The influence of carbon dioxide concentration in biogas on the composition of its reforming products has been studied to achieve optimal methanol synthesis conditions. A model of the biogas reforming stage is proposed. An optimal concentration of carbon dioxide in biogas essential for maximizing methanol production has been found. The effect of geometric characteristics of the mixing device on the homogeneity of the reacting flow, which is a critical for the process performance, has been established.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Biogas is a product of enzymatic processing of various biological raw materials and is a mixture containing mainly methane and carbon dioxide [1, 2]. A recent rapid development of the biogas industry [3] is associated with the increase in the price of traditional fossil raw materials and the strengthening of the fight against climate change due to greenhouse gas emissions. Since carbon dioxide produced by biogas burning participates in the rapid cycle of carbon in nature, it does not increase the carbon content in the atmosphere, unlike fossil fuels [4, 5].
The common field of biogas use is “green” energy, namely the cogeneration of electric current and thermal energy [6, 7]. Recently, however, biogas producers have been trying to convert biogas into higher value-added products. The conversion of biogas into methanol is the most attractive since methanol is a popular product on the market as a fuel and as a chemical raw material [8] with its further processing into chemical products, such as dimethyl ether [9], resins, plastics, varnishes, and other products. The development of these technologies is ensured by corresponding kinetic studies [10, 11]. The conversion of biogas into chemical products removes a certain amount of carbon from the cycle in nature, which reduces its content in the atmosphere and reduces the carbon pressure on the environment. Such processes become very attractive considering the “green” transition and sustainable development of the economy.
Most biogas plants produce from 1000 to 10000 Nm3 of biogas per day [12]. This corresponds to a methanol production capacity of about 4000 tons per year. Such a small production scale requires appropriate technical solutions. In particular, synthesis gas production can be based on methane partial oxidation [13, 14] with oxygen and steam over a nickel catalyst. Endothermic steam conversion reactions and exothermic oxidation reactions proceed in a reactor with a catalyst in this method. The process becomes auto-thermal at a certain gas/steam/O2 ratio. Autothermal reforming is more compact, easier, and cheaper than steam reforming [15], despite the need for an oxygen installation. It is more complex and expensive than free-volume non-catalytic partial oxidation with a much lower risk of soot formation.
This work aims to verify the positive role of carbon dioxide in the biogas conversion for methanol production. The research was conducted on a mathematical model of a synthesis gas plant for further compact production of methanol with a capacity of 5000 tons per year. The need for further biogas purification from CO2 in the range of O2/gas from 0.52 to 0.64 at steam/gas ratio = 1 and methane concentration from 60 to 100 vol.% was studied to minimize production costs. The technological pressure is 12 bar, and the temperature at the reactor outlet is 500°C. The role of geometric characteristics of the catalytic bed and reactor parameters was also studied. The mixing device was adopted according to the project developed at the Gas Institute of the National Academy of Sciences of Ukraine. The simulation was performed in the SATR-XTK computer software environment, as well as in the OpenFOAM environment [16].
Results and Discussions
Chemical conversions that take place during autothermal reforming over a nickel catalyst can be represented by the following general reactions:
The process is performed in a shaft-type reactor, which is a lined apparatus with a catalytic bed. The components of the initial mixture are heated to the maximum possible temperatures, mixed in a mixing device, and fed into the catalyst bed. The mixing device must ensure high mixing uniformity and a given flow rate in front of the catalyst bed to prevent the mixture from igniting in free space.
Flameless combustion occurs in the catalyst bed under standard process conditions. Oxygen burns out in the upper part of the layer, as a result, the temperature of the reaction gases rises to 950-1100°C, which provides the occurrence of an endothermic steam reforming reaction in the lower part of the catalyst with a decrease in temperature. Heat recovery and utilization promote further cooling of the converted gas. The composition of the gas is determined by the pressure of the process and the ratio of the initial components of the reaction mixture.
