Abstract
Proton pump inhibitors (PPIs) at low doses can effectively prevent gastrointestinal bleeding due to aspirin and are widely used in Japan for gastroprotection in patients taking anti-platelet agents. We examined the influence of different PPIs at low doses administered concomitantly or separately on anti-platelet functions of clopidogrel. In 41 healthy Japanese volunteers with different CYP2C19 genotypes who took clopidogrel 75 mg in the morning alone, or with omeprazole 10 mg, esomeprazole 10 mg, lansoprazole 15 mg, or rabeprazole 10 mg, either concomitantly in the morning or separately in the evening, we measured the inhibition of platelet aggregation (IPA, %) using VerifyNow P2Y12 assay at 4 h after the last clopidogrel dose on Day 7 of each regimen. IPA by clopidogrel with rabeprazole administered at lunchtime, approximately 4 h after clopidogrel, was also measured. Mean IPAs in those concomitantly receiving omeprazole, esomeprazole, lansoprazole or rabeprazole (47.2 ± 21.1%, 43.2 ± 20.2%, 46.4 ± 18.8%, and 47.3 ± 19.2%, respectively) were significantly decreased compared with those receiving clopidogrel alone (56.0%) (all ps < 0.001). This decrease was observed when PPIs were administered separately in the evening. However, IPA by clopidogrel with rabeprazole administered at lunchtime was 51.6%, which was markedly similar to that of clopidogrel alone (p = 0.114). All tested PPIs reduce the efficacy of clopidogrel when administered concomitantly. Our preliminary data suggest that administration of rabeprazole 4 h following clopidogrel may minimize potential drug–drug interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-platelet therapy is now widely used in patients with stroke, myocardial infarction or peripheral arterial disease. Clopidogrel is one of most important agents in therapies for these disorders. Clopidogrel is given as a single agent or concomitantly with other antiplatelet agent, such as aspirin [1, 2]. However, because this antiplatelet therapy carries an increased risk of gastrointestinal bleeding [3–7], a proton pump inhibitor (PPI) is often prescribed concomitantly with the anti-platelet agent. In 2008, the American College of Cardiology Foundation (ACCF), the American College of Gastroenterology (ACG) and the American Heart Association (AHA) published their statement on antiplatelet therapy, which recommends the prescription of a PPI to patients at risk of peptic ulcer and/or receiving two or more antiplatelet agents [8]. For this purpose, low doses of PPIs, such as lansoprazole 15 and 5 or 10 mg of rabeprazole are used in Japan [9, 10].
Against this background, drug–drug interaction between clopidogrel and PPIs has recently attracted attention. CYP2C19 is involved in the metabolism of clopidogrel to form its active metabolites [11], and the plasma level of the active metabolite of clopidogrel depends on the activity of CYP2C19 [12]. However, CYP2C19 is also the main metabolizing enzyme of PPIs [13], and the concomitant use of clopidogrel and a PPI induces a drug–drug interaction which results in the decreased activation of clopidogrel and attenuation of its anti-platelet effect. This could in turn lead to increased risks of re-infarction and stent thrombosis. PPIs are recognized as inhibitors of CYP2C19 [14], which would also contribute to attenuating the activation of clopidogrel. For example, Juurlink et al. [15] reported that concomitant therapy with a PPI other than pantoprazole was associated with an increased risk of re-infarction; Ho et al. [16] reported that concomitant use of clopidogrel and a PPI was associated with an increased risk of adverse outcomes compared with the use of clopidogrel alone; and Toth et al. [17] reported that the efficacy of clopidogrel was reduced if patients were concomitantly receiving a PPI such as omeprazole. In contrast, Siller-Matula et al. [18] reported that the intake of pantoprazole or esomeprazole was not associated with an impaired response to clopidogrel. The influences of PPIs on clopidogrel efficacy therefore appear controversial. However, prospective clinical studies have demonstrated that the concomitant use of a PPI does not increase the incidence of cardiovascular events in patients taking clopidogrel [19, 20]; on the contrary, the risk of gastrointestinal bleeding was increased in patients taking clopidogrel when they did not take a PPI [20]. A recent consensus has therefore stated that a PPI attenuates the antiplatelet function of clopidogrel to some extent but does not impair the clinical efficacy of clopidogrel, and that the risk of gastrointestinal bleeding is increased if a PPI is not administered concomitantly with clopidogrel. Coadministration of a PPI with clopidogrel is now considered preferable and recommended in patients at risk of gastrointestinal bleeding, while being careful about adverse events.
