Abstract
To analyze the cost-utility of oral dabigatran etexilate, enoxaparin sodium injection, and no intervention for venous thromboembolism (VTE) prophylaxis after total hip or knee replacement (THR/TKR) surgery among Thai patients. A cost-utility analysis using a decision tree model was conducted using societal and healthcare payers’ perspectives to simulate relevant costs and health outcomes covering a 3-month time horizon. Costs were adjusted to year 2014. The willingness-to-pay threshold of THB 160,000 (USD 4926) was used. One-way sensitivity and probabilistic sensitivity analyses using a Monte Carlo simulation were performed. Compared with no VTE prophylaxis, dabigatran and enoxaparin after THR and TKR surgery incurred higher costs and increased quality adjusted life years (QALYs). However, their incremental cost-effectiveness ratios were high above the willingness to pay. Compared with enoxaparin, dabigatran for THR/TKR lowered VTE complications but increased bleeding cases; dabigatran was cost-saving by reducing the costs [by THB 3809.96 (USD 117.30) for THR] and producing more QALYs gained (by 0.00013 for THR). Dabigatran (vs. enoxaparin) had a 98 % likelihood of being cost effective. Dabigatran is cost-saving compared to enoxaparin for VTE prophylaxis after THR or TKR under the Thai context. However, both medications are not cost-effective compared to no thromboprophylaxis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is a disease caused by a blood clot (thrombus) in a deep vein within the muscle. It has a clinical spectrum of conditions consisting of deep venous thrombosis (DVT) and pulmonary embolism (PE) [1, 2]. Patients undergoing total hip or knee replacement (THR or TKR), are at high risk of VTE [3, 4]. VTE is the third-rank cause of cardiovascular death among developed countries, behind myocardial infarction and stroke [5, 6] and it incurs a substantial financial burden for the health care system [7]. VTE has been perceived to be less common in Asia for a long time [1, 8]. However, it is increasingly recognized as a significant burden in Asian populations as its incidence (e.g. 0.3–3.9 % in Thailand) has been comparable or somewhat higher than 1.0−1.8 % in Western populations [5, 9].
Prevention of thrombi is commonly used for decreasing the risk of VTE for THR or TKR patients when pharmacological prophylaxis is feasible [5]. Low molecular weight heparins (LMWHs) such as enoxaparin sodium administered via subcutaneous (SC) injection is most widely used for VTE prophylactic therapy [2, 3, 5]. Although effective and safe, enoxaparin must be daily injected requiring administration skills by patients, or help from care givers. The injection can lead to pain, SC associated hematomas, or an infrequent serious complication known as type II heparin-induced thrombocytopenia (HIT) [6]. Dabigatran is a new oral anticoagulant which is easy to administer and it is currently approved for VTE prophylaxis in adult THR and TKR patients [2].
Previous economic evaluation studies suggested that dabigatran compared with enoxaparin for VTE prophylaxis was likely to provide a good value for money [6, 7, 10, 11]. However, there still remains a lack of economic evaluation conducted in Asian countries especially in Thailand where the healthcare system and costs differ from Western countries. The results of economic evaluation between dabigatran and enoxaparin will be required during decision making process for stakeholders in Thailand especially policy makers to choose pharmaceuticals to be in the National drug list. Therefore, this study aims to assess cost-utility of dabigatran, enoxaparin, and usual care for DVT and PE prophylaxis among Thai patients undergoing THR or TKR under the Thai healthcare system context.
Methods
Overall description
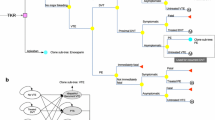
A cost-utility analysis was used to estimate cost and health outcomes of VTE (i.e. DVT and PE) prophylaxis among THR and TKR patients in Thailand. The intervention of interest was dabigatran and the comparators were generic and original enoxaparin, and no thromboprophylaxis (usual care). A hypothetical cohort of 1,000,000 individuals was simulated in a decision tree model (Fig. 1). The model captures five health states including incidences of symptomatic DVT, PE, major bleeding, minor bleeding, and HIT during the period of 3-month time horizon. This time horizon was chosen because the risk of VTE exists for only 3 months after the operation. Discounting is not required when it is less than 1 year according to the Thailand’s Health Technology Assessment (HTA) guideline [12]. The study was conducted under the societal and healthcare payers’ perspectives. Cost-effectiveness measures were presented as incremental costs per quality-adjusted life year gained (QALY) in Thai Baht (THB) and US dollars (USD). The foreign exchange rate of USD 1 = THB 32.48 [13].
