A method of montmorillonite modification with polar oligourethane containing reactive amino groups has been developed. It is shown that functionalized montmorillonite is capable of adding aromatic and aliphatic diisocyanates, as well as sequential addition of aromatic diisocyanates and glycerol, which is essential for montmorillonite application as filler for polymer materials. According to wide-angle X-ray scattering, interlayer distance d001 of montmorillonite in the obtained samples varies depending on nature of oligomer macromolecules in the interlayer space that is explained with different conformation and orientation of aromatic and aliphatic oligourethane fragments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The provision of organophilicity to the surface of silicate nanoparticles is an urgent task for the production of polymer organic/inorganic nanocomposites with montmorillonite (Mt) and other layered silicates. Recently, modification of the surface of Mt nanoparticles has been widely used due to their application in the production of organoclay nanocomposites [1]. These nanocomposites have increased mechanical strength in comparison with initial polymers. Modification of the surface of layered silicate nanoparticles by organic surfactants is required to increase the compatibility of a polymer with clay [2]. Better compatibility of the matrix with a filler can improve physical properties. Mt has a layered structure, where free cations of alkaline earth metals are placed in the interlayer space, which are easily replaced by organic cations. Common modifiers used for organic Mt modification include cations of organic quaternary ammonium salts [3, 4], cations of quaternary phosphonium salts [5, 6], diamines [7], and amino acids [8]. Mt modified with organic cations is dispersed in the polymer matrix, where it acts as a reinforcing agent, improving the properties of a polymer material. Sometimes, conventional alkylammonium cations do not provide sufficient affinity of the modified Mt with the polymer matrix. Modification with aliphatic surfactants does not lead to sufficient exfoliation in the polymer matrix in the case of polar polymers such as polyurethanes, polyamides, and polyepoxides. This is associated with the lack of sufficiently high-quality interaction between a nanofiller and the polar polymer matrix [9,10,11]. Mt modified with aliphatic surfactant is easily exfoliated in the polymer matrix in the case of non-polar polymers (polyethylene and polypropylene) [12]. This is explained with the nature of a modifier and the high interlayer distance ensured by sufficiently large hydrocarbon fragments of an organic modifier [13]. At the same time, molecules of polar monomers used in polycondensation reactions are not large sufficiently enough to significantly increase the interlayer distance in Mt [14,15,16,17]. Alkylammonium cations that contain polar hydroxyl groups are often applied in the production of organoclays, which are used in the development of polar polymer-based nanocomposites, in particular polyepoxides. For instance, bis(2-hydroxyethyl)-quaternary ammonium salts are often used. Hydroxyl groups provide increased affinity to the polar environment, however, they are sterically inaccessible for polycondensation or polyaddition reactions due to the proximity to a large hydrocarbon radical [18]. Layered silicate modification with high-molecular amino acids, for example, synthetic ω-aminodecanoic acid, is often used in the development of polar polymer-based nanocomposites, namely, polyamides [19]. At the same time, the presence of the terminal carboxyl group contributes to the affinity of polyamide with the polymer matrix.
Therefore, it can be concluded that the use of modifiers that contain sufficiently large fragments for an increase in the interlayer distance, as well as polar fragments for an increase in the polar polymer affinity is important for the production of polar polymer-based nanocomposites. Previously, cationic oligourethanes, which have two large fragments of polar urethane nature from 17 to 21 atoms in length [20], namely oligourethane ammonium chloride (OUAC) based on N-methyldiethanolamine (N-MDEA), 1,6-hexamethylene diisocyanate (GMDI), and isopropyl alcohol were synthesized. This cationically active oligourethane has two 17-atom long polar fragments that increase the interplanar distance and enable the exfoliation of the modified Mt in the environment of polar organic solvents. In addition, such a modifier provides high affinity to the polar polymer, forming nanocomposites with complete Mt exfoliation, which increases the strength of the polyurethane material by an average of 40%.
