Solid solutions K2Bi(PO4)(MoO4):Gd,Eu containing from 0.1 to 30 mole% of europium(III) and 0.1 mole% of gadolinium(III) have been prepared by conventional solid state reaction. The samples are characterized by X-ray powder diffraction method, IR and luminescence spectroscopies. It has been shown that doping with Eu3+ does not affect the symmetry of the layered framework. At room temperature, all the studied samples are characterized by intense red photoluminescence, which is associated with 5D0→7F0-4 emission transitions in Eu3+ ions. Due to the absence of concentration quenching and color characteristics, K2Bi(PO4)(MoO4):Gd,Eu has prominent prospects for application as a red phosphor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
LED lamps are widely used in everyday life and technology, namely for lighting residential and office spaces, in car headlights, floodlights, and street lights. Compact size, highly efficient electricity conversion into radiation, fast response time, and long service life are one of the advantages of white light-emitting diodes that are the main components of such lamps [1, 2]. They are the best ecological alternative to gas-discharge daylight lamps and incandescent lamps. At the same time, the energy efficiency and color characteristics of the radiation of modern white light-emitting diodes can be improved by selecting the optimal red phosphor. The search for such a material is an urgent issue of physical inorganic chemistry. The solution can be found by determining patterns and criteria of isovalent and heterovalent substitution of cations and anions in oxide frameworks with luminescent properties. The inevitability of the formation of defects and their decisive role in the luminescent properties of materials creates prerequisites for the controlled modification of a certain framework to functionalize its properties. Such an approach allows to introduce a controlled number of impurities and/or vacancies into the composition of the crystal framework and efficiently perform the controlled substitution of certain atoms within the lattice [3, 4].
Gd-Eu pair-based framework oxide compounds, which are characterized by high quantum luminescence yield, low cost, low toxicity, and high resistance to temperature and humidity changes, have become important considering the principles of “green” energy and energy-saving requirements [4, 5]. It was established that the Gd/Eu ratio not only determines the emission intensity of an activator, but also controls color coordinates of the obtained amorphous materials for phosphate-borate glass Li2O-ZnO-SrO-B2O3-P2O5 [6]. Increased interest in the Eu(III)-Gd(III) pair co-doping is also associated with the implementation of the sensitization effect of europium(III) luminescence by gadolinium(III) ions. For example, the intense red luminescence at 613 nm is attributed to the efficient energy transfer of Gd3+→Eu3+ in the case of excitation of the luminescence of Li2O-BaO-Gd2O3-SiO2-Eu2O3 glass at 275 nm [5], while the content of the Eu3+ activator does not exceed 2 mole %. At the same time, according to the sensitization efficiency, optimal ratios between Eu and Gd change depending on the matrix. In particular, enhancement of the luminescence of europium(III) ions due to gadolinium(III) co-doping was observed for ceramics in which the content of the latter varied from 0.1 mole %, which is similar to Y2P4O13:Gd3+,Eu3+ [7] and including cases where gadolinium is regular element of the crystal lattice, namely, K3Gd(PO4)2:Eu [8], Ba6Gd1.90Na2Eu0.10(PO4)6F2 [9], and Gd2MoO6:Eu3+ [10]. Therefore, gadolinium(III) doping can increase the emission intensity of europium(III)-based luminophores. K2Bi1–xEux(PO4)(MoO4) solid solutions are one of the interesting objects for such doping, which are characterized by a high quantum yield of red luminescence [11]. It was previously reported that the dependence of the luminescence intensity on the activator concentration in this system is characterized by a non-monotonic nature [12]. This indicates the presence of concentration quenching of Eu3+ luminescence, which can be affected by doping with Gd3+ ions [13].
This work aimed at obtaining a series of K2Bi0.99–xEuxGd0.01(PO4)(MoO4) solid solutions, where x = 0.01-0.30, and determining the effect of the activator concentration on the luminescence spectra and its excitation.
Experimental
Solid-phase interaction was used to obtain K2Bi0.99–xEuxGd0.01(PO4)(MoO4) powders, where x = 0.01-0.30. KPO3 (chemically pure), Bi2O3 (chemically pure), Gd2O3 (ultra-pure), Eu2O3 (chemically pure), K2CO3 (chemically pure), and MoO3 (chemically pure) were used as reagents without additional purification. The experimental method consisted of stepwise heating of thoroughly ground stoichiometric mixtures of the initial components from a temperature of 500 to 650°C with a step of 50°C. Heat treatment of solid solutions lasted 14 h at each annealing temperature. The interaction scheme corresponds to the following reaction equation:
where x = 1, 5, 10, 15, 20, and 30%. After each stage of heat treatment, intermediate products of the interaction were rearranged in the presence of a drop of ethyl alcohol. The phase composition of synthesized samples was studied by X-ray powder diffraction and IR spectroscopy. X-ray diffraction patterns were recorded using a Shimadzu XRD-6000 diffractometer with a graphite monochromator in front of the counter (method of 20 continuous scanning at a rate of 1 deg/min) in the range of 2θ = 5.0÷60.0°. Images of the surface were obtained on a JSM-6060 LA scanning electron microscope (JEOL, Japan) with a resolution of 3.5 nm, equipped with an energy-dispersive X-ray spectrometer OxfordXMAX-80T. Samples were covered with a layer of gold by the cathodic method on a JFC-1600 device.