Health technology assessment process
This study was undertaken according to the Thai HTA guideline emphasizing transparency, inclusiveness, and accountability [12]. Therefore, stakeholders were involved in our study process. They include (1) payer representatives from the national health security office, the comptroller general’s department, and the social security office, (2) policy makers from the national list of essential medicine and the university hospital network, (3) healthcare providers (i.e. hematologists and orthopedists), and (4) patient representatives. Two stakeholder meetings were conducted—one was to approve a proposed economic model, and to identify the study scope respecting its research question, population, intervention, comparators, and outcomes, and the other was to present the preliminary results. Some input examples from the stakeholder meetings that were applied in the study included confirmation of the model structure, input parameters, and assumptions, and the inclusion of “no thromboprophylaxis” as a comparator because it was the current usual care practice in Thailand.
Model input parameters
All base-case input parameters are shown in Table 1. Model input parameters mainly included incidences of the five health outcomes, case-fatality ratios of the outcomes, effectiveness of the intervention and comparators, utilities, and costs. These parameters were obtained from various sources. The main source was systematic review and meta-analysis which exhibit the highest reliability of evidence, and published articles. Local data were used when available.
Incidences
The incidences of patients developing DVT and PE receiving no thromboprophylaxis were obtained from systematic review and meta-analysis contextualized to Thailand or Asian population [9]. However, the incidences of patients developing major bleeding, minor bleeding, and HIT were based on data from a systematic review and meta-analysis among Western population [4] because local data were not available.
Case-fatality ratios
To reflect the local context, the case-fatality ratios related to DVT and PE were calculated from electronic hospital databases obtained from Ramathibodi and Buddhachinaraj hospitals. From the hospital databases, numbers of DVT and PE hospitalizations were calculated from their primary diagnoses of DVT [using the International Classification of Disease version 10 (ICD-10) codes of I82, I63.6, I67.6, I80.2, I81, and K55.0] and PE (using the ICD-10 codes of I26) from year 2011 to year 2014. There were 940 and 123 hospitalizations for DVT and PE, respectively. The retrospective data revealed that the case-fatality ratio of DVT was 8 % while that of PE was 22 %. However, based on the stakeholder meeting, it was commonly agreed based on their practice experience that the case fatality ratio of DVT should have been 0 % because the primary diagnosis from the databases may have been reflecting the primary healthcare resources used but not the actual mortality.
Effectiveness
The effectiveness of enoxaparin against no thromboprophylaxis for patients developing DVT and PE was derived from American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [5], while the effectiveness of enoxaparin against no thromboprophylaxis for patients developing bleeding was derived from a systematic review and meta-analysis [14]. Furthermore, the effectiveness of dabigatran against enoxaparin for patients developing DVT, PE, and bleeding was derived from systematic review and meta-analysis of a published article [4].
The incidences of DVT and PE in patients receiving enoxaparin and dabigatran were calculated based on the multiplication of the incidence rates of DVT and PE among patients without thromboprophylaxis and the relative risks of DVT and PE for THR and TKR patients receiving enoxaparin versus dabigatran.
Utility weights
The utility weights for THR and TKR patients without any complications were obtained from a local published study [15].
QALYs during hospitalization were calculated by multiplying utility values with the length of stay of DVT (8.5 days) and PE (10.5 days) estimated from Ramathibodi and Buddhachinaraj hospital databases. The lengths of stay for patients developing major bleeding (9 days) and HIT (15 days) were derived from the local publications [16, 17] while the length of stay for patients developing minor bleeding (1.5 days) was assumed and confirmed by the stakeholders.
Drug regimens for VTE prophylaxis after THR/TKR
Drug regimen durations were obtained from the registered indications for THR and TKR [4, 6, 18, 19]. The dosage regimen of dabigatran etexilate used in this study was 220 mg once daily, with a half dose on day 1 which was initiated 1–4 h after the surgery and then the full dose on the remaining duration of 33 days (minimum–maximum: 28–38) for THR. Similarly, for TKR, the initial dose was the same but the full dose onwards was prescribed only for 12 days (minimum–maximum: 10–14) [4, 6, 18].