A second major problem with clopidogrel is interindividual difference in its anti-platelet activity among the different CYP2C19 genotype groups [11, 12]. Because active metabolization of clopidogrel is decreased in subjects with intermediate or poor metabolizer genotypes of CYP2C19, these patients are at higher risk of stent thrombosis and re-infarction [21–24]. However, the influence of different PPIs on the anti-platelet function of clopidogrel with reference to CYP2C19 genotypes has not been fully elucidated.
Previous reports on the drug–drug interaction between PPIs and clopidogrel used standard doses (i.e., 20 mg of omeprazole) [25, 26], although low doses (e.g., rabeprazole 10 mg and lansoprazole 15 mg) are sufficient to prevent the mucosal injury induced by NSAIDs including aspirin [9, 10, 27–29]. However, it remains unclear whether PPIs at low doses have the same attenuating effect on the anti-platelet function of clopidogrel as they do at standard doses. Further, we also wondered whether low doses of PPIs administered separately would avoid attenuation of the anti-platelet function of clopidogrel.
Here, we prospectively examined the effect of low doses of four PPIs, omeprazole 10 mg, esomeprazole 10 mg, lansoprazole 15 mg and rabeprazole 10 mg, on the anti-platelet function of clopidogrel in relation to CYP2C19 genotype status. The reason why we tested 10 mg of esomeprazole and omeprazole were as follows: Firstly to compare the different PPIs, the doses of PPI should be made the same as much as possible; Secondly, 20 mg of omeprazole and esomeprazole have already been studied in several papers. In addition, we also examined whether the interaction between a PPI and clopidogrel could be avoided by administering the two drugs separately instead of taking them concomitantly in healthy volunteers in Japan in relation to CYP2C19 genotypes.
Materials and methods
Subjects
Forty-one healthy Japanese volunteers were enrolled in this study. This sample size was determined as follows: In our previous report [25], the means of changes in IPAs by PPIs was around 5% and the standard deviation of IPAs was around 20% when subjects were taking 75 mg of clopidogrel daily. The correlation of IPAs between before and after PPI dosing was 0.85. Therefore, when α and 1 − β were set 0.05 and 0.8, respectively, the sample size was calculated to be 40 [30]. All were non-smokers and had not taken any drug for at least 2 weeks before and during this study. Written informed consent had been obtained from each of subject before their participation. The protocol was approved before the enrollment of subjects by the Ethic Comity of Hamamatsu University School of Medicine, Hamamatsu, Japan.
Genotyping of CYP2C19
DNA was extracted from blood samples obtained from volunteers using a commercially available kit (Genomix, Talent, Trieste, Italy). DNA samples were genotyped for CYP2C19 as previously reported [31] to identify the CYP2C19 wild type (*1) gene and two mutant alleles, CYP2C19*2 (*2) in exon 5 and CYP2C19*3 (*3) in exon 4. Volunteers were then classified into three groups by genotype, namely rapid metabolizers (RMs) (*1/*1), intermediate metabolizers (IMs) (*1/*2 or *1/*3) and poor metabolizers (PMs) (*2/*2, *3/*3, or *2/*3) [31]. The presence of the CYP2C19*17 (*17) allele (ultra rapid metabolizer) was also determined for all DNA samples as previously reported [32].
