Abstract
The leech protein hirudin is a potent natural thrombin inhibitor. Its potential as an antithrombotic agent is limited by its promotion of bleeding. We attempted to modify this profile by positioning albumin and a plasmin cleavage site on its N-terminus, in recombinant protein HSACHV3 [comprising hirudin variant 3 (HV3) fused to the C-terminus of human serum albumin (HSA) via a plasmin cleavage site (C)], Previously we showed that HSACHV3 inhibited thrombin in a plasmin-dependent manner, and that, unlike HV3, it did not increase bleeding in vivo when administered to mice. Here we tested HSACHV3 for the ability to reduce thrombosis and assist enzymatic thrombolysis in animal models. Intravenous administration of HSACHV3, but not a control protein lacking the plasmin cleavage site (HSAHV3), reduced thrombus weight by 2.1-fold in the ferric chloride-injured mouse vena cava. Similarly, thrombi formed in a rabbit jugular vein stasis model were 1.7-fold lighter in animals treated with HSACHV3 compared to those receiving HSAHV3. Administration of 60 mg/kg body weight HSACHV3 prolonged the time to occlusion in the ferric chloride-injured mouse carotid artery by threefold compared to vehicle controls, while equimolar HSAHV3 had no effect. HSACHV3 had no ability to restore flow to the murine carotid arteries occluded by ferric chloride treatment, but combining HSACHV3 (60 mg/kg) with recombinant mutant tissue plasminogen activator (TNKase) significantly reduced the time to restore patency to the artery compared to TNKase alone. Unlike unfused HV3, HSACHV3 did not increase bleeding in a mouse liver laceration model. Our results show that HSACHV3 acts as an antithrombotic agent that does not promote bleeding and which speeds the time to flow restoration when used as an adjunct to pharmacological thrombolysis in animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombin is the final effector of the coagulation system. This versatile enzyme promotes coagulation through a variety of mechanisms, including activation of: precursor fibrinogen to clottable fibrin; resting platelets to activated platelets competent for adhesion and aggregation; cofactors V and VIII to Va and VIIIa; and precursor factor XIII to the active transglutaminase FXIIIa [1]. Thrombin also links coagulation and fibrinolysis by converting thrombin-activated fibrinolysis inhibitor (TAFI) from inactive precursor protein to active carboxypeptidase TAFIa (reviewed in [2]). TAFIa removes C-terminal lysines from partially degraded fibrin, disrupting binding sites for plasminogen and plasmin [3], and attenuating fibrinolysis in a positive feedback mechanism that indirectly promotes clot extension by opposing clot lysis [4]. To attenuate coagulation and promote clot lysis by opposing clot extension, by analogy we sought to create a plasmin-activatable coagulation inhibitor by protein engineering of hirudin, for potential use as an antithrombotic agent.
The hirudins are a family of closely related small proteins of 65–66 amino acids in length, found in nature in the salivary secretions of leeches [5, 6]. Hirudins inhibit thrombin by tight binding, with reported binding affinities in the sub-picomolar range [7]. Two recombinant hirudins [8], lepirudin and desirudin, are direct thrombin inhibitors used clinically when heparin becomes contraindicated in patients who develop heparin-induced thrombocytopenia [9]. Use of the hirudins in clinical settings affecting large numbers of patients, for instance in unstable angina, has been hampered by a narrow therapeutic window and observations of increased bleeding risk compared to other agents [10].
The biochemical details of the hirudin-thrombin interaction suggested a way to engineer a molecular switch in modified hirudins, one acting to convert them from latent to active thrombin inhibitors. The crystallographic finding that the N-terminus of hirudin penetrates to the active site canyon of thrombin [11] implied that transient blockage of this end of the molecule might provide such a control point [12, 13]. We and others have shown that blocking the N-terminus with either fused polypeptides or fused peptides renders hirudin essentially inactive, with the block to thrombin inhibition being lifted if these additional residues can be removed by specific proteolysis [14–17]. Incorporation of factor Xa, factor XIa, thrombin, or plasmin cleavage sites has been employed to activate such latent hirudins.
Previously, our laboratory described HSACHV3, a recombinant fusion protein in which hirudin variant 3, the most potent of the known hirudin variants, was positioned C-terminal to a cleavage site specifically recognized by the fibrinolytic protease, plasmin [14]. The blocked hirudin was fused to the C-terminus of recombinant human serum albumin (HSA). We showed that HSACHV3 inhibited both free and clot-bound thrombin in a plasmin-dependent manner, and that in contrast to free HV3, it did not provoke bleeding in a mouse tail vein transection model [14]. In the present study, we tested HSACHV3 in animal models of thrombosis and pharmacological thrombolysis. We report that intravenous HSACHV3 reduces fibrin deposition and vascular occlusion in rabbit and mouse models of thrombosis, and that it reduces the time to restoration of blood flow when administered with a plasminogen activator in a murine carotid artery thrombolysis model. Furthermore, we report that, unlike unfused HV3, HSA does not promote bleeding in a second model, one involving liver laceration in the mouse.