The dosage regimen of enoxaparin for THR was 30 mg subcutaneously (SC) every 12 h for 12 days, and 40 mg SC once daily for the next 21 days (minimum–maximum: 16–26), whereas that for TKR was 30 mg SC every 12 h for 12 days (minimum–maximum: 10–14) [4, 6, 19].
Costs
Societal and healthcare payers’ perspectives were applied in the analysis. The societal perspective included only direct medical and non-medical costs. Indirect cost was excluded to prevent double counting because the loss of productivity would be counted in the disutility of QALY, based on the Thailand’s HTA guideline [12]. For analysis using healthcare payers’ perspective, only direct medical cost was included.
The prices of medications were acquired from Drug And Medical Supply Information Center, Ministry of Public Health [20]. We calculated drug cost per THR/TKR course by multiplying drug price per unit with the thromboprophylaxis duration. The minimum and maximum of drug costs were multiplied by the lower and upper bounds of thromboprophylaxis days, respectively (Table 1 presents the final costs). The unit price of dabigatran per capsule (110 mg) was THB 56.18 (USD 1.73) [20]. The unit price of generic enoxaparin in a 60 mg/0.6 ml pre-filled syringe was THB 229.59 (USD 7.07), whereas that of a 40 mg/0.4 ml pre-filled syringe was THB 198.04 (USD 6.1) [20]. The price of generic enoxaparin 60 mg/0.6 ml pre-filled syringe was used for the dosage regimen of 30 mg every 12 h. The same method was used for original enoxaparin where the unit price was THB 241.72 (USD 7.44) for a 60 mg/0.6 ml pre-filled syringe and it was THB 201.14 (USD 6.19) for a 40 mg/0.4 ml pre-filled syringe [20].
Direct medical costs of prophylaxis were collected from two different costing approaches—Cost to Charge Ratio (CCR) for base-case analysis and Diagnostic Related Group (DRG) for sensitivity analysis. The former was used for the societal perspective taking into account that real cost incurred from resources consumed, whereas the latter was applied for the healthcare payers’ perspective estimating expected costs from similar patients and diagnoses. The CCR approach utilized charge data multiplied by cost to charge ratios of 0.8 and 1.37 for Buddhachinaraj and Ramathibodi hospital, respectively. The DRG approach was based on DRG using the National Health Security Office database. The direct non-medical cost included costs related to travelling and food estimated from Health Intervention and Technology Assessment Program (HITAP) costing database in Thailand [21]. All costs were adjusted to cost values in 2014 using medical-care consumer price index [22].
Base-case analysis
Primary outcomes in this analysis were the number of DVT, PE, major bleeding, minor bleeding, and HIT cases among hypothetical THR/TKR Thai patients; incremental costs, QALYs gained, and incremental cost-effectiveness ratio (ICER). The base-case analysis used the societal perspective.
The interpretation of cost-effectiveness of the findings was based on the willingness-to-pay threshold of a cost-effective intervention at THB 160,000 (USD 4926) per QALY gained in 2013 set by the Sub-committee of Thai Working Group on Health Technology Assessment year 2013 [23–26].
Sensitivity analysis
One-way sensitivity analysis was performed to evaluate the uncertainties surrounding the input parameters within plausible ranges of 95 % confidence intervals. Those parameters included incidences of complications, effectiveness, costs and utilities.
In addition, a probabilistic sensitivity analysis (PSA) was conducted to examine the effects of all parameters uncertainty using a Monte Carlo simulation. The distributions of each probability were assigned as follows [27]: (a) beta distribution for probability and utility parameters, in which their values ranged between zero and one, (b) gamma distribution for costs and length of stay where their values were positively skewed and above zero, and (c) log-normal distribution for the effectiveness and relative risks. A Monte Carlo simulation was run for 1000 iterations to provide a distribution of values for total costs, outcomes, and ICERs. Results of the PSA were presented as a cost-effectiveness acceptability curve. The expected net monetary benefit (NMB) was performed to highlight the probability whether dabigatran was cost-effective.
