantly tempered at 37 °C.
Chandler loop
As previously reported, precipitation thrombi were made in the Chandler loop under standardized conditions [13]. A polymeric vessel-phantom (length = 20 mm, diameter = 6 mm) was used to obtain standardized thrombus configuration from the chandler loop. A series of pre-tests (n = 150) confirmed stable and reproduceable thrombus dimensions of 0.501 ± 0.022 g and 18.914 ± 0.24 mm2 (SD <10%). The thrombi were extracted from the vessel-phantom, measured and immediately transferred into the circulation model.
Construction of the catheter prototype
The shape of the catheter prototype was designed using the CAD software CATIA V6 (Dassault Systems, Vélizy-Villacoublay, France). The catheter comprised a circle of fiberglass with a maximal outer diameter of 12.3 Fr, a minimal inner tip diameter of 8.1 Fr and a length of 55 cm (Fig. 1). Arranging the glass fibers around a Y-shaped inner scaffold with the central OCT-imaging catheter ensures simultaneous application of laser-thrombolysis and OCT imaging. The first design study of the catheter was originally based on the SLS II 16 F laser catheter (Spectranectics Inc., Colorado Springs, Colorado).
Thrombolysis with alteplase
The pharmacological lysis was performed with alteplase (Actilyse®; Boehringer Ingelheim, Ingelheim am Rhein, Germany) following a standard high-dose protocol for thrombolysis [14]. First, the initial dose of 10 mg alteplase was applied followed by a 60 min course of continuous 10 mg alteplase infusion. Simultaneously, intravenous unfractionated heparin (500 IU/h) was administered.
Laser ablation
Ablation of thrombi was performed with an excimer-laser CVX-300 (Spectranectics Inc., Colorado Springs, Colorado). As published before, we have used a protocol for maximal tissue protection [15]. Ultraviolet laser pulses were applied with wavelength of 308 nm, flux of 30 mJ/mm2, and a pulse repetition rate of 80 Hz and 125 ns pulse duration. The laser catheter was guided via OCT and placed 0.5 cm in front of the thrombus. Ablation rate was determined as 40 shots with the excimer laser [4]. The temperature measurement was performed 0.5 cm proximal and distal to the thrombus and was realized at baseline and during ablation.
OCT imaging
The frequency-domain OCT imaging (Ilumien Optis system®; St. Jude Medical, Saint Paul, USA) was performed with a wavelength of 1310 nm and a lateral pullback mechanism. The imaging-catheter (Dragonfly®; St. Jude Medical, Saint Paul, USA) enabled B-scan rates of 100 Hz and a maximum viewing area of 11 mm (Fig. 2).
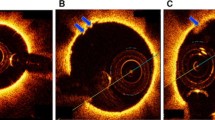
OCT images from the thrombi made at intervals of 20 min (a), thrombus from the alteplase + laser group (white outline) at 20 min (b), the same thrombus at 40 min (c) and at 60 min (white star) (d), Illustration of the lateral pullback mechanism displayed in the sagittal plane (white arrows mark the transition point between the flow model and the artery)
Analysis of thrombus size
The effect of thrombolysis was first assessed by OCT imaging. The maximum extent of the thrombus formation was sized at baseline and at 20-min intervals. Measurements were performed by using the implemented software (Ilumien Optis system®; St. Jude Medical, Saint Paul, USA). Second, extent of thrombolysis was assessed by using a modification of a previously reported method with the free ImageJ-software (http://imagej.nih.gov/ij/, version 1.49). The pixel number of the thrombus was determined at intervals of 20 min and relative to the image area not involved in the thrombosis [16]. Changes in thrombus size were calculated according to a slight modification of a previously reported formula: relative thrombus size = IPNn ÷ IPNo (IPNn = integrative pixel number at various time intervals during thrombolysis, IPNo = integrative pixel number after stabilization of the thrombus). The extent of thrombolysis was expressed as a percentage of the initial thrombus formation [16]. Moreover, after the experiments all thrombi were weighted using a precision scale (Cubis®; Sartorius, Göttingen, Germany).