Study protocol
The primary endpoint of the study was to assess whether different types of PPI at low doses dosed concomitantly or separately influenced the antiplatelet function of clopidogrel. The secondary endpoint was to assess the influence of CYP2C19 genotypes on the interaction between clopidogrel and different types of PPIs at low doses. The study was conducted under an open-label single-arm crossover design. First, all 41 subjects took 75 mg of clopidogrel at 8 AM for 7 days. Inhibition of platelet aggregation (IPA), a representative index of the anti-platelet function of clopidogrel, was measured with the VerifyNow P2Y12 kit before the first dose to exclude subjects with abnormal platelet function and at 4 h (at noon) after the last dose of clopidogrel on day 7 to obtain the baseline data of antiplatelet function of clopidogrel. Next, all subjects participated in a crossover study of dosing of clopidogrel 75 mg with a different PPI for 7 days, in which they took 75 mg of clopidogrel with 10 mg of omeprazole (Omepral® AstraZeneca K.K., Osaka, Japan), 10 mg of esomeprazole (Nexium®, AstraZeneca K.K., Osaka, Japan), 15 mg of lansoprazole (Takepuron®, Takeda Pharmaceutical Co Ltd. Osaka, Japan) and 10 mg of rabeprazole (Pariet®, Eisai Co. Ltd., Tokyo, Japan), administered concomitantly in the morning or separately in the evening of the day before. Antiplatelet function was measured at 4 h after the last dose of clopidogrel on day 7 of each regimen. The order of the four PPIs administered in the morning or evening was randomized by the block method (Fig. 1). A clinical research coordinator (J. K.) managed the study schedule of each subject. Clopidogrel was taken once daily at 8 AM. PPIs were taken at 8 AM or 8 PM. A washout period of at least 14 days was provided between different dosing terms. Compliance was confirmed by sending reminder e-mail every morning and evening and by receiving a response from each subject confirming the completion of their drug protocol for the day.
Of 41 subjects, 37 (11 RMs, 22 IMs and 4 PMs) participated in the second study to examine whether separate administration of rabeprazole 10 mg at 20 h prior to clopidogrel could further prevent the drug–drug interaction. They took 10 mg of rabeprazole at 12 PM and 75 mg of clopidogrel at 8 AM in the next morning for 7 days. Antiplatelet function of clopidogrel was measured at 4 h after the last dose of clopidogrel on day 7, as noted above.
Data analysis
All numerical data are given as mean ± standard deviation (SD). Statistically significant differences in means of age and body weight between the CYP2C19 genotype groups were assessed by one-way ANOVA. Male/female ratios between two genotype groups were assessed by Fisher’s exact test. Statistically significant differences in changes in IPA with different regimens between the CYP2C19 genotype groups were assessed by repeated measures ANOVA and Scheffe’s multiple comparison test. Genotyping and allele frequencies of CYP2C19 have been calculated by counting. Expected genotype frequencies were calculated using the Hardy–Weinberg equation from allele frequencies (p2 + 2pq + q2 = 1), where p was the frequency of the CYP2C19*1 allele and q was the combined allele frequency of CYP2C19*2, CYP2C19*3 and CYP2C19*17. Hardy–Weinberg equilibrium was performed. All p values were two-sided, and p < 0.05 indicated statistical significance. All statistical calculations were performed using SPSS (IBM, SPSS, Statistics ver 20).
Results
CYP2C19 genotypes of subjects
All of the 41 subjects complete the study according to the study protocol. They consisted of 12 RMs, 25 IMs and 4 PMs CYP2C19. There were no subjects who had CYP2C19*17 allele. The allele frequencies was 0.60 for CYP2C19*1 (0.63), 0.33 for CYP2C19*2 and 0.07 for CYP2C19*3 and 0.00 for CYP2C19*17. All genotype distributions were in Hardy–Weinberg equilibrium. This distribution was almost the same as those reported in our ethnic groups [33, 34].
There were no significant differences in mean age, body weight, and male/female ratio among three genotype groups (Table 1).
Influence of CYP2C19 genotype on the IPA by clopidogrel alone
Before clopidogrel dosing, there were no differences in IPA among the three genotype groups (2.5 ± 5.0% for RMs, 4.0 ± 5.5% for IMs and 9.5 ± 6.5% PMs). After dosing of clopidogrel 75 mg for 7 days, mean (±SD) IPA was highest in RMs (73.2 ± 15.2%), followed in order by the IMs (52.4 ± 18.0%) and PMs (26.5 ± 7.6%) (Fig. 2). This observation is compatible with previous reports [25].