This work aims at producing a new N-MDEA and GMDI-based modifier, namely oligourethane aminoammonium chloride (OUAC) with terminal amino groups. This will provide new opportunities in the field of organoclay and the development of organic/inorganic nanocomposites, namely, the ability to perform step-by-step addition on the surface of modified Mt nanoparticles by polyaddition and polycondensation. The paper provides several examples of such additions with aliphatic and aromatic diisocyanate, as well as an example of sequential addition of aromatic diisocyanate, glycerol, and repeated aromatic diisocyanate.
EXPERIMENTAL
Natural montmorillonite from the Cherkasy clay deposit (Ukraine) was used for research. N-methyldiethanolamine (Aldrich) and 1,6-hexamethylene diisocyanate (Aldrich) were used for the OUAC modifier synthesis. Glycerol (Gl) (Aldrich) and diphenylmethane diisocyanate (DPMDI) (Aldrich) were used for functionalization. Dimethylformamide (DMF) (Aldrich) was used as a solvent. An HCl aqueous solution with a concentration of 0.1 mol/L obtained from a standard titer (Kharkivreakhim, Ukraine) was used for the formation of an ammonium cation. A methylene blue indicator (MB) (China) was used for the determination of the Mt exchange capacity.
The sodium form of Mt (Na-Mt) was obtained in the following way: a suspension of initial (natural) Mt (5%) in distilled water was boiled with sodium carbonate at a weight ratio of Mt:Na2CO3 = 100:1 for 1 h. Separation of the formed Na-Mt from the sodium carbonate solution was performed by four-time centrifugation and washing with distilled water. The content of dry matter in the Na-Mt suspension was determined by the gravimetrical method.
The Mt surface exchange capacity was determined by the MB indicator adsorption on the Na-Mt surface. Photocalorimetric analysis was used for the calculation of the modifier:Mt ratio [21]. The MB adsorption on the surface of Mt was evaluated for the determination of the Mt exchange capacity. The dependence of the adsorption value on the MB equilibrium concentration (solution concentration when adsorption equilibrium is reached) was determined. Eight samples of the same Mt mass were filled with solutions of the same volume but different MB concentrations. The equilibrium concentration was determined after two days. Adsorption (a, mmol/g) was calculated as the difference between the initial amount of MB added to the suspension of Mt in a solution with a concentration of C0, and the amount of MB remaining after adsorption per1g of Mt according to the formula
where C0 is the initial MB concentration; Ceq is the equilibrium concentration of MB (per 1 g of Mt) after adsorption; V is the volume of the sample aqueous solution, L; g is the mass of Mt sample, g.
The equilibrium concentration increases sharply after the adsorption value exceeds 0.65 mmol/g indicating that the exchange capacity of the used Mt is 0.65 mmol/g. The MB concentration in the solution was measured with a CK-2PM photocolorimeter after separating Mt from the aqueous solution. The value of the Mt exchange capacity enables the calculation of the required amount of the modifier, as well as the determination of its theoretical content after adsorption.
The modified Mt structure was studied by wide-angle X-ray scattering (WAXS) [22]. The X-ray optics of a DRON-4-07 diffractometer are produced according to the Debye–Scherrer (transmission) method. CuKα radiation was monochromatized with a Ni filter. A scintillation counter was used to register scattered X-rays in the automated step-by-step scanning mode. The measured values of the scattering intensity were corrected for the attenuation of the incident X-ray beam of the studied samples and the subsequent deviation of the background intensity of X-ray scattering by the collimator system. Scattering intensity values were normalized to the scattering volume. Finely dispersed Mt powders were placed in unit cells. Scattering intensity was recorded during conditional scanning of a scintillation detector at a scattering angle from 2 to 40°. The Bragg law was used for the determination of the distance (d) between layers of particles in Mt [23]
where n is the serial number of the diffraction peak on the X-ray diffraction patterns (n = 1); Α is the wavelength (for CuKα Α = 0.154 nm); 0 is the X-ray scattering angle.