The presence of orthophosphate and molybdate anions in crystalline phases was confirmed by IR spectra obtained for samples in tablets with KBr using a Perkin Elmer FTIR Spectrum BX-II spectrometer.
The luminescent properties of samples were studied by a DFS-12 spectrometer at room temperature. A diode-pumped laser (λirrad = 473 nm) and a DKsEL-1000 xenon lamp with an MDR-4 monochromator were used as radiation sources for luminescence excitation.
Results and Discussion
The K2Bi(PO4)(MoO4) framework is a promising luminescent material for doping that can have a targeted effect on the color coordinates of the resulting luminophore. According to the results of previous studies, it has been shown that doping with europium(III) ions produces red luminophores with color coordinates close to the CIE standard [11]. Doping with Sm3+ ions [14] also shows significant prospects in adjusting color coordinates due to the concentration of the activator.
The selection of the matrix is associated with several factors. The K2Bi(PO4)(MoO4) framework is a unique layered structure, where BiO8 dodecahedra are linked into chains forming common edges and vertices, while formed one-dimensional chains create a network with phosphate tetrahedra [15]. Molybdate tetrahedra, which are responsible for the formation of corrugated layers where potassium cations are located in the structure, are located above and below each layer. Therefore, according to the crystallographic point of view, the framework optimally corresponds to the isovalent substitution scheme of bismuth(III) with europium/gadolinium(III) in a wide range of concentrations with minimal deformations of the corresponding oxygen polyhedron.
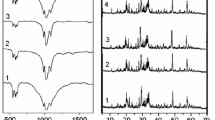
The K2Bi0,99Gd0,01(PO4)(MoO4) framework is substituted with europium(III) in the range of x = 1-30 mole% under the conditions of solid-phase sintering. At the same time, IR spectroscopy confirmed the formation of isolated orthophosphate and molybdate groups (Fig. 1a). There are three bands at 520, 557, and 592 cm–1 in the range of 500-650 cm–1, which are responsible for the deformation vibrations of the orthophosphate group. Their position and intensity coincide with the corresponding values for the K2Bi(PO4)(MoO4) framework [15]. A set of lines in the range of 735-900 cm–1 is a superposition of valence symmetric vibrations of \({\mathrm{MO}}_{4}^{2-}\) and \({\mathrm{PO}}_{4}^{3-}\) tetrahedra. It should be noted that there is a shift of only one band in the spectrum (Fig. 1a, dashed line) from 1052 to 1067 cm–1 with an increase in the concentration of europium(III) to 30 mole %, which corresponds to the valence asymmetric vibration of \({\mathrm{PO}}_{4}^{3-}\). This feature is associated with the fact that only the orthophosphate group has a direct connection with the substituted dodecahedron (Bi/Eu)O8 among two tetrahedral groups, which are present in the structure of \({\mathrm{MO}}_{4}^{2-}\) and \({\mathrm{PO}}_{4}^{3-}\). Therefore, the band at 1052 cm–1 is sensitive to isovalent substitution in the cation sublattice.
Samples obtained at 650°C are characterized by a developed surface, while crystallites have an elongated prismatic shape with an average size of 5×1×1 μm (Fig. 1b). A similar morphology of samples is typical for K2Bi(PO4)(MoO4) at a sintering temperature of 600-650°C [16]. A further increase in the sintering temperature is irrelevant due to the partial melting of phosphate molybdates of the given composition [15, 16].
X-ray diffraction of the powder (Fig. 1c) confirms the formation of solid solutions, which are isostructural to K2Bi(PO4)(MoO4) that crystallizes in orthorhombic syngonia in the Ibca space group [15].
K2Bi1–x–yEuxGdy(PO4)(MoO4) solid solutions show bright red luminescence upon excitation in the ultraviolet and visible ranges of the spectrum. According to the analysis of excitation spectra (Fig. 2a), there are several ways of excitation of this photoluminescence. Direct excitation through electronic absorption transitions from the 7F0 ground level to excited levels is the most effective for low (x = 0.01) and high (x = 0.30) concentrations of Eu3+ ions. There is an increase in the intensity of the band in the range of 250-300 nm when the europium(III) content is increased, which is associated with charge transfer from O2– to Eu3+. This result is consistent with the literature data [12]. There is a set of bands in the range of 310-335 nm (marked by arrows in Fig. 2a), which are associated with absorption transitions in Eu3+ ions (F0→5HJ) [17] and Gd3+ ions (8S7/2→6P7/2, 5/2) [8].