Inhibition of platelet aggregation (IPA) after administration of 75 mg clopidogrel in CYP2C19 RMs (rapid metabolizers), IMs (intermediate metabolizers) and PMs (poor metabolizers). There were statistically significant differences in the IPA among three CYP2C19 genotype groups. Data are shown as means ± SD (errors bars)
Effect of low doses of PPIs on the anti-platelet function of clopidogrel
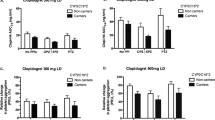
Compared with administration of clopidogrel alone, IPA was significantly decreased when omeprazole 10 mg, esomeprazole 10 mg, lansoprazole 15 mg or rabeprazole 10 mg was administered with clopidogrel 75 mg in the morning for 7 days. These PPIs had almost the same influence on mean IPA when administered separately in the evening as when administered concomitantly, showing no advantage for separate dosing [Figs. 3a–c, 4c: total (left panels)].
Inhibition of platelet aggregation (IPA) (%) after administration of clopidogrel (CLO) 75 mg dosed in the morning (Mor) with or without four types of PPI [a omeprazole (OPZ) 10 mg, b esomeprazole (EPZ) 10 mg, c lansoprazole (LPZ) 15 mg and d rabeprazole (RPZ) 10 mg] administered concomitantly in the morning (Mor) or separately in the evening (Eve) in the total (n = 41) and different CYP2C19 genotype groups [RMs rapid metabolizers (n = 12), IMs intermediate metabolizers (n = 25) and PMs poor metabolizers (n = 4)]. When PPIs were dosed concomitantly in the morning, IPAs were decreased by any of four PPIs in the total, RMs and IMs of CYP2C19. When PPIs were dosed separately in the evening, IPAs were decreased by omeprazole 10 mg, esomerapzole 10 mg and lansoprazole 15 mg in the total, RMs and IMs, but not statistically by rabeprazole 10 mg. No statistically significant influences of PPIs on the IPAs were not observed in PMs of CYP2C19. Data are shown as means ± SD (errors bars)
Percent changes of IPA by different PPIs, omeprazole (OPZ) 10 mg, esomeprazole (EPZ) 10 mg, lansoprazole (LPZ) 15 mg or rabeprazole (RPZ) 10 mg, administered concomitantly in the morning (Mor) or separately in the evening (Eve) in comparison with clopidogrel alone (%). Influences of RPZ 10 mg dosed separately in the evening appeared smallest of the eight dosing schemes. Data are shown as means ± SD (errors bars)
When subjects were stratified by CYP2C19 genotypes, IPA was significantly decreased in the RMs when omeprazole or esomeprazole was administered concomitantly in the morning or administered separately in the evening [(Fig. 3a, b: RM (2nd panel from the left)]. In cases of lansoprazole 15 mg and rabeprazole 10 mg, IPAs were significantly decreased by the concomitant dosing, while such significant decrease was not observed by the separate dosing in the evening [Fig. 3c, d: RM (2nd panels from the left)].
In IMs, IPA was significantly decreased when any of four PPIs were administered both concomitantly in the morning. When dosed separately in the evening, significant decreases in IPA were observed in cases of omeprazole, esomeprazole and lansoprazole, but not in case of rabeprazole [Fig. 3a–d: IM (2nd panel from the right)].
In PMs, the numbers of subjects with these genotypes were so limited that the effects of PPIs could not be determined [Fig. 3a–d: PM (the right panels)].
Percentage changes in IPA by four PPIs administered differently
Percentage changes in IPA by four different PPIs administered concomitantly in the morning or separately in the evening are compared in Fig. 4. A significant difference was observed between esomeprazole 10 mg and rabeprazole10 mg. Of the four PPIs, rabeprazole 10 mg appeared to have the smallest effect on the IPA by clopidogrel 75 mg.