Results
Base-case analysis
Out of 1,000,000 hypothetical THR patients, the use of dabigatran resulted in the highest number of patients (932,676 patients) who did not have any health complications, compared with the use of enoxaparin (927,720 patients) and no thromboprophylaxis (922,300 patients) (Table 2). When compared to no thromboprophylaxis, the thromboprophylaxis therapies resulted in a greater reduction in DVT and PE complications but with a larger number of bleeding cases. Dabigatran (vs. no thromboprophylaxis) reduced 20,448 cases of DVT and 1828 cases of PE while it incurred additional 11,900 cases of major and minor bleeding. Similarly, but in a lesser extent, enoxaparin (vs. no thromboprophylaxis) reduced 12,870 DVT cases and 1350 PE cases, but it caused additional 6800 major and minor bleeding cases. Compared with enoxaparin, dabigatran reduced additional 7578 DVT cases and 264 PE cases but it resulted in additional 5100 bleeding cases. Moreover, only found in patients receiving enoxaparin, there were total of 2000 cases of patients developing HIT. The trend of the clinical outcomes was the same for TKR patients.
For both THR and TKR, under the societal perspective, the total costs of both thromboprophylaxis medications were higher and the QALYs were increased compared to no thromboprophylaxis while dabigatran was cost-saving compared to enoxaparin (Tables 3, 4). For example, the incremental costs of dabigatran (vs. no prophylaxis) for treating THR were THB 2582.19 (USD 79.50) and its QALYs gained was 0.00015 resulting in ICER of THB 17,503,661 (USD 538,906). Enoxaparin (vs. no prophylaxis) for treating THR had the incremental costs of THB 6392 (USD 196.81), the QALYs gain of 0.00002, and the ICER of THB 339,690,120 (USD 10,458,440). When comparing with enoxaparin, dabigatran for treating THR was cost-saving by lowering total costs by THB 3809.96 (USD 117.30) and producing more QALYs gain by 0.00013.
When using the healthcare payers’ perspective (Online Appendix Tables A1 and A2), the results for TKR and THR were very similar to those of the base case using the societal perspective.
Sensitivity analyses
The results of a series of one-way sensitivity analyses of dabigatran against no thromboprophylaxis were different from that between enoxaparin against no thromboprophylaxis. All sensitivity analysis results of dabigatran against no thromboprophylaxis for THR fall in the northeast quadrant (higher QALYs and higher cost) of a cost-effectiveness plane. The incidence of symptomatic PE was the most influential parameter. The ICERs varied from THB 4,918,671 (USD 151,437) to THB 70,201,621 (USD 2,161,380) per QALY (Online Appendix Fig A1). On the other hand, the one-way sensitivity analyses of enoxaparin against no thromboprophylaxis for THR under the societal perspective showed that several parameters affected the results widely. The results fall in the range of the northwest quadrant (dominated: lower QALYs and higher cost) and the northeast quadrant (higher QALYs and higher cost) [up to THB 61,309,382 (USD 1,887,604) per QALY] in a cost-effectiveness plane. The incidence of symptomatic DVT was the most influential parameter. Other influential parameters were the utility of minor bleeding and the relative risk of bleeding with use of enoxaparin compared to placebo. One-way sensitivity analysis of dabigatran against enoxaparin for THR showed that the results were quite robust across all changes of parameters except one which was relative risk of dabigatran against enoxaparin for DVT and PE. The ICERs varied from cost-saving to THB 10,706,206 (USD 329,625) per QALY. All of the results of one-way sensitivity analysis for TKR were similar to those in THR.
From PSA, both dabigatran and enoxaparin were estimated to have higher cost and lower QALYs compared with no thromboprophylaxis, shown in the scatter plot (Online Appendix Fig A2). To be cost-effective at the cut-off level of THB 160,000 (USD 4926) per QALY, for THR, the price of dabigatran should be reduced by 67 % [from THB 3724 (USD 115) to THB 1205 (USD 37) per course]. Likewise, the price of enoxaparin must be decreased by 93 % [from THB 6913 (USD 213) to THB 524 (USD 16) per course]. For TKR, the cost-effective price of dabigatran and enoxaparin for TKR should be as low as THB 1053 (USD 32) [25 % reduction of THB 1404 (USD 43)] per course and THB 450 (USD 14) [84 % reduction of THB 2755 (USD 85)], respectively. The results of cost-effective prices under the healthcare payers’ perspective were presented in Online Appendix Table A3.