Spectra were recorded by a Tensor 37 (Bruker) Fourier transform infrared spectrometer in the wavelength range of 4500-500 cm–1. Irtran glass was used for the production of a modifier sample from an aqueous solution. The obtained spectra were interpreted according to [24].
Thermogravimetric studies were performed on a Q-1000 derivatograph (MOM) to determine the content of the organic part in the modified Mt. The measurements were performed in a ceramic cone-shaped crucible made of Al2O3 in an air atmosphere at a temperature of 25-800°C and a heating rate of 10 deg-min–1.
RESULTS AND DISCUSSION
A two-stage simple and at the same time effective synthesis of cationically active oligourethane OUAC has been developed. A characteristic feature of the synthesis of this modifier is the presence of functional amino groups, which occur simultaneously during the formation of a tertiary ammonium cation and are accompanied by the formation of an OUAC solution of a given concentration. The target product appears in the form of a modifier solution of a given concentration, ready to be added to the Na-Mt aqueous dispersion due to such a unique and simple process. All products formed as a result of the synthesis contain amino groups in their composition and are water-soluble compounds capable of adsorbing on the Mt surface.
N-MDEA and GMDI were introduced into DMF in a ratio of 1:2 at the first stage of synthesis. The DMF content was 70 wt.%. in the reaction mixture. The synthesis was performed in an adiabatic reactor filled with an inert gas. The temperature was maintained at 60°C under gentle stirring of the reagent solution for 1.5 h. A product with a tertiary amine and terminal isocyanate groups was obtained after the interaction of all N-MDEA hydroxyl groups with half of all GMDI isocyanate groups:

where n = 1, 2.
The resulting mixture mainly contains the product with n = 1, but there is a small possibility of the formation of the product with n = 2, which also implies the presence of a small amount of unreacted GMDI.
An OUAC aqueous solution of a given concentration was obtained in the second stage. The product obtained at the first stage was diluted 10 times with DMF. Then, the solution was cooled to a temperature of 25-30°C and introduced into a large volume of an aqueous solution of hydrochloric acid, the amount of which corresponds to the amount of N-MDEA used in the first stage, namely 1 mol of N-MDEA per 1 mol of HCl with correction on the loss of the oligomer solution on walls of the vessel where the first stage of synthesis occurred. Losses on the walls of the vessel were calculated by determining the amount of solvent (DMF) remaining on the walls of the vessel after draining 30 mL of DMF. This amount of DMF is approximately 0.36 g, and the required volume of 0.1 N of HCl solution for neutralization is approximately 0.75 mL in the case of an oligomer solution of the same mass.
The amount of distilled water for the production of HCl solution was calculated in such a way as to obtain an OUAC aqueous solution with a concentration of 4.5-10–3 mol/L. The HCl aqueous solution was heated to a temperature of 25-30°C, and the oligomer solution synthesized in the first stage was added to it under intensive stirring for 1-2 sec. Since the neutralization reaction occurs almost instantaneously, much faster than the reaction of isocyanate groups with water, an ammonium cation is formed at first, which dissolves oligomer molecules in water, and then isocyanate groups, being in a huge excess of water, are transformed into amino groups:

A solution of the OUAC modifier with a given concentration of ionic groups (4.5-10–3 mol/L) was obtained in this way. Amino groups formed from isocyanate groups were determined by acid–base titration using 0.1 mol/L of HCl solution and bromophenyl blue indicator. An OUAC solution in the presence of an indicator provides a clear basic reaction. The determination of basic amino groups was performed by adding HCl until the change in the color of the solution, which corresponds to a neutral reaction. The content of the main amino groups was 7.2·10–3 g-eq/L. The yield of amino groups is 80% of the theoretical one, which can be associated with the formation of urea bonds in the reactions of newly formed amino groups with isocyanate groups that did not have time to react with water.