Only photoluminescence of europium(III) ions is observed at all excitations, which is associated with transitions from the 5D0 level to the 1FJ level (Fig. 2b). The absence of Gd3+ luminescence bands is caused by their location in the UV range of the spectrum [18]. As for the intrinsic photoluminescence of the K2Bi(PO4)(MoO4) matrix, it is very weak when compared to the luminescence of REE ions, even at low temperatures [19]. There is a weak band at 580 nm, which corresponds to the forbidden transition of 5D0→7F0 at a europium concentration of 10 mole% and higher. The presence of this band confirms the crystallographic data on the absence of inversion symmetry for the Eu3+ position in the K2Bi(PO4)(MoO4) matrix. Table 1 shows the degree of asymmetry R = I(7F2)/I(7F1), which was calculated based on the integral intensities of PL spectra in 580-600 (5D0→7F1) and 600-640 nm (5D0→7F2) regions. Its relatively high value (R > 1) also indicates a rather low point symmetry of the position of Eu3+ ions. The photoluminescence spectra for different concentrations of Eu(III) in the K2Bi0.99Gd0.01(PO4)(MoO4) matrix differ mainly in intensity. As for the role of gadolinium in the K2Bi0.99–xEuxGd0.01(PO4)(MoO4) system, a very weak shift of radiation color coordinates towards the NTSC standard for red color (X = 0.66; Y = 0.33) and a change in dependence of the peak intensity of red photoluminescence (I615) is observed when the europium content is increased. According to the literature data [12], the intensity of I615 (λex = 394 nm) increases by 10% when the Eu3+ content is increased in the K2Bi0.99–xEux(PO4)(MoO4) system from x = 0.10 to 0.30. At the same time, an almost threefold increase in intensity is observed in our studies with the same changes in Eu3+ concentrations. It can be stated that concentration quenching of photoluminescence of Eu3+ ions is weaker in the K2Bi0.99Gd0.01(PO4)(MoO4) matrix than in the K2Bi(PO4)(MoO4) matrix.
Therefore, K2Bi1–xEuxGd0.01(PO4)(MoO4) samples, where x = 1, 5, 10, 15, 20, or 30%, are synthesized by solid-phase interaction at relatively low annealing temperatures of 500-650°C. It is found that the crystallization of compounds occurs in the structure of the K2Bi(PO4)(MoO4) matrix regardless of the content of the europium(III) activator. All studied samples are characterized by intense red photoluminescence at room temperature, which is associated with 5D0→7F0-4 radiation transitions in Eu3+ ions. The absence of concentration attenuation and color characteristics of samples indicate the prospects of using the K2Bi(PO4)(MoO4):Eu,Gd compound as a red phosphor.
References
Q. Wang, M. Liao, Q. Lin, et al., J. Alloys Compd., 850, 156744 (2021).
P. Dang, D. Liu, G. Li, et al., Adv. Opt. Mater., 8, 1901993 (2020).
G. B. Nair, H. C. Swart, and S. J. Dhoble, Prog. Mater. Sci., 109, 100622 (2020).
A. G. Bispo-Jr, L. F. Saraiva, S. A. Lima, et al., J. Lumin., 237, 118167 (2021).
I. Khan, G. Rooh, R. Rajaramakrishna, et al., Spectrochim. Acta A., 210, 21-29 (2019).
P. Ramakrishna, R. K. Padhi, D. K. Mohapatra, et al., Opt. Mater., 125, 112060 (2022).
B. Fan, S. Qi, W. Zhao, et al., J. Lumin., 196, 520-524 (2018).
K. V. Terebilenko, V. P. Chornii, A. V. Lysenko, et al., Theor. Exp. Chem., 57, 121-125 (2021).
M. Xie, H. Liang, Y. Huang, et al., J. Solid State Chem., 201, 18-23 (2013).
Y. Chen, Y. Lan, G. Zhang, et al., Dalton Trans., 50, 6281-6289 (2021).
J. Grigorjevaite and A. Katelnikovas, ACS Appl. Mater. Interfaces, 8, 31772-31782 (2016).
X. He, M. Guan, N. Lian, et al., J. Alloys Compd., 492, 452-455 (2010).
J. G. Li, X. Li, X. Sun, and T. Ishigaki, J. Phys. Chem. C., 112, 11707-11716 (2008).
J. Grigorjevaite, E. Ezerskyte, J. Paterek, et al., Mater. Adv., 1, 1427-1438 (2020).
I. V. Zatovsky, K. V. Terebilenko, N. S. Slobodyanik, et al., J. Solid State Chem., 179, 3550-3555 (2006).
H. Huang, G. Chen, S. Wang, et al., Mater. Res. Bull., 51, 455-459 (2014).
F. Baur and T. Jbstel, Opt. Mater. X., 1, 100015 (2019).
V. Singh, B. R. V. Rao, A. S. Rao, et al., Optik, 206, 164020 (2020).
Y. Hizhnyi, V. Chornii, S. Nedilko, et al., Radiat. Meas., 90, 314-318 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 59, No. 2, pp. 97-101, March-April, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zozulia, V.O., Terebilenko, K.V., Nedilko, S.G. et al. Luminescence Properties of K2Bi(PO4)(MoO4):Gd,Eu Solid Solutions. Theor Exp Chem 59, 107–111 (2023). https://doi.org/10.1007/s11237-023-09769-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-023-09769-2