Based on the PSA comparing dabigatran with enoxaparin shown in Fig. 2, the majority of simulated ICERs (86.8 %) were found in the lower right quadrant indicating that dabigatran tended to be less costly and more effective than enoxaparin (dominant). The cost-effectiveness acceptability curve showed that at a threshold value of THB 160,000 (USD 4926) per QALY, dabigatran had a 98 % likelihood of being cost effective when compared with enoxaparin.
Discussion
To the best of our knowledge, this is the first study assessing the cost-effectiveness of dabigatran for the prevention of VTE after THR and TKR in an Asia-Pacific region. Dabigatran offered a good value for money, when compared to enoxaparin for VTE prevention, in patients undergoing THR and TKR under Thai context. In addition, we also estimated cost-effectiveness of the prevention of VTE (dabigatran or enoxaparin) compared to no VTE prevention. Interestingly, VTE prophylaxis with these two medications was not cost-effective, when compared to ‘no thromboprophylaxis’ with the current context. These findings will be very important information to be considered for policy and clinical decision making by relevant stakeholders including policy makers, public health organizations, and healthcare professionals.
The present cost-utility analysis revealed that the use of dabigatran was cost-saving compared to enoxaparin for the prevention of VTE after THR and TKR under both societal and healthcare perspectives. This is due to several reasons. Dabigatran reduced VTE complications in a greater extent compared to enoxaparin, although the bleeding was moderately higher than that of enoxaparin. However, the costs of VTE complications were larger than the bleeding costs. Also, enoxaparin caused HIT complications whose treatment was costly. Furthermore, dabigatran had lower cost than enoxaparin. The last two reasons were the main factors driving dabigatran to be cost-saving.
The findings in this study are consistent with the results from three previous studies evaluating oral anticoagulants versus enoxaparin. First, a study was conducted in Spain to evaluate oral anticoagulants (i.e. dabigatran, rivaroxaban, and apixaban) compared with enoxaparin for the prophylaxis of VTE by using separated decision tree models for THR and TKR during a 3-month time horizon [6]. The second study conducted an economic evaluation of dabigatran compared with enoxaparin in the United Kingdom using a decision tree model for the 10-week acute phase after the surgery, and a Markov model with a lifetime horizon for the chronic phase [10]. Next, an Irish study assessed oral anticoagulants versus enoxaparin using decision tree models for THR and TKR separately for the 6-month post-surgery period [7]. The results showed that the oral anticoagulants were less costly than enoxaparin under the Spanish National Health System and the UK National Health Service perspectives and dabigatran was cost-effective compared to enoxaparin with the Irish health-payer perspective.
This study estimated that the prevention of VTE using dabigatran and enoxaparin compared with no thromboprophylaxis in patients undergoing THR and TKR was not cost-effective using the willingness to pay in Thailand. This is because the costs of dabigatran and enoxaparin were much higher than that of no thromboprophylaxis whereas the QALYs gained from both medications were very minimal.
In Asian countries, thromboprophylaxis therapy is not a common practice for patients undergoing THR or TKR. Nowadays, pharmacoprophylaxis prescription among eligible THR or TKR patients in Thailand as well as in Asian countries has solely depended on individual clinician judgment. The potential reason is mostly due to the belief of low VTE incidence in Asian countries [1, 8]. However, current evidence reveals that VTE incidence rates in Asian countries and especially in Thailand with the incidence rate of 0.3–3.9 % were generally comparable or considerably higher to those in Western countries (1.0–1.8 %) [5, 9]. This suggests that stakeholders need to realize the importance of VTE prophylaxis.
Furthermore, our current knowledge through global literature review found no study evaluating the cost-effectiveness of VTE prevention compared to no thromboprophylaxis. Rather, what was found about thromboprophylaxis was only the first documented clinical practice guideline in 1990 [5]. From a global perspective, prevention of VTE has been a standard of care worldwide [5, 28, 29]. This suggests that a decision on adoption of VTE prevention in Western countries might be driven by clinical practice.