Bands corresponding to NH bonds of the urethane part are visible in the IR spectrum of OUAC, namely, ν(amide I) = 1695 cm–1 and ϖ(amide II) = 1540 cm–1. A band of deformation vibrations of amino groups –NH2 (v(NH2) = 1645 cm–1) is visible between them. A band of the trans-associated form of the NH group (v(NH) = 3300 cm–1) that is connected to an amino group band is also visible. An absorption band of C–N vibrations (ϖ(C–N) = 1260 cm–1) is visible. The covalent coordinate bond of NH+ in the (CH3)–NH+= fragment confirms the presence of a characteristic broad shoulder at 2640 cm–1. All other absorption bands correspond to the presence of aliphatic fragments (–(CH2)6–, –(CH2)2–, and –CH3).
Mt was modified by adding an OUAC solution to the Na-Mt suspension. The amount of the modifier substance corresponded to the exchange capacity of Mt. The process was performed according to the ion exchange mechanism of free cations Na+ for organic cations OUA+.
The resulting suspension was diluted with distilled water to a ratio of1g of Mt per 500 mL of water after adding the OUAC solution. The adsorption process lasted two days, then the modified Mt precipitate was filtered, dried in an oven at a temperature of 60°C to a constant mass, and ground to form a dispersed powder.

The content of the organic component of modified Mt, determined by thermogravimetric study, is 23 wt.% This value is 1.5% higher than the theoretical value of the modifier proportion in the composition of modified with OUA/Mt, which was calculated based on the exchange capacity of Mt, which was determined above (0.65 mmol/g).
The addition of functional compounds on the surface of modified OUA/Mt nanoparticles was performed in DMF. The OUA/Mt dispersion (1-1.5 wt.%) in DMF was obtained by treating a mixture of modified Mt and DMF with ultrasound (with cooling) for 30 min. Diisocyanates were added to the obtained OUA/Mt dispersion in DMF, then it was kept at a temperature of 60°C for 1 d after ultrasonic treatment (30 min). All processes occurred in an inert gas environment. Then the dispersion was centrifuged, washed twice, and centrifuged again until complete purification of diisocyanate.
Therefore, functionalized GMDI, OUA/Mt was treated with a fourfold excess of GMDI to obtain modified Mt. Such an excess of GMDI was used to achieve optimal functionalization of the Mt surface, which is connected with the minimization of reactions of isocyanate groups of attached GMDI molecules with amino groups of neighboring OUA molecules. An increase in the excess of GMDI leads to a change in the nature of the solution and a worsening of the dispersion of modified Mt in it.
Some precipitation of modified Mt in DMF and the formation of a thick suspension were observed after ultrasonic treatment of the dispersion mixture of OUA/Mt and GMDI for 30 min. The obtained dispersion was centrifuged many times and washed with DMF after aging for1d at a temperature of 60°C.

Functionalization of OUA/Mt with aromatic DPMDI was carried out similarly.

A bulk gel in DMF was obtained after repeated centrifugation and washing of DPMDI-functionalized OUA/Mt.
The obtained products were dried at a temperature of 60°C for further research.
Thermogravimetric analysis of functionalized GMDI OUA/Mt showed an increase in the organic component up to 32 wt.%. This value corresponds to the addition of GMDI to all amino groups of the modifier. However, thermogravimetric analysis of OUA/Mt functionalized with DPMDI showed an organic component content of 46 wt.%. This value exceeds the amount of DPMDI, which is equivalent to the number of amino groups of the modifier. The higher amount of added DPMDI can be explained by the fact that, unlike the aliphatic diisocyanate GMDI, the aromatic diisocyanate DPMDI is more reactive and can participate in reactions of addition to urethane groups –CO–NH– with the formation of biuret bonds accompanied by the branching of macromolecules. The opportunity for DPMDI addition to urethane groups was sufficient considering the use of a four-fold excess of DPMDI similar to the use of GMDI. However the high energy threshold of this reaction and the limited time hinder the complete process of such reactions, and only partial addition of DPMDI to urethane groups occurs. The addition of a larger amount of DPMDI only contributes to higher surface functionalization of Mt.