Materials and methods
Methods
Fusion protein expression, purification, and characterization
Expression plasmids directing the synthesis of His-tagged recombinant hirudin variant 3 (HV3), HSACHV3 and HSAHV3 [14] were purified using commercial DNA plasmid preparation columns (Qiagen, Chatsworth, CA, USA) from lysed E. coli DH5α, linearized with SacI, and used to transform Pichia pastoris strain X33 cells to Zeocin (Invitrogen, Carlsbad, CA, USA) resistance. Clonally transformed cell lines were cultured and induced with methanol, and secreted recombinant HV3 or HSA fusion proteins were purified from media conditioned by transformed Pichia pastoris cells as previously described [18–20] using Ni–NTA agarose chromatography (Qiagen).
Functional tests of HV3 released from HSA to HV3 fusion proteins in vitro
As previously described [14], a two-stage assay was used to follow the liberation of HV3 from fusion proteins in the presence of plasmin, and then to quantify the effects of the liberated HV3 on thrombin cleavage of a chromogenic substrate. Purified HSACHV3 (376 nM) was incubated with 600 nM human plasmin, thrombin, factor XIa, factor XIIa (Enzyme Research Laboratories, South Bend, IN, USA) or recombinant factor VIIa in 20 mM HEPES pH 7.4, 150 mM NaCl, and 2 mM calcium chloride for varying times up to 240 min. The reaction mixture was then diluted into 7 nM thrombin for 1 min, and subsequently diluted into 0.1 mM S2238 chromogenic substrate (Chromogenix, Lexington, MA, USA); colour generation was then monitored for 5 min to determine the residual reaction velocity. Control reactions containing proteases but not HSACHV3 in the first stage of the assay showed that they did not subsequently cleave S2238, except for thrombin, whose results were normalized to time zero of the second stage.
In vitro clot lysis
The formation and dissolution of clots in diluted normal human pooled plasma (NHPP) was followed by recording changes in turbidity, using an ELx808 plate reader (BioTek Instruments, Winooski, VT, USA) set to take absorbance readings at 340 nm every 30 s for 4 h, and quantified as the area under the curve. NHPP was supplemented with tPA (tissue-type plasminogen activator (Alteplase; Genentech, South San Francisco, CA, USA) to 0.5 nM final concentration, purified human thrombin to 5 nM, and with either purified HV3 or purified HSACHV3 at 0.01–1,000 nM; each reaction contained, by volume, two parts NHPP and three parts appropriately diluted HV3-related protein, tPA, and thrombin in Tris-buffered saline. Clotting was initiated by simultaneous addition of thrombin and recalcification.
Thrombus formation in mouse vena cava treated with ferric chloride
Thrombus formation was induced by topical application of 10 % (w/vol) ferric chloride on the surgically exposed murine vena cava, as described in previous reports from this laboratory [21, 22]. Briefly, CD1 mice(Charles River, Wilmington, MA, USA) were anesthetized, the vessel exposed, and recombinant protein or vehicle was injected intravenously 2 min prior to application of the ferric chloride-saturated filter paper to the vessel. The time of vessel exposure to ferric chloride was 3 min. Thirty minutes after removal of the filter paper, the vessel was excised and the clot weight determined [21, 22]. These experiments as well as all other animal experimentation described in this communication were carried out under the terms of an Animal Utilization Protocol reviewed, approved, and monitored by the Animal Research Ethics Board of the Faculty of Health Sciences, McMaster University.
Rabbit jugular vein thrombosis model
Clotting rabbit blood was introduced into a jugular vein segment and held in stasis in New Zealand White rabbits, in a modified Wessler procedure [23] as previously described by this laboratory [18]. Briefly, rabbits (2.5–3.0 kg body weight) were cannulated via the carotid artery and the jugular veins exposed. Two cm long sections of the right and left jugular veins were emptied of blood, and clamped. Anticoagulated autologous citrated whole blood was combined with purified HSA fusion protein in sterile saline and warm (37 °C) human thromboplastin reagent Thromborel S (Dade Behring, Mississauga, ON, Canada) in a 10:4:5 volume ratio, and 0.15 ml of this mixture was re-introduced into the isolated segments; the concentration of purified HSA fusion protein in the clotting blood was 1.5 mg/ml. After 30 min of stasis the clamps were removed and blood flow restored for 60 min prior to recovery and weighing of the blood clots.