With the evidence of VTE incidence rates in Asian countries being contrary to conventional wisdom and justification of thromboprophylaxis supported by clinical purposes, consideration of thromboprophylaxis practice in Asian countries should be influenced by multiple factors. First, other health outcomes such as the number of preventable VTE and bleeding cases should be considered. Second, the implication of potential lawsuits involved for preventable cases of possible complications should be brought into the table for discussion. In addition to incremental costs per QALY, other important factors (used in the UK, Australia, and Canada), include uncertainty regarding cost effectiveness, net cost to the relevant jurisdiction, burden of disease, availability of alternative treatments, and specific factors indicated by the jurisdiction, the willingness to negotiate price, and approach to generic drugs [30, 31]. All relevant stakeholders should make informed decisions together using current evidence with full understanding of potential consequences associated with decisions made.
Several key strengths of this study were the following. First, the findings should have high validity because the effectiveness of thromboprophylaxis was derived from recent systematic reviews and meta-analysis of randomized controlled trials comparing oral anticoagulants and enoxaparin for the prevention of VTE after THR and TKR. This reflects the highest reliable source of evidence of efficacy and effectiveness [4]. Second, the results were contextually relevant because some incidences, mortality, utility, and cost data were mostly derived from the local data to reflect the Thai population. These parameters especially costs were reported to have very high impact on making economic evaluation specific to local settings [32]. Moreover, the stakeholders including payers, policy makers, healthcare providers, physicians, and patient representatives were involved throughout the study process to develop valid input parameters and increase transparency.
A number of limitations are acknowledged. First, some of the input parameters, especially utility weights, were derived from European studies [33, 34] because of the limited availability of local data. However, the European utility weights were used as the adjusting factors only and the baseline utility values were obtained from the local study [15]. Second, costs of PE and DVT, and the case-fatality ratio of PE were derived from solely two electronic local hospital databases. Although they may not be the representative of the whole population in Thailand, they were the best available data. Also, even though the Thai data may limit generalizability to other settings, some settings with similar context to Thailand may be able to apply the findings directly. Lastly, we have not assessed other oral anticoagulants (i.e., apixaban and rivaroxaban) in this analysis. Further study can be conducted not only comparison between oral anticoagulants and enoxaparin or no prophylaxis but also comparison among all marketed oral anticoagulants.
Conclusions
On the basis of current best evidence and input data, this economic evaluation suggests that dabigatran is preferably a cost-saving option compared with enoxaparin under the Thai context, when thromboprophylaxis is prescribed. However, both treatments are not cost-effective compared to no thromboprophylaxis but thromboprophylaxis may be necessary when considering other aspects such as reduction of VTE complications. Policy making on thromboprophylaxis among eligible patients in Asian countries including Thailand should take other aspects into account, other than considering only economic values.
References
Angchaisuksiri P (2011) Venous thromboembolism in Asia–an unrecognised and under-treated problem? Thromb Haemost 106(4):585–590
Liew NC et al (2012) Asian venous thromboembolism guidelines: prevention of venous thromboembolism. Int Angiol 31(6):501–516
Geerts WH et al (2008) Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 133(6 Suppl):381S–453S
Gomez-Outes A et al (2012) Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. Bmj 344:e3675
Falck-Ytter Y et al (2012) Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e278S–325S
Gomez-Outes A et al (2014) Pharmacoeconomic evaluation of dabigatran, rivaroxaban and apixaban versus enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement in Spain. Pharmacoeconomics 32(9):919–936
McCullagh L et al (2009) A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the irish healthcare setting. Pharmacoeconomics 27(10):829–846
Rojnuckarin P (2010) Venous thromboembolism: an important emerging problem in Thailand. J Hematol Transfusion Med 20(4):253–254