Autothermal reforming is a heterogeneous catalytic process that occurs in a fixed catalytic bed, which is a set of catalytic randomly located particles of the same size with a highly developed inner surface. Chemical processes proceed in the porous structure of particles and are accompanied by heat and mass exchange processes inside the grain and between the flow volume and the grain surface. There are transfer phenomena in the catalytic bed in radial and axial directions and heat exchange with the external environment under standard conditions.
Several physical models can describe the processes in the catalytic bed. They are widely represented in scientific literature [17, 18]. The quasi-homogeneous model is often used to analyze processes in the stationary bed of industrial reactors. In this case, the catalyst is a permeable continuous medium through which a flow of gas or liquid passes. The actual location of individual catalyst grains, their configuration, and the shape of the passages between the grains are not considered.
The following model was used to analyze the problem in this study:
with boundary conditions
where w is the flow rate; ρ is density; cp is heat capacity; Ci is concentration of i-component; Ci0 is the initial concentration of i-component: l is axial coordinate; Q is heat of reaction; T is fluid temperature; T0 is the initial fluid temperature;Wi (C, T) is the reaction rate per unit volume of the catalyst; Vc is the catalyst volume.
This model is valid since the high gas flow rate leads to the absence of external diffusion restrictions. At the same time, the thermal conductivity and particle size are such that temperature and concentration gradients along the radius can be neglected. This assumes the following: 1) steady heat exchange and flow movement; 2) the layer is homogeneous and isotropic in all directions; 3) the physical characteristics of the flow are constant throughout the layer; 4) the layer size is much larger than the grain diameter; 5) grain temperature is equal to the flow temperature at the same point of the layer.
The composition of the reacting mixture is calculated from the known ratio in a state of equilibrium:
where Keq is the equilibrium constant; S is the stoichiometric reaction vector; pi is the partial pressure of i-reagent.
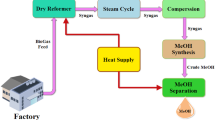
The design flow diagram of the synthesis gas production unit by the autothermal reforming of biogas is shown in Fig. 1. It was used to study the process parameters’ dependence on the initial composition, the ratio of steam:CH4 , and the ratio of O2 :CH4 . The flow diagram consists of an autothermal reforming reactor R101 where catalytic partial steam-oxygen oxidation of the source mixture; a system of heat exchange equipment for utilizing reaction heat for generating steam in a boiler E101 with a steam drum D101; heating the vapor-gas mixture, source gas and oxygen in heat exchangers E102-E104; heating the feed and demineralized water in heat exchangers E102, E104; and final cooling of the converted gas in a water cooler E107 occur. The condensate formed during the cooling of the converted gas is separated in the separators V101 and V102. The boiler water treatment system includes deaerator D102 and feed water pump P101 to balance water consumption and steam production.
Routes (1)-(3) were selected as a linearly independent basis of chemical reactions to describe a chemical transformation.
The rate of steam reforming of methane on a nickel catalyst (Eq. (1)) was calculated using the kinetic equation described in [19]. Reactions (2) and (3) were described in the equilibrium approximation. Due to the symmetry of the catalytic bed, a one-dimensional problem was considered in the adiabatic approximation. The temperature dependence of the equilibrium constant was calculated by the Temkin–Schwartzmann method.
The heat capacity was approximated by the following equation:
The coefficients presented in Table 1 were obtained from the experimental data [20]. Cp and T values are in J/(mol·K) and K/1000, respectively.
Comparisons with experimental data and equation
showed that Eq. (9) can be used up to temperatures of ~1100 K, which is a little more than 800°C.