Effect of rabeprazole 10 mg administered separately at 4 h after clopidogrel on the antiplatelet function of clopidogrel
Because the active metabolization of clopidogrel begins immediately after absorption and active metabolites of clopidogrel are eliminated from the systemic circulation within 4 h after an oral dose [12], a PPI administered at 4 h after clopidogrel has the lowest theoretical chance of interaction with clopidogrel. Moreover, Fig. 4 indicated that the influence of rabeprazole on clopidogrel was smaller than those of the other PPIs. We therefore tested the influence of 10 mg of rabeprazole administered after lunch on the antiplatelet function of clopidogrel administered after breakfast. When rabeprazole 10 mg was dosed at 20 h prior to (=4 h after) clopidogrel 75 mg, the IPA by clopidogrel, which was decreased by rabeprazole dosed concomitantly in the morning from 55.1 ± 20.6 to 47.6 ± 19.4%, was significantly restored to 51.6 ± 19.6% (p = 0.048) (Fig. 4a, total). When stratified based on CYP2C19 genotypes, significant restoration of IPA was observed by dosing of rabeprazole at 20 h before clopidogrel in IMs (from 45.6 ± 16.9 to 48.3 ± 18.6%, p = 0.048) (Fig. 5a, IM). We found that the percent decrease in IPA when rabeprazole was administered at lunch, 20 h before the next dosing of clopidogrel, was significantly smaller than that by the concomitant administration in the morning (−1.9 ± 28.5% vs. −10.6 ± 24.4%, p = 0.036) (Fig. 5b).
Effects of rabeprazole 10 mg administered concomitantly in the morning or separately in the evening or at noon. a Inhibition of platelet aggregation (IPA) in different CYP2C19 genotypes after administration of clopidogrel 75 mg in the morning (Mor) for 7 days with or without rabeprazole 10 mg administered concomitantly in the morning (Mor) or separately in the evening (Eve) or at noon (Noon). b Percent changes of IPA by rabeprazole administered concomitantly in the morning (Mor) or separately in the evening (Eve) or at noon (Noon). When rabeprazole 10 mg was dosed at noon, anti-platelet function of clopidogrel attenuated by rabeprazole dosed concomitantly in the morning was significantly restored. Data are shown as means ± SD (errors bars)
Individual changes in IPA by PPI dose
Figure 6 shows individual changes in IPA by different PPIs on administration in the morning or evening in the three CYP2C19 genotype groups. At 20% of IPA, we drew the line for descriptive purposes. IPAs of RMs of CYP2C19 were rarely decreased to less than by PPIs, except in one case administered with lansoprazole in the evening. However, IPAs of IMs of CYP2C19 were likely to be lowered to <20% by any of the PPIs on either administration. All IPAs of PMs of CYP2C19 became <20% by some kind of PPI.
IPA of each individual with or without the four types of PPIs (Omperazole 10 mg: OPZ, esomeprazole 10 mg: EPZ, lansoprazole 15 mg: LPZ and rabeprazole 10 mg: RPZ) administered concomitantly in the morning (Mor) or separately in the evening (Eve) in different CYP2C19 genotypes. The line of IPA of 20% was drawn for descriptive purposes
Discussion
In this study, we found that all four types of PPIs at the low doses influenced the antiplatelet function of clopidogrel, although we expected that low doses of PPIs would not interact with clopidogrel. We also found that the PPIs differed in their inhibitory effects on the antiplatelet function of clopidogrel. Interestingly, it appeared that interaction could be avoided by administration of rabeprazole 10 mg around 4 h after (20 h before) clopidogrel dosing. We also re-confirmed that the antiplatelet function of clopidogrel depended on CYP2C19 genotype.