Histological analysis
Six proximal, mean and distal vessel specimens were routinely fixed in 4% formalin solution and embedded in paraffin. Paraffin-embedded vascular tissue Sect. (4 μm) were then stained with hematoxylin-eosin (HE) or Elastica van Gieson (EvG) to define the intimal and medial layers by light microscopy. The intima media thickness ratio was quantified in ten random vascular sections by an image analyzer (Samba 2000®; North Sioux City, Iowa, USA).
Surface analysis via scanning electron microscopy (SEM)
Scanning electron microscope (SEM) was used to analyze the vascular surface after laser ablation and describe the degree of ultrastructural damage. Vessel specimen were fixed in 2.5% glutaraldehyde and postfixed in 1% osmium tetroxide, irrigated with distilled water and then dehydrated in series of 50–100% ethanol. They were then placed in tertiary-butyl alcohol and dried. The specimens were mounted onto SEM slabs and sputter-coated with platinum-vanadium. Examination of the vessel specimens was performed with a MERLIN Scanning Electron Microscope (Zeiss, Jena, Germany) at ×1000 magnification. Specimen of patients underwent femoral endarterectomy were used as a control group.
Statistical analysis
All values were expressed as mean ± standard deviation (SD). The curves were analyzed with the aid of Graph Pad Prism version 7.0 for Windows (GraphPad Software®; San Diego, California, USA). The sample size calculation for the experimental design was performed by the free G*power 3.1-software (http://www.gpower.hhu.de) and authorised by the Institute for Biometrics of the University Kiel and the ethics committee. Statistical analysis of the results was performed by ANOVA-test for repeated measures and the Tukey’s test or the Students t-test, when appropriate. Equality of group variances was analyzed by the Brown-Forsythe test. A P value less than 0.05 was considered significant.
Results
Evaluation of the catheter design
Four initial design strategies were used during the development of the catheter prototype. The final catheter design used for the present study comprised the best energy output of 42.6 mJ/mm² and a low level of interferences during simultaneous laser ablation and OCT imaging (Fig. 1c). All thrombi were clearly identified and sized by OCT imaging (SD <10%), as shown in Fig. 4. Insertion of the catheter prototype as well as the laser ablation of the thrombus formation was easy and rapid to perform. No penetration of the outer vascular surface was observed. Macroscopic inspection of the outer and inner vascular surface revealed no thermal damage.
Spontaneous thrombolysis in the placebo group
The stable thrombi made by the Chandler Loop showed only marginally spontaneous thrombolysis, and at 60 min thrombi weight remained stable at 0.493 ± 0.028 g. Thrombus size by OCT imaging also retained at 18.691 ± 0.20 mm2, respectively 96.4 ± 5.3% of the initial thrombus size (Fig. 4).
Thrombolysis induced by alteplase
The sole application of alteplase was associated with the highest thrombus weight after thrombolysis at 60 min but decreased significantly in contrast to the placebo group (0.250 ± 0.036 g; p < 0.0001). Accordingly, the OCT imaging showed a reduction of initial thrombus size at 40 min (15.531 ± 0.423 mm2) and a significant reduction at 60 min (14.495 ± 0.526 mm2; p < 0.0001). Relative thrombus size also decreased to 79.4 ± 4.5% at 40 min and to 73.6 ± 4.1% at 60 min (p < 0.0001).
Thrombolysis induced by laser ablation
The thrombus weight after treatment with laser ablation was 0.145 ± 0.028 g (p < 0.0001) and significantly decreased in contrast to the placebo and alteplase group. After 60 min the thrombus size (11.559 ± 1.034 mm2; p < 0.0001) and the relative thrombus size (53.6 ± 5.2%; p < 0.0001) were significantly lower than the initial thrombus size (Fig. 4). The temperature range was 37.9 ± 0.39 °C during laser ablation.