The simplest model of heat exchange processes was used to obtain balance ratios for modeling heat exchange processes. Either the heat load or the required temperature of the environment is set in this model. The thermodynamic model was used to simulate steam-liquid separators, a steam collector, and a deaerator. The non-ideal behavior of the phases was described by the Redlich–Kwong equation of state in the Soave modification. Binary interaction parameters were used for a better description of the solubility of gases in the condensate and the correct calculation of volumes of steam and liquid. Binary parameters were obtained from experimental data [21]. The temperature dependence of the interaction parameters for a pair of components i, j was approximated as
where T is the absolute temperature, K. The values of the binary coefficients are given in Table 2.
The study examined the mixing unit because the mixing device is an important part of the reactor since autothermal reforming requires homogeneous mixing of the initial components. It is crucial because self-ignition of the mixture outside the catalyst bed can occur due to local changes in concentration. The probability of this phenomenon increases due to the high temperatures of the incoming gases. Ignition of the mixture in the free volume of the mixing device leads to the formation of soot and a decrease in the productivity of the catalyst.
The mixing device of the reactor was designed according to the project developed at the Gas Institute of the NAS of Ukraine. The mixing of the initial components of the gas occurs in the mixing pipes, where one of the components is fed axially, and the other components are fed radially into the tubes. The design of the device is shown in Fig. 2a.
A device model was developed in the OpenFOAM environment [16] to calculate the characteristics of the device that ensure homogeneous mixing. Figures 2b-d shows the simulation area of one mixing tube with 6 holes.
During a parametric study, the dependence of mixing homogeneity (the maximum value of the concentration gradient) on the parameters of the device, the diameter and number of mixing holes, the angle of inclination of the axes of the holes, and the length of the mixing tube was studied. Their optimal values for specific conditions are determined.
The required degree of biogas purification was determined to obtain the optimal composition of synthesis gas for methanol production. The dependence of the composition of biogas was studied to minimize production costs in the range of O2/CH4 ratio from 0.52 to 0.64 and methane concentrations from 60 to 100 vol.%. The calculations were performed at a steam:gas ratio of 1.0. The two-component composition of biogas was considered, namely CH4 and CO2 . The process pressure is 12 bar, and the reactor inlet temperature is 500 °C.
Figure 3a shows the dependence of the ratio of H2 and CO on the degree of biogas purification. The most acceptable values for methanol synthesis lie in the range of 2.2-2.4, which corresponds to the CO2 content in biogas of 5-18 vol.% The synthesis gas rate dependence on the degree of purification of biogas is shown in Fig. 3b. According to the above dependence, the purification of biogas from carbon dioxide leads to a decrease in the yield of synthesis gas per unit volume of biogas. This characteristic, together with the H2/CO ratio, is the main indicator of the predicted productivity of a methanol plant using such synthesis gas. Graphs in Fig. 3a and 3b are given for steam/gas = 1 and O2/CH4 = 0.64.
The calculations show an increase in the H2/CO criterion with a decrease in O2/CH4 , which can produce an acceptable composition at a lower degree of purification. However, this simultaneously leads to an increase in the concentration of unreacted methane, which results in losses and a decrease in the productivity of the methanol synthesis cycle. Therefore, the working area is 0.6-0.64 for the O2/CH4 ratio with a residual CH4 of no more than 0.5 vol.% (Fig. 3c).
The conversion degree distribution along the reaction zone was obtained. According to these data and considering the predicted mileage of the catalyst and the decrease in its activity during operation, the real amount of the catalyst was estimated as 0.2 m3 at a space velocity of 9850 h–1.
During a series of calculations, the dependence of mixing homogeneity (the maximum value of the concentration gradient) on the parameters of the device was studied. Optimal values of diameter and number of mixing holes, the angle of inclination of the axes of the holes, and the length of the mixing tube were determined for specific technological conditions of the reactor operation. The results are as follows: the minimum length of the mixing tube is 0.15 m, the number of holes is 5 pcs with a diameter of 2 mm, and the optimal angle of inclination of the hole axis is 15°.