Sequential addition of DPMDI, glycerol, and again DPMDI was performed to study the possibility of step-by-step addition of functional compounds on the surface of OUA/Mt nanoparticles. The previously obtained gel, functionalized with DPMDI OUAA/Mt in DMF, was treated with a four-fold excess of glycerol using ultrasound in an inert gas atmosphere for 30 min. The resulting mixture was aged at a temperature of 50°C for 1 day. The product obtained after the reaction with glycerol was re-centrifuged and washed with DMF. A gel of modified Mt in DMF, purified from glycerol residues, was treated with a fourfold excess of DPMDI. The above-mentioned addition was performed according to the scheme:

The resulting addition product forms a thick gel with a content of 1.5 wt.%. in DMF. This indicates significant swelling of the organic layer formed on the surface of Mt nanoparticles and the formation of a dense physical network.
Modified Mt after repeated centrifugation and washing with DMF was dried at a temperature of 60°C for further studies.
Thermogravimetric analysis of modified Mt, obtained after three-stage addition on the surface of OUA/Mt nanoparticles, showed an organic matter content of 56 wt.%
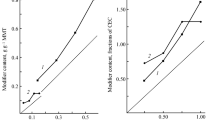
The results of the X-ray examination of all four types of modified Mt and the initial Na-Mt show a gradual increase in the interplanar distance d001 with an increase in the content of the organic component (Fig. 1).
Analysis of the change in the interlayer distance d001 of modified Mt and comparison of TGA data leads to the following conclusions. GMDI OUA/Mt has a larger interplanar distance d001 = 1.54 nm than OUA/Mt (d001 = 1.45 nm), which is quite natural. However, the DPMDI OUA/Mt sample has d001 = 1.47 nm, which is smaller than that of the GMDI OUA/Mt and close to the value of OUA/Mt, although the amount of organic component in the DPMDI OUA/Mt sample is higher than in both other samples. This fact can be explained by the rigid and relatively flat molecular conformation of DPMDI, which enables the formation of a compact organic layer on the surface of nanoparticles. This assumption is confirmed by a slight increase in d001 = 1.84 nm in the OUA/MB sample (DPMDI+ Gl + DPMDI) in which the content of organic matter is 2.4 times higher than that of OUA/Mi. Table 1 lists values of the content of organic matter and the interplanar distance d001.
According to Table 1, it is clear that an increase in the content of the organic component is not proportional to an increase in the interplane distance. However, it is caused by the nature of oligomer macromolecules in the interlayer space due to different conformation and orientations of aromatic and aliphatic oligourethane fragments. The sample of DPMDI OUA/Mt suspension is significantly different from the similar OUA/Mt sample with the same content of modified Mt (1.5 wt.%) in the DMF medium. The DPMDI OUA/Mt sample forms a thick suspension, while the OUA/Mt is a liquid dispersion, and the (DPMDI+ Gl + DPMDI) OUA/Mt sample forms a thick gel.
CONCLUSIONS
A modification method of montmorillonite surface with cationically active oligourethane containing available terminal amino groups and a simple and effective scheme for the synthesis of cationically active oligourethaneaminoammonium chloride have been developed, which contributed to the production of a modifier solution of a given concentration. It is established that montmorillonite modified with oligourethaneaminoammonium chloride has amino groups available for addition reactions on the surface of nanoparticles. The addition of functional compounds on the surface of nanoparticles of modified montmorillonite in a polar solvent environment promotes the functionalization of the surface and increases the content of the organic component and the interplane distance. The possibility of synthesizing an oligomer in terms of a regular structure on the surface of nanoparticles is shown with the example of a step-by-step addition of monomers.