Effects of HSA fusion proteins on the ferric chloride-treated murine carotid artery
The effect of intravenously injected HSA fusion proteins on thrombus formation elicited by ferric chloride application to the murine carotid artery was also determined, using methods previously described by this laboratory [22]. Briefly, blood flow through the artery was determined using a Doppler ultrasound flow meter and a 0.5 mm diameter flow probe, using a system purchased from Transonic Systems (Ithaca, NY, USA). 10 % (w/vol) ferric chloride was applied in the same manner and with the same timing as described above for the vena cava model. The time to occlusion (TTO) of the artery was taken as the interval between application of the filter paper and reduction of flow to less than 0.10 ml/min; each experiment was terminated at 60 min, and that time scored for vessels that did not occlude during the observation period [22].
Thrombolysis of the occluded murine carotid artery
The restoration of flow through the occluded murine carotid artery used the approach of Machlus et al [24]. with minor modifications. The surgically exposed murine carotid artery was subjected to 10 % (w/vol) ferric chloride treatment for 3 min, and flow through the artery monitored. After removal of the ferric chloride-impregnated filter paper, the artery was washed three times with saline, and kept moist throughout. Once flow was observed to have been stably and continuously occluded for 5 min, purified HSACHV3 or HSAHV3 or saline vehicle was injected intravenously via a jugular vein catheter. Two minutes later, Tenecteplase (TNKase, Genentech, South San Francisco, CA, USA) was given via the jugular vein catheter at a dose of 17.5 mg/kg body weight. Flow was monitored and the time to restore flow stably to pre-ferric chloride levels was noted; experiments were stopped at 60 min post-TNKase if no prior restoration in flow was noted. In some experiments C57Bl6 mice were compared to CD1 mice in this model (Charles River, Wilmington, MA, USA).
Effects of fusion proteins on bleeding from the lacerated liver
The effect of recombinant protein administration on blood loss subsequent to liver laceration was assessed as previously described [25]. Briefly, the left lobe of surgically exposed livers of anesthetized mice was exteriorized, and a 5 mm long, perforating transverse incision was made 1 cm from the lobe’s inferior edge using a number ten scalpel blade. Shed blood was captured into a tared plastic weigh boat for 30 min post-liver laceration. Two minutes prior to liver laceration, 0.2 ml of purified HV3, HSAHV3, HSACHV3, or saline vehicle, was injected intravenously via the tail vein in saline. Mice were euthanized at the close of this and all other in vivo experiments described above without regaining consciousness.
Statistical analysis
Statistical tests were performed using GraphPad Instat version 4 (GraphPad Software). Multiple comparisons used one-way parametric analysis of variation (ANOVA) with Tukey–Kramer post-tests, since all data sets compared in this study met the required criteria of being normally distributed and having similar standard deviations; comparisons of only two data sets used an unpaired t test. All numerical values reported herein are the mean ± one standard deviation (SD).
Results
Reaction of HSACHV3 with plasmin and coagulation proteases
Prior to investigating the effects of HSACHV3 in thrombosis and thrombolysis models, we exposed the fusion protein to excess protease (plasmin, thrombin, factor VIIa, factor XIa, or factor XIIa) and looked for release of functional hirudin in discontinuous thrombin inhibition assays. As shown in Fig. 1, plasmin treatment led to the release of clearly increasing thrombin inhibitory capacity over time, culminating in abrogation of thrombin activity by 240 min. In contrast, no reduction in thrombin activity ensued following exposure to thrombin (normalized to take into account its presence in both stages of the assay), factor VIIa, or factor XIIa. For factor XIa, some HV3 appeared to be cleaved, but only after prolonged (180–240 min) incubation.
Effects ofexposure to different proteases on the liberation of thrombin inhibitory activity from chimeric protein HSACHV3. Purified HSACHV3 (376 nM) was incubated with the proteases indicated to the right of the tracings (600 nM) for varying times, then diluted into 7 nM thrombin for 1 min prior to further dilution into chromogenic substrate-containing buffer. The rate of amidolysis was determined and expressed as a percentage of the mean reaction velocity of controls lacking HSACHV3 in the initial step. NA no addition. The mean of 3 (thrombin) or 6 (all other proteases or NA) determinations is shown ± the standard error of the mean
Effects of HSACHV3 on thrombus weight in venous thrombosis models
We next examined the effects of injecting HSA-hirudin proteins into the murine circulation just prior to eliciting thrombus formation on the surgically exposed vena cava using transient exposure to ferric chloride. As shown in Fig. 2a, clots that formed when either saline vehicle or non-activatable fusion protein HSAHV3 was administered did not differ statistically in weight (0.53 ± 0.15 vs. 0.54 ± 0.06 mg, n = 9). In contrast, administration of plasmin-activatable HSACHV3 led to a 2.1- to 2.2-fold, statistically significant reduction in thrombus weight (0.25 ± 0.13 mg, p < 0. 001 with respect to either other group).