Thrombolysis induced by alteplase and laser ablation
Thrombus weight decreased significantly after combined treatment with alteplase and laser ablation (0.051 ± 0.026 g; p < 0.0001) in contrast to the placebo, alteplase and laser group. The maximum thrombus size was significantly lower at 60 min (9.622 ± 0.582 mm2; p < 0.0001) compared to sole treatment with alteplase and laser ablation (Fig. 4). Likewise, the relative thrombus size decreased significantly to 55.2% ± 3.9 at 40 min and 47.4 ± 6.1% at 60 min (p < 0.0001).
Histological analysis and surface analysis via SEM
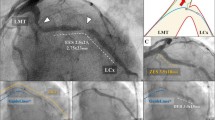
The specimens of arteries and veins were stained with HE and EvG to examine potential damage of the vascular layers. No morphologic differences were found after laser ablation in contrast to the placebo group. The histological examination of arteries (745.8 ± 5.5 μm vs. 748.6 ± 4.4 μm; p = 0.69) and veins (448.3 ± 4.5 μm vs. 440.6 ± 4.6 μm; p = 0.27) showed no relevant destruction of Tunica intima or media after laser ablation (Fig. 3b, d). The intima media thickness ratio did not differ significantly between the study groups and the vascular stratum appeared intact in every sample probe (Fig. 5). Analogous to the histological findings, no ultra structural damage was observed via SEM. In contrast to the control group, the intima and media appeared intact after laser ablation (Fig. 3e, f).
Histological analysis and SEM-images of the arteries and veins used in the flow model (a) HE staining of an artery and thrombus from the alteplase group (original magnification 100x) (b) EvG staining of a vein segment from the laser group (original magnification 100x) (c) the corresponding OCT image (d) and at original magnification 400x for analyzing the vascular wall (Quantification of the intima media thickness ratio were made in six proximal, mean and distal vessel segments and ten random vascular sections were analyzed to get obtained the mean for each vessel, next the mean of each experimental group (n = 9) was determined) (e) SEM-image of an artery after laser ablation without any obvious damage and f SEM-image of an femoral artery after endarterectomy
Discussion
Although pharmacological lysis and catheter directed thrombolysis has been established as standard treatments for thrombotic vascular occlusions during the last three decades, residual occlusions, the post-thrombotic syndrome and bleeding complications have remained unsolved problems [17–19]. In the course of developing new endovascular techniques, a growing number of mechanical tools for pharmacomechanical thrombectomy (PMT) have been introduced to the vascular field but yet without a dominant impact on the international guidelines [18, 19]. However, Blackwood and colleagues (2016) reported on current evidence supporting the use of PMT over catheter directed thrombolysis or simple anticoagulation [20]. Accordingly, shorter procedure times and lower urokinase dosage were observed for patients underwent PMT [21]. Moreover, former studies have assumed a positive effect for complete and rapid thrombus elimination in occluded vessels [17, 20].
In the present study, a novel catheter for laser thrombolysis under OCT guidance was designed for recanalization of thrombotic vascular occlusions. The final prototype comprised a laser sheath with an inner OCT channel. The positioning of the glass fibers around a Y-shaped inner scaffold enabled simultaneous application of laser thrombolysis and OCT imaging. The additional working channel presented in Fig. 1b, c can be used for the aspiration of residual clots. The catheter prototype was inserted in the flow model using a 12 Fr introduction sheath. Fully rotation for 360° could be performed even when it was fully inserted and the OCT catheter could easily move in and out through the working channel. Especially improvements in the configuration and isolation of the fiberglass and consequently heat dissipation may enable a much smaller catheter design. Pre-tests for different design strategies have shown that the actual prototype achieved the best results with regard to energy loss of only 13%. Further optimized manufacturing under industrial conditions may also improve the power performance of the laser sheath and lead to a smaller catheter design.
Moreover, no obvious penetration or dissection of the vascular wall was observed by macroscopic inspection. Contrary to mechanical devices, laser energy and respectively laser irradiation can be controlled and applied much more precisely during interventional procedures. Whereas the therapeutic success of mechanical tools is often bound to the interventionalist’s skills, laser ablation could potentially be an easy-to-use method.