The influence of the PPIs on the anti-platelet function of clopidogrel appears to differ by PPI. Zvyaga et al. [35] compared the inhibitory effects of different PPIs on CYP2C19 and found that two members of the PPI class (esomeprazole and omeprazole) were more likely to serve as clinically relevant inhibitors of CYP2C19. Ishizaki et al. [36] reported that omeprazole significantly decreased the mean clearance of diazepam, whereas rabeprazole had no effect on this variable, indicating that rabeprazole is a weaker substrate of CYP2C19 and has very little or no potential to interact with diazepam compared with omeprazole. However, in some report, rabeprazole was reported to influence the antiplatelet function of clopidogrel [37], whereas others reported that rabeprazole 20 mg as well as esomeprazole 20 or 40 mg did not influence the antiplatelet function of clopidogrel [38, 39]. Although we cannot offer appropriate explanation for these controversy, as reported by Funck-Brentano et al. [40], the influence of PPIs on pharmacokinetics of clopidogrel is not always so large, that such small changes in pharmacokinetics of clopidogrel might not be always reflected to the changes in antiplatelet function of clopidogrel.
In our present study, rabeprazole had significantly weaker influence on the anti-platelet function of clopidogrel than esomeprazole, which is compatible with previous reports on the effects of PPIs on cytochromes P450 [35, 41]. Because rabeprazole appeared to have the weakest inhibitory effect of the four PPIs and the least interaction with clopidogrel when administered separately, we conducted an additional study with administration of rabeprazole 4 h after clopidogrel. Because clopidogrel is metabolized to the active form within 4 h after administration [12], PPI administered at 4 h after clopidogrel is expected to have least chance to interact with clopidogrel. Moreover, the inhibitory effect of rabeprazole on the activity of P450 is the lowest of the 4 PPIs [35]. The dosing of rabeprazole at 4 h after clopidogrel is equal to the dosing of rabeprazole at 20 h before clopdidogrel dosing. The duration of 20 h is enough for most PPIs disappears from systemic circulation. In our previous reports, low levels of omeprazole, omeprazole-sulphone, lansoprazole, rabeprazole and thioether rabeprazole, which were substrates of CYP2C19, were detected in plasma at 10 h but none or very low at 24 h after the last dosing of omerapzole, lansoprazole or rabeprazole [42–44]. This timing (at 20 h prior to Clopidogrel) therefore was assumed to allow us to avoid interaction of rabeprazole with clopidogrel.
The overall changes of IPAs by PPIs were small, individual values greatly changed (Fig. 6). Interestingly, we found that IPAs of RMs of CYP2C19 were hardly decreased to very low levels (e.g., <20%) after concomitant use of a PPI. In contrast, IPAs of IMs of CYP2C19 were easily lowered to <20%. Therefore, in this study, we could reconfirm that CYP2C19 genotypes had great influence on the antiplatelet function of clopidogrel. However, as shown in Fig. 6, there were very wide interindividual differences in IPAs in each CYP2C19 genotype group, suggesting that an individual IPA level is very difficult to estimate and that although the overall changes in IPAs by a PPI is small, it is necessary to be careful for the presence of some subjects whose IPAs on clopidogrel are greatly influenced by a PPI.
In the clinical practice, clopidogrel is often used with low dose of aspirin, for example, in patients who underwent percutaneous coronary intervention. Since anti-platelet function of low dose of aspirin is not attenuated by a PPI [29], influence of a PPI on clopidogrel is assumed to be compensated by aspirin to some extent. However, patients with ischemic cerebrovascular disease and/or peripheral arterial diseases are often treated with clopidogrel alone. We should carefully review the concomitant use of a PPI in such patients.
Finally, our results must be interpreted within the following limitations of the study. First, our study subjects were all healthy Japanese volunteers, not patients or not non-Asians. There are ethnic differences in the incidence of CYP2C19 *2 and *3 alleles. Therefore, we are not sure whether our results can be extrapolated to European and US patients. Secondly, the sample size seems small to study the combined effects of CYP2C19 genotypes and different PPIs on clopidogrel in one study. Third, study duration was 7 days, although patients take clopidogrel and PPI for longer times. Forth, we did not measure the time-course of changes in plasma levels of clopidogrel, its metabolites or PPIs. Finally, we measured the anti-platelet function by only one test (e.g., VerifyNow P2Y12). For these reasons, our results should be considered preliminary and cannot be extrapolated to clinical practice. Nevertheless, we wish to emphasize that PPIs at low doses can attenuate the antiplatelet function of clopidogrel.