Effects of injected recombinant proteins on clot weight in mouse and rabbit venous thrombosis models. a Weights of clots formed in response to ferric chloride injury in the murine vena cava are shown for mice treated with saline vehicle (squares) or 60 mg/kg HSACHV3 (inverted triangles) or HSAHV3 (circles) (n = 9). Horizontal lines and descriptions above them indicate the extent of statistical differences between the groups (by one-way ANOVA with Tukey post-tests); n.s. not significant”, p > 0.05. Each symbol represents a single mouse. b Weights of clots formed in response to stasis in isolated rabbit jugular veins for animals treated with HSAHV3 (circles) or HSACHV3 (inverted triangles). The extent of statistically significant difference (by unpaired t test, Welch corrected) is shown above the horizontal line connecting the groups. Each symbol represents a single rabbit
Similar results were obtained in a stasis model of venous thrombosis in rabbits, as shown in Fig. 2b. Whole blood was combined with a source of tissue factor and either non-activatable fusion protein HSAHV3 or plasmin-activatable HSACHV3 and introduced into the isolated jugular vein. Following clamp removal and flow restoration, the mean thrombus weight in the group of rabbits treated with HSACHV3 was significantly less than that from the HSAHV3-treated group (45 ± 10 mg vs. 26 ± 7 mg, n = 12, p = 0.008).
Effects of HSACHV3 on thrombus weight in arterial thrombosis models
Figure 3 shows the results of experiments analogous to those shown in Fig. 2a, except that thrombus formation was provoked in the murine carotid artery, as opposed to the murine vena cava, and the time to occlusion (TTO) determined using an ultrasound flow meter. As before, administration of HSAHV3 had no significant effect on the TTO, as compared to saline vehicle (both 10 ± 3 min, n = 5–8). In contrast, the TTO in mice treated with HSACHV3 at the same 1.5 mg dose per mouse (60 mg/kg body weight) was prolonged threefold, to 29 ± 10 min (p < 0. 05 vs. saline). Doubling the dose of HSACHV3 increased the TTO to 44 ± 10 min, but the increased TTO was not statistically significantly different from the lower dose.
Effects of injected recombinant proteins on time to occlusion in the ferric chloride-injured murine carotid artery. The time in minutes (min) to occlusion of arteries is shown, for groups of n mice treated either with saline vehicle or HSAHV3 or HSACHV3. Protein doses, in mg/kg, are shown below the x axis. Horizontal lines and descriptions above them indicate the extent of statistical differences between the groups (by non-parametric ANOVA with Dunn’s post-tests); n.s. not significant, p > 0.05
Effects of HSACHV3 on restoration of occluded carotid artery flow
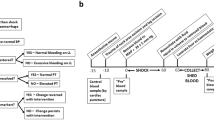
We next employed a mouse thrombolysis model to determine if HSACHV3 had any innate or adjunctive thrombolytic capacity. The protocol is summarized on the timeline presented in Fig. 4a. Prior to selecting the TNKase dose that was employed, we tested lower doses of 5 and 9 mg/kg in both C57BL6 and CD1 mice, finding neither sufficient to restore blood flow in less than 60 min (data not shown); 17.5 mg/kg TNKase, however, led to full artery patency in all mice tested within the observation period. As shown in Fig. 4b, 60 mg/kg HSACHV3 alone had no ability to restore flow through the occluded artery; 0/7 arteries were patent at the end of the observation period of 60 min. In contrast, all treated arteries (14 per group) exhibited restored flow within 60 min of administration of 17.5 mg/kg TNKase alone or 17.5 mg/kg TNKase plus 60 mg/kg HSACHV3; the mean time to restoration of flow was reduced from 27 ± 9 to 15 ± 3 min (p = 0.005).
Effects of TNKase and/or HSACHV3 on time to restore flow in the occluded murine carotid artery. a Schematic diagram of the murine carotid artery model employed, in which occlusion was provoked by ferric chloride exposure. A timeline (not to scale) is shown, with elapsed time in minutes (min) below and actions above: TTO time to occlude, PTN recombinant protein, TTRF time to restore flow. b Flow restoration time for mice with ferric chloride-occluded carotid arteries treated either with 17.5 mg/kg TNKase, 60 mg/kg HSACHV3, or both, as indicated on the x axis. The number of mice per group is indicated below the x axis. Each symbol indicates one mouse. Arteries that remained occluded at the end of the 60 min observation period were scored “>60” as shown above the break in the x axis. The extent of statistically significant difference (by unpaired t test, Welch corrected) is shown above the horizontal line connecting the first two groups, which exhibited continuous responses
Promotion of fibrinolysis in vitro byHV3 versus HSACHV3
To gain some understanding of how much hirudin was being liberated from HSACHV3 during clot lysis, we followed the change in turbidity of clots that formed rapidly in recalcified human plasma, and then lysed slowly in the presence of 0.5 nM tPA. Figure 5a shows representative clot lysis profiles from such experiments, in which omitting the exogenous tPA eliminated clot lysis (compare tracings 1 and 2) and adding increasing amounts of either HSACHV3(tracing 3) or HV3 (tracing 4) reduced and, at high enough doses (tracing 5), eliminated the clot represented by the area under the turbidity tracing curve. Figure 5b shows a dose response experiment in which increasing amounts of either HV3 or HSACHV3 were titrated into the lysing clots. The HV3 curve is clearly left-shifted relative to that of HSACHV3; 75 % clot reduction was achieved with approximately 15 nM HV3 and 900 nM HSACHV3. Taken together with the parallel shape of the dose response curves, this finding suggests that only 1–2 % of the HSACHV3 released active HV3 in these experiments.