To our knowledge, our preclinical study is the first experimental trial to evaluate the potential efficacy of laser ablation plus alteplase in treating thrombosis. In the placebo group, low levels of spontaneous thrombolysis were observed after 60 min. In comparison, the thrombus weight and size of the alteplase group were significantly decreased at 60 min in contrast to the placebo group (Fig. 4). Further, the alteplase+laser ablation group showed significant enhanced levels of thrombolysis compared to the placebo, the alteplase and sole laser group. The enhanced thrombolysis at 40 min mediated by alteplase + laser ablation was even significant higher to that mediated by alteplase at 60 min, despite the high-dose lysis protocol used in the current study (Fig. 4). These observations suggest that alteplase in combination with laser ablation was most effective in the reduction of thrombus size. Accordingly, laser irradiation has the potential to reduce alteplase levels during thrombolytic therapy possibly resulting in fewer bleeding complications. Further experiments with various levels of alteplase in combination with laser thrombolysis could possibly clarify this aspect.
Time course of thrombolysis quantified by the maximal diameter of the thrombi in the OCT images at various time points. a Quantification made by the ImageJ-software: decrease of thrombus size is expressed as the pixel number relative to the pixel number of the initial thrombus. b Quantification of the thrombus size made by the implemented software of the OCT platform (Ilumien Optis system®; St. Jude Medical, Saint Paul, USA); data are presented as mean ± SD; ***p < 0.0001 (ANOVA-test)
As previously described, for the cleavage of peptide bonds an electromagnetic spectrum of 190–300 nm is needed [4]. Accordingly, the laser ablation was performed with a wavelength of 308 nm and is mainly based on the vaporizing of the intracellular water [22, 23]. The effect of this photothermal interaction is the fracture of molecular bonds and cells [4, 23]. Thus, the heating of the thrombotic formation is accompanied by the denaturation of fibrinogen and consequently with an improved exposure of the remaining thrombus for pharmacological lysis. Whereas the differences of the ambient temperature ranged between 0.9 and 1.29 °C in our experiments, the blood cells close to the laser-tip absorb the power and denature to carbon particles of high temperatures, typically over 200 °C [23]. The layer of carbonized blood on the fiber tip enables heat transfer to the vascular wall by direct contact, heat conduction and Plancks’s black body radiation [23]. However, heat transfer to distal vessel segments caused by steam bubbles were also reported [23]. Putatively, the greater efficiency of treatment with alteplase under hyperthermia may also have influenced the present results and correlates with the significant decreased thrombus size for combined treatment with alteplase and laser ablation, as shown in Fig. 4 [14, 24].
Although laser revascularization has been performed and described in small series during the last two decades, mostly for peripheral arterial stenosis, remarkably little is known about laser-tissue interactions, especially in vascular procedures [4, 23, 25]. In this current study, we found no statistically significant differences in intima media thickness ratio after laser ablation compared to native vessels of the placebo group (Fig. 5). Despite, the present findings suggest that the photo thermal effects caused no substantial damage during temporary laser application. The catheter prototype only allowed energy output of 30 mJ/mm2 and a single repetition rate of 80 Hz. Hence, no prediction about these technical parameters is possible and how varying these may have influenced the results. Additionally, this should be tested prior to clinical trials.
To our knowledge, OCT imaging of peripheral vessels and thrombotic occlusions has not been performed yet. In particular, OCT has been applied to complex coronary or carotic stenosis and in the ophthalmologic field [6, 8–10]. Spatial resolution of intraluminal OCT imaging ranges between 4 and 20 µm and the depth of penetration amounts approximately 2 mm in scattering media [26]. According to these parameters, the application of the actual OCT technology is still limited to peripheral vessels and areas of a few cm². High optical scattering of erythrocytes typically results in a limited imaging depth [6]. Hence, saline solution is usually injected through the OCT catheter to create a blood free environment during coronary intervention. But otherwise than highly scattering coronary plaques, all thrombi formations could be clearly identified and measured by OCT in the present study (Figs. 2, 4). The reduction of thrombus size could be precisely displayed by OCT and differed significantly between the experimental groups. Measurements made by the implemented software and external evaluation made by the ImageJ-software presented equal results for sizing and demonstrated no substantial differences between both methods (Fig. 4). The reproducibility of OCT imaging and measurement accuracy was accordingly high (SD <10%).