In conclusion, our study not only showed a positive role of CO2 in biogas for methanol production but also revealed that the optimal concentration of CO2 in biogas is about 15 vol.%. Moreover, simulations highlighted an important effect of flow homogeneity on the performance of the process indicating the optimal conditions for reacting flow homogeneity. These results open new avenues not only for the design of a compact methanol production unit from biogas but also for biogas utilization in other processes.
References
P. Ghosh, G. Shah, S. Sahota, et al., Bioreactors, Sustainable Design and Industrial Applications in Mitigation of GHG Emissions, Eds. L. Singh, A. Yousuf, and D. M. Mahapatra, Amsterdam, Elsevier (2020).
S. Liu, Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design, Amsterdam, Elsevier (2020).
H. Singh, T. Padhi, A. Kashyap, and S. Taneja, Mater. Today. Proc., In press (2023), DOI: https://doi.org/10.1016/j.matpr.2023.04.484.
A. Dahiya, Bioenergy: Biomass to Biofuels and Waste to Energy, Elsevier (2020).
R. Feiz, M. Johansson, E. Lindkvist, et al., J. Clean. Prod., 378, 134536 (2022).
M. J. B. Kabeyi and O. A. Olanrewaju, Energy Rep., 8, No. 16, 774-786 (2022).
S. Abanades, H. Abbaspour, A. Ahmadi, et al., Energy Sci. Eng., 10, No. 2, 630-655 (2022).
S. Sarp, S. G. Hernandez, C. Chen, and S. W. Sheehan, Joule., 5, No. 1, 59-76 (2021).
T. Nakyai and D. Saebea, J. Clean. Prod., 241, 118334 (2019).
A. Trypolskyi, A. Zhokh, V. Gritsenko, et al., Chem. Pap., 75, No. 7, 3429-3442 (2021).
A. Zhokh, A. Trypolskyi, V. Gritsenko, et al., Asia-Pac. J. Chem. Eng., 17, No. 1, e2722 (2022).
S. Abanades, H. Abbaspour, A. Ahmadi, et al., Int. J. Environ. Sci. Technol., 19, No. 4, 3377-3400 (2022).
J. G. Speight, Synthesis Gas: Production and Properties, John Wiley & Sons, New Jersey (2020).
M. Ruoshui, X. Bang, and Z. Xiao, Catal. Today, 338, 18-30 (2019).
J. J. Caballero, I. N. Zaini, and W. Yang, App. Energy Combust. Sci., 10, 100064 (2022).
M. Klevs, V. Geza, A. Jakovius, and L. Rodin, Book of abtr. of CONECT 2023 XVI International Scientific Conference of Environmental and Climate Technologies, Riga, Riga Technical University, 69 (2023), https://doi.org/10.7250/CONECT.2023.049.
J. B. Rawlings and J. G. Ekerdt, Chemical Reactor Analysis and Design Fundamentals, Nob Hill Publishing, LLC (2020).
J. A. Conesa, Chemical Reactor Design: Mathematical Modelling and Application, Wiley-VCH, Weinheim, Germany (2020).
F. Maqbool, S. Z. Abbas, S. Ramirez-Solis, et al., Int. J. Hydrog. Energy, 46, No. 7, 5112-5130 (2021).
J. G. Speight, Lange’s Handbook of Chemistry, McGraw Hill (2016).
CRC Handbook of Chemistry and Physics, Ed. J. R. Rumble, CRC Press (2021).
Acknowledgements
This work was funded by the European Regional Development Fund under the contract “Development of Syngas Production Method for Innovative Methanol Obtainment in Compact Plant Using Mathematical Modelling of Technological Processes” (project number: 1.1.1.1/20/A/110).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 59, No. 3, pp. 179-184, May-June, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shorokhov, M.M., Olabin, V.M., Klevs, M. et al. Effect of Co2 Content on Biogas-to-Syngas Conversion for Methanol Production. Theor Exp Chem 59, 207–213 (2023). https://doi.org/10.1007/s11237-023-09780-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-023-09780-7