Comparison of effects of HV3 and HSACHV3 on clot formation and lysis in recalcified diluted plasma. a The change in turbidity over time in minutes (min) in recalcified plasma supplemented with thrombin and tPA is shown. Each tracing represents an individual reaction whose optical density at 340 nm was measured every 30 s for up to 240 min. Tracing number labels refer to: 1 no tPA, no recombinant protein, 2 no recombinant protein, 3 300 nM HSACHV3, 4 3 nM HV3, 5 30 nM HV3. b The observed area under the turbidity curve, normalized to Condition 2 internal controls, was calculated from experiments like those shown in Panel A for reactions containing varying concentrations of HV3 or HSACHV3. Data points represent the mean ± SD of triplicate determinations
Blood loss subsequent to HV3 versus HSACHV3 administration
We had previously shown that HV3, but neither HSACHV3 nor HSAHV3 promoted bleeding when the fusion proteins were administered prior to tail transection in mice [14]. To substantiate this finding, we treated mice with 0.8 µmol of HSACHV3 or HSAHV3 (1,500 µg) or equimolar HV3 (183 µg) or saline vehicle and measured blood loss in a different bleeding model, one involving liver laceration. Figure 6 shows that the weight of shed blood did not differ significantly among groups of mice receiving saline vehicle (170 ± 40 mg) or fusion proteins (180 ± 50 mg for HSACHV3 or 160 ± 50 mg for HSAHV3) but was significantly increased when unfused HV3 was administered, to 720 ± 130 mg (p < 0.001).
Effects of fused or unfused hirudin on blood loss in a liver laceration model. The weight of shed blood collected from the lacerated, exteriorized liver is shown for groups of mice receiving saline vehicle (open bar) or 0.8 µmol of HSACHV3 or HSAHV3 (1,500 µg) or 0.8 µmol of HV3 (183 µg) (closed bars). The mean of five determinations ± the standard deviation is shown for each group. Horizontal lines and descriptions above them indicate the extent of statistical differences between the groups (by one-way ANOVA with Tukey post-tests): n.s. not significant; ***p < 0.001
Discussion
Previously we demonstrated that HSACHV3 released active hirudin in vitro when challenged with plasmin but not thrombin, that it inhibited clot formation and clot lysis in plasmin-containing systems in vitro, and that it, unlike equimolar HV3, did not promote bleeding in a murine tail transection model [14]. However, our initial study left unresolved the question of whether or not HSACHV3 had antithrombotic activity in vivo. Our findings in the present study strongly supported both of our previously untested hypotheses: that HSACHV3 would inhibit thrombosis in vivo; and that HSACHV3 would assist pharmacological thrombolysis in vivo, when administered with a plasminogen activator.
HSACHV3 reduced thrombus size in mouse models of both arterial and venous thrombosis employing ferric chloride as a prothrombotic stimulus. Ferric chloride treatment leads to endothelial damage via strongly oxidant mechanisms [26] in which erythrocytes are important mediators [27, 28]. Both endothelial damage and thrombin stimulation release tPA from endothelial cells, which would be expected to generate local plasmin [29]. Plasmin generation over the relatively short time span of these ferric chloride in vivo experiments is also consistent with the results of previous studies with plasminogen and α2-antiplasmin (α2-AP) knockout mice in venous and arterial settings. Plasminogen −/− mice exhibited shortened mean times to occlusion in the photochemically injured carotid artery compared to wild-type mice (from 12.1 to 5.9 min), suggesting that plasmin activity opposes clot extension in normal animals [30]. Similarly, α2-AP −/− mice exhibited prolonged time to occlusion compared to their wild-type counterparts (from 7.2 to 27.1 min) in the photochemically injured jugular vein, suggesting that α2-AP ordinarily opposes plasmin activity in this setting [30]. The antithrombotic activity demonstrated by HSACHV3 in a rabbit model of venous thrombosis provides further confidence in the generalizability of our findings, since a second experimental animal species and a prothrombotic stimulus, stasis and tissue factor infusion, was employed.