Conclusion
The present results demonstrate that laser thrombolysis in combination with OCT imaging is feasible with the novel catheter prototype evaluated in this preclinical study. Moreover, the prototype provides easy operation during evaluation, effective laser thrombolysis, and continuous success monitoring by OCT. The current results indicate that the combination of alteplase and laser ablation appears as the most efficient method of thrombolysis. Minor improvements in the design of the prototype should be made prior to clinical studies. However, the present results are encouraging; indicating that laser thrombolysis under OCT guidance can offer a significant impact on the treatment of thrombotic vascular occlusions.
References
Vandvik PO, Lincoff AM, Gore JM, Gutterman D D, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH, Spencer FA (2012). Primary and secondary prevention of cardiovascular disease: antithrombotic therapy and prevention of thrombosis. American College of Chest Physicians evidence-based clinical practice guidelines. CHEST J, 141(2_suppl), e637S–e668S
Meissner MH, Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Lohr JM, McLafferty RB, Murad MH, Padberg F, Pappas P, Raffetto JD, Wakefield TW (2012) Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J vasc surg 55(5):1449–1462
Dave RM, Patlola R, Kollmeyer K, Bunch F, Weinstock BS, Dippel E, Jaff MR, Popma J, Weissman N (2009) Excimer laser recanalization of femoropopliteal lesions and 1-year patency: results of the CELLO registry. J Endovasc Ther 16(6):665–675
Bidinger J, Ackermann R, Cattaneo G, Kammel R, Nolte S (2014) A feasibility study on femtosecond laser thrombolysis. Photomed Laser Surg 32(1):17–22
Shammas NW, Weissman NJ, Coiner D, Shammas GA, Dippel E, Jerin M (2012) Treatment of subacute and chronic thrombotic occlusions of lower extremity peripheral arteries with the excimer laser: a feasibility study. Cardiovasc Revasc Med 13(4):211–214
Soest CG, Goderie T, Regar E, Koljenovic S, van Leenders GL, Gonzalo N, van Noorden S, Okamura T, Bourma BE, Tearny GJ, Oosterhuis JW, Serruys PW, van der Steen AF (2010) Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. J Biomed Opt 15(1):011105
Jorge E, Baptista R, Calisto J, Faria H, Monteiro P, Pan M, Pêgo M (2016) Optical coherence tomography of the pulmonary arteries: a systematic review. J Cardiol 67(1):6–14
Koustenis A, Harris A, Gross J, Januleviciene I, Shah A, Siesky B (2016). Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br J Ophthalmol, doi:10.1136/bjophthalmol-2016-309389.
Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, Kozuma K, Kitabata H, Shinke T, Habara M, Saito Y, Hou J, Suzuki N, Zhang S (2013). OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC, 6(10), 1095–1104.