Conclusion
We found that the antiplatelet function of clopidogrel was attenuated by low doses of PPIs. Our preliminary data suggest that administration of rabeprazole 4 h following clopidogrel may minimize potential drug–drug interactions. We reconfirmed that the antiplatelet function of clopidogrel is dependent on CYP2C19 genotype. Clinicians treating patients with clopidogrel and a PPI should take these pharmacological characteristics of these agents into consideration.
Abbreviations
- CYP2C19:
-
Cytochrome p450 2C19
- CYP3A4:
-
Cytochrome p450 3A4
- IM:
-
Intermediate metabolizer
- IPA:
-
Inhibition of platelet aggregation
- PM:
-
Poor metabolizer
- RM:
-
Rapid metabolizer
References
Milionis H, Michel P (2013) Acute ischemic cerebrovascular events on antiplatelet therapy: what is the optimal prevention strategy? Curr Pharm Des 19:3788–3794
Gouya G, Arrich J, Wolzt M et al (2014) Antiplatelet treatment for prevention of cerebrovascular events in patients with vascular diseases: a systematic review and meta-analysis. Stroke 45:492–503
Derry S, Loke YK (2000) Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. Bmj 321:1183–1187
Chan FK, Ching JY, Hung LC et al (2005) Clopidogrel versus aspirin and esomeprazole to prevent recurrent ulcer bleeding. N Engl J Med 352:238–244
Hallas J, Dall M, Andries A et al (2006) Use of single and combined antithrombotic therapy and risk of serious upper gastrointestinal bleeding: population based case-control study. Bmj 333:726
Awtry EH, Loscalzo J (2000) Aspirin. Circulation 101:1206–1218
Nakayama M, Iwakiri R, Hara M et al (2009) Low-dose aspirin is a prominent cause of bleeding ulcers in patients who underwent emergency endoscopy. J Gastroenterol 44:912–918
Bhatt DL, Scheiman J, Abraham NS et al (2008) ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 118:1894–1909
Iwakiri R, Higuchi K, Kato M et al (2014) Randomised clinical trial: prevention of recurrence of peptic ulcers by rabeprazole in patients taking low-dose aspirin. Aliment Pharmacol Ther 40:780–795
Sugano K, Matsumoto Y, Itabashi T et al (2011) Lansoprazole for secondary prevention of gastric or duodenal ulcers associated with long-term low-dose aspirin therapy: results of a prospective, multicenter, double-blind, randomized, double-dummy, active-controlled trial. J Gastroenterol 46:724–735
Hulot JS, Bura A, Villard E et al (2006) Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 108:2244–2247
Umemura K, Furuta T, Kondo K (2008) The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics in an active metabolite of clopidogrel in healthy subjects. J Thromb Haemost 6:1439–1441
Furuta T, Shirai N, Sugimoto M et al (2004) Pharmacogenomics of proton pump inhibitors. Pharmacogenomics 5:181–202
Stedman CA, Barclay ML (2000) Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther 14:963–978
Juurlink DN, Gomes T, Ko DT et al (2009) A population-based study of the drug interaction between proton pump inhibitors and clopidogrel. CMAJ 180:713–718
Ho PM, Maddox TM, Wang L et al (2009) Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA 301:937–944
Toth PP, Armani A (2009) Thienopyridine therapy and risk for cardiovascular events in secondary prevention. Curr Atheroscler Rep 11:364–370
Siller-Matula JM, Spiel AO, Lang IM et al (2009) Effects of pantoprazole and esomeprazole on platelet inhibition by clopidogrel. Am Heart J 157:148e1–e5
O’Donoghue ML, Braunwald E, Antman EM et al (2009) Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 374:989–997
Bhatt DL, Cryer BL, Contant CF et al (2010) Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med 363:1909–1917
Malek LA, Kisiel B, Spiewak M et al (2008) Coexisting polymorphisms of P2Y12 and CYP2C19 genes as a risk factor for persistent platelet activation with clopidogrel. Circ J 72:1165–1169
Simon T, Verstuyft C, Mary-Krause M et al (2009) Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 360:363–375