Our initial selection of plasmin as the activator for our latent albumin-hirudin construct was also based on the idea that HSACHV3 could provide a well-controlled and safe adjunct to thrombolytic therapy. To test HSACHV3 as an adjunct to thrombolysis, we followed the approach of Machlus et al., who employed TNKase to lyse experimental thrombi formed in the ferric chloride-injured murine carotid artery [31]. TNKase is less rapidly cleared than first generation recombinant tPA, primarily due to elimination of a site of high mannose N-linked glycosylation, eliminating the need to provide it by continuous infusion [32]. We found it necessary to administer TNKase at 17.5 mg/kg rather than the 5 mg/kg employed by Machlus et al., in order to achieve restoration of blood flow in approximately 30 min, and eliminated mouse strain difference as a possible explanation. However, Machlus et al. did not quantify the time to blood flow, but rather reported that 5/5 normal mice receiving the 5 mg/kg dose had open arteries 60 min post-infusion. This methodological difference, combined with different routes of TNKase administration and the possibility of differing specific activities in the TNKase used by our laboratories may contribute to this apparent discrepancy. Our data are nevertheless internally consistent and importantly, in our adaptation of this model, HSACHV3 had no independent thrombolytic activity. Its presence, co-administered with TNKase, was associated with a 1.8-fold reduction in the time to restore flow compared to TNKase alone. Since hirudin is exquisitely specific for thrombin [33], our results are consistent with the presence of active thrombin in the lysing clot, which would have extended the clot and potentially re-occluded the artery had it not been inhibited by released hirudin. Our findings suggest that HSACHV3 could be used to improve the efficacy of thrombolytic therapy, joining antiplatelet agents [34] and heparin and heparinoid [35] anticoagulants in this role, potentially provoking less associated bleeding.
HSACHV3 was designed for preferential if not exclusive activation by plasmin, and incorporates a GSGIYR-I cleavage site originally selected in a peptide screening study that sought superior peptide substrates for plasmin [36]. However, it should be noted that we cannot exclude the possibility of activation by other proteases in vivo. In this regard, we found no evidence of activation of HSACHV3 by thrombin, factor VIIa, or factor XIIa in vitro, using extreme conditions in which the concentration of protease exceeded that of HSACHV3, circumstances that likely never came close to being repeated in our vivo work. We did note weak reactivity with factor XIa under identical conditions. If factor XIa did contribute in small part to HSACHV3 activation, this would likely not be counterproductive because factor XI deficiency protects mice from thrombosis [37–39] and fluid-phase factor XIa is subject to inhibition by circulating protease inhibitors [40–42]. Therefore, factor XIa is likely localized to the thrombus surface, like plasmin, leaving the location of HSACHV3 activation unchanged if some factor XIa also contributes to its predominant plasmin-dependence. The effectiveness of HSACHV3 contrasted with the lack of effect of HSAHV3 in all models, reinforcing the absolute need for proteolysis to liberate activity.
Others have reported the production and characterization of hirudin derivatives with blocked N-termini, and their investigation in rat models of thrombosis. Zhang et al. produced recombinant hirudins in P. pastoris with 3–5 amino acid N-terminal extensions cleavable by FXIa (EH), FXa (GH), or thrombin (LH) [17]. In a rat carotid artery thrombosis model, in which thrombosis was elicited by electrical injury, the highest dose tested (8 mg/kg) increased the time to occlusion by 1.5- to 2.0-fold (from 10–12 min to 16–20 min). In the mouse model of arterial thrombosis that we employed, we obtained similar results for HSACHV3 doses of 60 mg/kg (comparable to 6 mg/kg unfused hirudin due to the tenfold difference in molecular mass), with a similar, approximately 10 min baseline and 2- to 3- fold prolongations of the time to occlusion. At equivalent doses, however (6 mg/kg for EH, GH, or LH versus 60 mg/kg for HSACHV3) Zhang et al. reported a statistically significant elevation of bleeding in the mouse tail transection bleeding assay [17], whereas we saw no such elevation in bleeding for the fusion protein. Such equivalent efficacy with decreased bleeding risk may suggest a superior safety profile for HSACHV3 versus the less extensively N-terminally capped hirudins.
Previously we demonstrated that HSACHV3 administration did not increase blood loss relative to vehicle controls in a tail transection model of moderate bleeding; HV3-treated mice lost approximately 7 % of their blood volume during the observation period, as opposed to 1.7 % for HSACHV3- or vehicle-treated mice [14]. In the current study, we addressed bleeding in a different anatomical setting. Following a standardized liver laceration injury, mean blood losses in HV3-treated animals were 46 % of blood volume (assuming a blood density of 1060 kg/m3) compared to 10 % for HSACHV3- or vehicle-treated mice. In both the moderate and severe bleeding models, HV3 promoted approximately fourfold more bleeding than HSACHV3 or vehicle. These similar results in two models of bleeding support the enhanced safety of HSACHV3 arising from its latency and specific activation and should diminish any concerns arising from the limitations of the tail vein transection model [43].