Caiazzo G, Longo G, Giavarini A, Kilic ID, Fabris E, Serdoz R, Mattesini A, Foin N, Secco GG, De Rosa S, Indolfi C, Di Mario C (2016) Optical coherence tomography guidance for percutaneous coronary intervention with bioresorbable scaffolds. Int J Cardiol 221:352–358
Van der Steenhoven TJ, Bosman PF, Tersteeg C, Jacobs MJ, Moll FL, de Groot PG, Heyligers JM (2012) Thrombogenicity of a new injectable biocompatible elastomer for aneurysm exclusion, compared to expanded polytetrafluoroethylene in a human ex vivo model. Eur J Vasc Endovasc Surg 43(6):675–680
Tien WH, Chen HY, Berwick ZC, Krieger J, Chambers S, Dabiri D, Kassab GS (2014) Characterization of a bioprosthetic bicuspid venous valve hemodynamics: implications for mechanism of valve dynamics Eur J Vasc Endovasc Surg 48(4):459–464
Diamond, Scott L. (1999) Engineering design of optimal strategies for blood clot dissolution. Annu Rev Biomed Eng 1(1):427–461
Tsetis DK, Katsamouris AN, Giannoukas AD, Hatzidakis AA, Kostas T, Chamalakis K, Ioannou C, Gourtsoyiannis NC (2003) Potential benefits from heating the high-dose rtPA boluses used in catheter-directed thrombolysis for acute/subacute lower limb ischemia. J Endovasc Ther 10(4):739–744
Marczynski-Bühlow M, Gro J, Berndt R, Röcken C, Wedel T, Böttner M, Cremer J, Lutter G, Petzina R (2014) Comparison of different resection tools for human calcified aortic valves. Innovations 9(4):312–316
Yamashita T, Sato T, Sakamoto K, Ishii H, Yamamoto J (2015) The free-radical scavenger edaravone accelerates thrombolysis with alteplase in an experimental thrombosis model. Thromb Res 135(6):1209–1213
Lee K, Istl A, Dubois L, DeRose G, Forbes TL, Wiseman D, Mujoomdar A, Kribs S, Power AH (2015) Fibrinogen Level and bleeding risk during catheter-directed thrombolysis using tissue plasminogen activator. Vasc Endovascular Surg 49(7):175–179
Du GC, Zhang MC, Zhao JC (2015). Catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone in the treatment of proximal deep vein thrombosis—a meta-analysis. Vasa 44(3): 195–202.
Kahn SR, Galanaud JP, Vedantham S, Ginsberg JS (2016) Guidance for the prevention and treatment of the post-thrombotic syndrome. J Thromb Thrombolysis 41:144–153
Blackwood S, Dietzek AM (2016) Pharmacomechanical thrombectomy: 2015 update. Expert Rev Cardiovasc Ther 14(4):463–475
Park KM, Moon IS, Kim JI, Yun SS, Hong KC, Jeon YS, Cho SG, Kim JY (2014) Mechanical thrombectomy with Trerotola compared with catheter-directed thrombolysis for treatment of acute iliofemoral deep vein thrombosis. Ann Vasc Surg 28(8):1853–1861
Wilkoff BL, Byrd CL, Love CJ, Hayes DL, Sellers TD, Schaerf R, Parsonnet V, Epstein LM, Sorrentino RA, Reiser C (1999) Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol 33(6):1671–1676
Malskat WS, Poluektova AA, van der Geld CW, Neumann HM, Weiss RA, Bruijninckx CM, van Gemert MJ (2014) Endovenous laser ablation (EVLA): a review of mechanisms, modeling outcomes, and issues for debate. Lasers Med Sci 29(2):393–403
Mumme A, Heinen W, Geier B, Maatz W, Barbera L, Walterbusch G (2002) Regional hyperthermic fibrinolytic perfusion after unsuccessful venous thrombectomy of extensive deep venous thrombosis. J Vasc Surg 36(6):1219–1224
Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC, Mena-Hurtado C, Beasley R, Soukas P, Colon-Hernandez PJ, Stark MA, Walker C (2015). Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in-stent restenosis: initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In-Stent Restenosis). JACC 8(1):92–101.
Huang C, Liu B, Brezinski ME (2008) Ultrasound-enhanced optical coherence tomography: improved penetration and resolution. J Opt Soc Am A 25(4):938–946
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Rouven Berndt and Rene Rusch have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Berndt, R., Rusch, R., Hummitzsch, L. et al. Development of a new catheter prototype for laser thrombolysis under guidance of optical coherence tomography (OCT): validation of feasibility and efficacy in a preclinical model. J Thromb Thrombolysis 43, 352–360 (2017). https://doi.org/10.1007/s11239-016-1470-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1470-0