The use of montmorillonite modified with oligourethaneaminoammonium chloride provides new opportunities in the field of production of hybrid organic/inorganic nanocomposites based on polar polymers, in particular, the design of an organic layer on the surface of nanoparticles using diisocyanates and other functional compounds.
References
Y. F. Huang, P. C. Wang, J. H. Lee, et al., Polym. Plast. Technol. Eng., 54, 433-439 (2015).
B. Esteki, H. Garmabi, M. R. Saeb, et al., Polym. Plast. Technol. Eng., 52, 1626-1636 (2013).
E. M. M. Mohamed, R. Marya, R. Denis, et al., Appl. Clay Sci., 185, 105417 (2020).
S. Gul, A. Kausar, B. Muhammad, et al., Polym. Plast. Technol. Eng., 55, 684-703 (2016).
J. L. Alves, P. D. V. E. Rosa, and A. R. Morales, Mater. Chem. Phys., 218, 279-288 (2018).
M. Vikas, Appl. Clay Sci., 56, 103-109 (2012).
G. Pircheraghi, H. Nazockdast, and M. Salehi, Polym. Plast. Technol. Eng., 50, 1109-1117 (2011).
C. B. Liu, T. Tang, D. Wang, et al., J. Polym. Sci., 41, 2187-2196 (2003).
A. Steele, I. Bayer, and E. Loth, J. Appl. Polym. Sci., 125, 446-452 (2012).
N. Aranburu, J. I. Eguiazabal, and G. Guerrica-Echevarria, Polym. Eng. Sci., 58, 830-838 (2018).
M. Tomic, B. Dunjic, and M. S. Nikolic, Appl. Clay Sci., 154 52-63 (2018).
G. L. Xiahou, W. Q. Liu, J. Q. Tan, et al., Acta Polym. Sin., 4, 444-450 (2015).
V. Mittal, Philos. Mag., 90, 2489-2506 (2010).
S. Pavlidoua and C. D. Papaspyridesb, Prog. Polym. Sci., 33, 1119-1198 (2008).
S. S. Ray and M. Okamoto, Prog. Polym. Sci., 28, 1539-1641 (2003).
S. Zulfiqar, I. Lieberwirth, Z. Ahmad, et al., Acta Mater., 56, 4905-4912 (2008).
D. Briesenick and W. Bremser, Prog. Org. Coat., 82, 26-32 (2015).
H. Miyagawa, M. Rich, and L. Drzal, J. Polym. Sci., 42, 4384-4390 (2004).
G-M. Kima, D-H. Lee, and B. Hoffmannc, Polymer, 42, 1095-1100 (2001).
Y. V. Savelyev, A. N. Gonchar, and T. V. Travinskaya, Am. J. Nano Res. Appl., 1, 87-93 (2013).
J. Sanchez, A. Beltran, J. Alonso, et al., Anal. Chim. Acta., 382, 157-164 (1999).
M. A. Moharram and M. M. Osama, J. Appl. Polym. Sci., 105, 2978-2983 (2007).
R. A. Meyers (ed.), Encyclopedia of Analytical Chemistry. Applications, Theory and Instrumentation, Tarzana, USA, John Wiley & Sons (2007), https://doi.org/10.1002/9780470027318.
J. L. Koenig, Spectroscopy of Polymers, New York, Elsevier (1999), https://shop.elsevier.com/books/spectroscopy-of-polymers/koenig/978-0-444-10031-3.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 59, No. 3, pp. 185-191, May-June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gonchar, O.M., Nesin, S.D. & Saveliev, Y.V. Functionalization of the Surface of Montmorillonite with Cationic Oligourethane Capable of Diisocyanate Addition. Theor Exp Chem 59, 214–221 (2023). https://doi.org/10.1007/s11237-023-09781-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-023-09781-6