In conclusion, our results suggest that the protease-dependent “switch” incorporated into the design of HSACHV3 is thrown not only in vitro, but also functions in vivo to reduce experimentally induced venous and arterial thrombosis and, in combination with plasminogen activators, to assist thrombolysis by blocking rapid clot re-growth. Its relative resistance to activation clearly contributes to its safety profile and its lack of bleeding promotion [14]. Whether or not this extreme latency is optimal could be tested by incorporating spacer regions N-terminal to the cleavage site, obtaining HSACHV3 derivatives activated by lower concentrations of plasmin, and performing dose response experiments in the same models employed here.
References
Weitz JI (2011) Factor Xa and thrombin as targets for new oral anticoagulants. Thromb Res 127(Suppl 2):S5–S12
Mann KG, Brummel K, Butenas S (2003) What is all that thrombin for? J Thromb Haemost 1(7):1504–1514
Redlitz A, Tan AK, Eaton DL, Plow EF (1995) Plasma carboxypeptidases as regulators of the plasminogen system. J Clin Invest 96(5):2534–2538
Cesarman-Maus G, Hajjar KA (2005) Molecular mechanisms of fibrinolysis. Br J Haematol 129(3):307–321
Dodt J, Machleidt W, Seemuller U, Maschler R, Fritz H (1986) Isolation and characterization of hirudin isoinhibitors and sequence analysis of hirudin PA. Biol Chem Hoppe Seyler 367(8):803–811
Scharf M, Engels J, Tripier D (1989) Primary structures of new ‘iso-hirudins’. FEBS Lett 255(1):105–110
Stone SR, Hofsteenge J (1986) Kinetics of the inhibition of thrombin by hirudin. Biochemistry 25(16):4622–4628
Nutescu EA, Shapiro NL, Chevalier A (2008) New anticoagulant agents: direct thrombin inhibitors. Cardiol Clin 26 (2):169–187; (v–vi)
Yoon JH, Jang IK (2011) Heparin-induced thrombocytopenia in cardiovascular patients: pathophysiology, diagnosis, and treatment. Cardiol Rev 19 (3):143–153
Warkentin TE (2004) Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol 17(1):105–125
Grutter MG, Priestle JP, Rahuel J, Grossenbacher H, Bode W, Hofsteenge J, Stone SR (1990) Crystal structure of the thrombin-hirudin complex: a novel mode of serine protease inhibition. EMBO J 9(8):2361–2365
Lazar JB, Winant RC, Johnson PH (1991) Hirudin: amino-terminal residues play a major role in the interaction with thrombin. J Biol Chem 266(2):685–688
Betz A, Hofsteenge J, Stone SR (1992) Interaction of the N-terminal region of hirudin with the active-site cleft of thrombin. Biochemistry 31(19):4557–4562
Sheffield WP, Eltringham-Smith LJ, Gataiance S, Bhakta V (2009) A long-lasting, plasmin-activatable thrombin inhibitor aids clot lysis in vitro and does not promote bleeding in vivo. Thromb Haemost 101(5):867–877
Peter K, Graeber J, Kipriyanov S, Zewe-Welschof M, Runge MS, Kubler W, Little M, Bode C (2000) Construction and functional evaluation of a single-chain antibody fusion protein with fibrin targeting and thrombin inhibition after activation by factor Xa. Circulation 101(10):1158–1164
Peter K, Gupta A, Nordt T, Bauer S, Runge MS, Bode C (2003) Construction and in vitro testing of a novel fab-hirudin-based fusion protein that targets fibrin and inhibits thrombin in a factor xa-dependent manner. J Cardiovasc Pharmacol 42(2):237–244
Zhang C, Yu A, Yuan B, Dong C, Yu H, Wang L, Wu C (2008) Construction and functional evaluation of hirudin derivatives with low bleeding risk. Thromb Haemost 99(2):324–330
Sheffield WP, Eltringham-Smith LJ, Gataiance S, Bhakta V (2009) Addition of a sequence from alpha2-antiplasmin transforms human serum albumin into a blood clot component that speeds clot lysis. BMC Biotechnol 9:15
Sheffield WP, Smith IJ, Syed S, Bhakta V (2001) Prolonged in vivo anticoagulant activity of a hirudin-albumin fusion protein secreted from Pichia pastoris. Blood Coagul Fibrinolysis 12(6):433–443
Sheffield WP, McCurdy TR, Bhakta V (2005) Fusion to albumin as a means to slow the clearance of small therapeutic proteins using the Pichia pastoris expression system: a case study. Methods Mol Biol 308:145–154
Sheffield WP, Eltringham-Smith LJ (2011) Incorporation of albumin fusion proteins into fibrin clots in vitro and in vivo: comparison of different fusion motifs recognized by factor XIIIa. BMC Biotechnol 11:127
Sheffield WP, Eltringham-Smith LJ, Bhakta V, Gataiance S (2012) Reduction of thrombus size in murine models of thrombosis following administration of recombinant alpha1-proteinase inhibitor mutant proteins. Thromb Haemost 107(5):972–984
Wessler S (1962) Thrombosis in the presence of vascular stasis. Am J Med 33:648–666
Machlus KR, Lin FC, Wolberg AS (2011) Procoagulant activity induced by vascular injury determines contribution of elevated factor VIII to thrombosis and thrombus stability in mice. Blood 118(14):3960–3968