Mega JL, Close SL, Wiviott SD et al (2009) Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 360:354–362
Collet JP, Hulot JS, Pena A et al (2009) Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 373:309–317
Furuta T, Iwaki T, Umemura K (2010) Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol 70:383–392
Gilard M, Arnaud B, Cornily JC et al (2008) Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol 51:256–260
Nishino M, Sugimoto M, Kodaira C et al (2011) Preventive effects of lansoprazole and famotidine on gastric mucosal injury induced by low-dose aspirin in Helicobacter pylori-negative healthy volunteers. J Clin Pharmacol 51:1079–1086
Graham DY, Agrawal NM, Campbell DR et al (2002) Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs. lansoprazole. Arch Intern Med 162:169–175
Uotani T, Sugimoto M, Nishino M et al (2012) Ability of rabeprazole to prevent gastric mucosal damage from clopidogrel and low doses of aspirin depends on CYP2C19 genotype. Clin Gastroenterol Hepatol 10:879–885e2
Nagashima K (2013) A sample size estimation tool for the paired t test (internet). http://nshi.jp/contents/js/pairedmean/ (in Japanese)
Furuta T, Sagehashi Y, Shirai N et al (2005) Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H. pylori Infection. Clin Gastroenterol Hepatol 3:564–573
Sim SC, Risinger C, Dahl ML et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79:103–113
Kubota T, Chiba K, Ishizaki T (1996) Genotyping of S-mephenytoin 4′-hydroxylation in an extended Japanese population. Clin Pharmacol Ther 60:661–666
Furuta T, Shirai N, Takashima M et al (2001) Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther 69:158–168
Zvyaga T, Chang SY, Chen C et al (2012) Evaluation of six proton pump inhibitors as inhibitors of various human cytochromes P450: focus on cytochrome P450 2C19. Drug Metab Dispos 40:1698–1711
Ishizaki T, Chiba K, Manabe K et al (1995) Comparison of the interaction potential of a new proton pump inhibitor, E3810, versus omeprazole with diazepam in extensive and poor metabolizers of S-mephenytoin 4′-hydroxylation. Clin Pharmacol Ther 58:155–164
Siriswangvat S, Sansanayudh N, Nathisuwan S et al (2010) Comparison between the effect of omeprazole and rabeprazole on the antiplatelet action of clopidogrel. Circ J 74:2187–2192
Sibbing D, Morath T, Stegherr J et al (2009) Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost 101:714–719
Wu J, Jia LT, Shao LM et al (2013) Drug–drug interaction of rabeprazole and clopidogrel in healthy Chinese volunteers. Eur J Clin Pharmacol 69:179–187
Funck-Brentano C, Szymezak J, Steichen O et al (2013) Effects of rabeprazole on the antiplatelet effects and pharmacokinetics of clopidogrel in healthy volunteers. Arch Cardiovasc Dis 106:661–671
Li XQ, Andersson TB, Ahlstrom M et al (2004) Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 32:821–827
Furuta T, Ohashi K, Kosuge K et al (1999) CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther 65:552–561
Shirai N, Furuta T, Xiao F et al (2002) Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment Pharmacol Ther 16:837–846
Shirai N, Furuta T, Moriyama Y et al (2001) Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther 15:1929–1937
Acknowledgements
This work was supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590718). We thank the staff at the Translational Research Unit, Ms. Takako Toyoda, Ms. Junko Kuroki, Ms. Yoko Akahori, Ms. Yumi Kiyama, Ms Keiko Arasawa, Ms. Saori Oikawa and Ms. Naomi Hashimoto for their help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors had any conflict of interest related to this study.
Rights and permissions
About this article
Cite this article
Furuta, T., Sugimoto, M., Kodaira, C. et al. Influence of low-dose proton pump inhibitors administered concomitantly or separately on the anti-platelet function of clopidogrel. J Thromb Thrombolysis 43, 333–342 (2017). https://doi.org/10.1007/s11239-016-1460-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1460-2