Kanchanabat B et al (2011) Systematic review and meta-analysis on the rate of postoperative venous thromboembolism in orthopaedic surgery in Asian patients without thromboprophylaxis. Br J Surg 98(10):1356–1364
Wolowacz SE et al (2009) Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clin Ther 31(1):194–212
Wolowacz SE et al (2010) Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism in patients aged over 75 years or with moderate renal impairment undergoing total knee or hip replacement. Thromb Haemost 103(2):360–371
Chaikledkaew U Thailand’s National Health Technology Assessment Guidelines 2009
Rates of Exchange of Commercial Banks in Bangkok Metropolis (2002-present). 3 July 2015 [cited 2015 4 July]; REFERENCE RATE: US DOLLAR (USD)]. Available from: http://www2.bot.or.th/statistics/ReportPage.aspx?reportID=123&language=th
Dranitsaris G, Jelincic V, Choe Y (2011) Meta regression analysis to indirectly compare dalteparin to enoxaparin for the prevention of venous thromboembolic events following total hip replacement. Thromb J 9(1):3
Pakpianpairoj C (2012) Perception of leg length discrepancy after total hip replacement and its impact on quality of life. J Med Assoc Thai 95(Suppl 10):S105–108
Sruamsiri R et al (2014) A cost-effectiveness study of intravenous immunoglobulin in childhood idiopathic thrombocytopenia purpura patients with life-threatening bleeding. Pharmacoeconomics 32(8):801–813
Saokaew S et al (2013) Cost-effectiveness of pharmacist-participated warfarin therapy management in Thailand. Thromb Res 132(4):437–443
A Summary of Product Characteristics of Pradaxa 110 mg hard capsules. Retrieved 6 Jul, 2015 from http://www.boehringer-ingelheim.com/content/dam/internet/pm/pradaxaglobal/com_EN/documents/pdf_new/pradaxa_smpc.pdf
A Summary of Product Characteristic of Clexane Syringe. Retrieved 6 Jul, 2015 from http://www.sanofi-aventis.co.uk/products/Clexane_SPC.pdf
Drug And Medical Supply Information Center, Ministry of Public Health. (2015). Retrieved 10 Apr, 2015 from http://dmsic.moph.go.th/dmsic/index.php?&p=1&type=3&t=3&id=1
Standard Cost List for Health Technology Assessment. Retrieved 10 Apr, 2015 from http://www.hitap.net/costingmenu/
Bureau of Trade and Economic Indices and Ministry of Commerce Thailand. Report for consumer price index of Thailand, base year 2011. Retrieved 4 Jul, 2015 from http://www.indexpr.moc.go.th/price_present/tableIndexCpi_bot.asp
Sub-committee of Thai Working Group on Health Technology Assessment. (2013) Meeting report of 2nd annual meeting
Teerawattananon Y et al (2014) The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwes 108(7):397–404
Saokaew S et al (2014) Cost-effectiveness analysis of HLA-B*5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PLoS One 9(4):e94294
Bamrungsawad N et al (2015) Cost-utility analysis of intravenous immunoglobulin for the treatment of steroid-refractory dermatomyositis in Thailand. Pharmacoeconomics 33(5):521–531
Limwattananon S (2008) Handling uncertainty of the economic evaluation result: sensitivity analysis. J Med Assoc Thai 91(Suppl 2):S59–S65
Jacobs J (2011) American Academy of Orthopaedic Surgeons clinical practice guideline on preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty
Greaves M (2011) Prevention and management of venous thromboembolism. A national clinical guideline
Devlin N, Parkin D (2004) Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Econ 13(5):437–452
Clement FM et al (2009) Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia, and Canada. JAMA 302(13):1437–1443
Sculpher MJ et al (2004) Generalisability in economic evaluation studies in healthcare: a review and case studies. Health Technol Assess 8(49):1–192
Rasanen P et al (2007) Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop 78(1):108–115
Hogg K et al (2013) Estimating quality of life in acute venous thrombosis. JAMA Intern Med 173(12):1067–1072
Acknowledgments
This study is funded by Boehringer Ingelheim (Thai) Ltd. We would like to thank all stakeholders who shared their perspective in our meetings and Puttarin Kulchaitanaroaj, Ph.D., who assisted in editing the manuscript. SK, BC, and NC performed the research, NC and SK designed the research study, SK, BC and NC analyzed the data, and BC, NC, and SK wrote the manuscript. The authors have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kotirum, S., Chongmelaxme, B. & Chaiyakunapruk, N. A cost-utility analysis of dabigatran, enoxaparin, and usual care for venous thromboprophylaxis after hip or knee replacement surgery in Thailand. J Thromb Thrombolysis 43, 252–262 (2017). https://doi.org/10.1007/s11239-016-1433-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1433-5