Bajaj MS, Ogueli GI, Kumar Y, Vadivel K, Lawson G, Shanker S, Schmidt AE, Bajaj SP (2011) Engineering kunitz domain 1 (KD1) of human tissue factor pathway inhibitor-2 to selectively inhibit fibrinolysis: properties of KD1-L17R variant. J Biol Chem 286(6):4329–4340
Kurz KD, Main BW, Sandusky GE (1990) Rat model of arterial thrombosis induced by ferric chloride. Thromb Res 60(4):269–280
Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C (2011) Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost 9(4):779–789
Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG (2013) Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 121(18):3733–3741
van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ (1995) Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood 85(12):3510–3517
Matsuno H, Kozawa O, Okada K, Ueshima S, Matsuo O, Uematsu T (2002) Plasmin generation plays different roles in the formation and removal of arterial and venous thrombus in mice. Thromb Haemost 87(1):98–104
Machlus KR, Cardenas JC, Church FC, Wolberg AS (2011) Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 117(18):4953–4963
Tanswell P, Modi N, Combs D, Danays T (2002) Pharmacokinetics and pharmacodynamics of tenecteplase in fibrinolytic therapy of acute myocardial infarction. Clin Pharmacokinet 41(15):1229–1245
Chang JY (1983) The functional domain of hirudin, a thrombin-specific inhibitor. FEBS Lett 164(2):307–313
Greenbaum AB, Ohman EM, Gibson CM, Borzak S, Stebbins AL, Lu M, Le May MR, Stankowski JE, Emanuelsson H, Weaver WD (2007) Preliminary experience with intravenous P2Y12 platelet receptor inhibition as an adjunct to reduced-dose alteplase during acute myocardial infarction: results of the Safety, Tolerability and Effect on Patency in Acute Myocardial Infarction (STEP-AMI) angiographic trial. Am Heart J 154(4):702–709
Quinlan DJ, Eikelboom JW (2009) Low-molecular-weight heparin as an adjunct to thrombolysis in ST elevation myocardial infarction. Arch Intern Med 169(12):1163–1164
Hervio LS, Coombs GS, Bergstrom RC, Trivedi K, Corey DR, Madison EL (2000) Negative selectivity and the evolution of protease cascades: the specificity of plasmin for peptide and protein substrates. Chem Biol 7(6):443–453
Rosen ED, Gailani D, Castellino FJ (2002) FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost 87(4):774–776
Wang X, Cheng Q, Xu L, Feuerstein GZ, Hsu MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D (2005) Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost 3(4):695–702
Kleinschnitz C, Stoll G, Bendszus M, Schuh K, Pauer HU, Burfeind P, Renne C, Gailani D, Nieswandt B, Renne T (2006) Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med 203(3):513–518
Knauer DJ, Majumdar D, Fong PC, Knauer MF (2000) SERPIN regulation of factor XIa. The novel observation that protease nexin 1 in the presence of heparin is a more potent inhibitor of factor XIa than C1 inhibitor. J Biol Chem 275(48):37340–37346
Wuillemin WA, Hack CE, Bleeker WK, Biemond BJ, Levi M, ten Cate H (1996) Inactivation of factor Xia in vivo: studies in chimpanzees and in humans. Thromb Haemost 76(4):549–555
Huang X, Rezaie AR, Broze GJ Jr, Olson ST (2011) Heparin is a major activator of the anticoagulant serpin, protein Z-dependent protease inhibitor. J Biol Chem 286(11):8740–8751
Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM, Animal Models Subcommittee of the S, Standardization Committee Of The I (2010) Towards a standardization of the murine tail bleeding model. J Thromb Haemost 8(12):2820–2822
Acknowledgments
The generous gift of tissue-plasminogen activator (tPA; Alteplase, Genentech, South San Francisco, California, USA) from Dr. Ed Pryzdial, Canadian Blood Services and University of British Columbia Centre for Blood Research, is gratefully acknowledged. The authors thank Dr. Jeffrey I. Weitz, McMaster University and the Thrombosis and Atherosclerosis Research Centre (TaARI), for helpful discussions and assistance in obtaining Tenecteplase (Genentech, South San Francisco, California, USA) for research use. This work was made possible by Grant-In-Aid T6588from the Heart and Stroke Foundation of Ontario to WPS.
Conflict of interest
The authors declare that they have no conflicts of interest, financial or otherwise, in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheffield, W.P., Eltringham-Smith, L.J., Gataiance, S. et al. A plasmin-activatable thrombin inhibitor reduces experimental thrombosis and assists experimental thrombolysis in murine models. J Thromb Thrombolysis 39, 443–451 (2015). https://doi.org/10.1007/s11239-014-1157-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-014-1157-3