Steam reforming of ethanol (SRE) over complex magnesium, manganese, iron, and zinc oxides at 823 K was investigated. It was shown by X-ray phase analysis that the catalytically active phase consists of the ferrites of the respective metals with spinel structure. The yield of hydrogen over manganese and magnesium ferrites is greater than 80% with the absence of CO in the reaction products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Steam reforming of ethanol (SRE) is today regarded as a promising method for the production of hydrogen for subsequent use as a motor fuel or in fuel cells [1–4]. Hydrogen produced in the SRE process is a renewable energy source since ethanol can be obtained by the treatment of plant material (biomass).
Supported metals predominate among the studied SRE catalysts, and only a limited number of catalysts of different type, including simple and complex oxides, have been studied in this reaction [1–4]. The catalytic properties of MgO, Al2O3, ZnO, V2O5, La2O3, CeO2, and Sm2O3 were investigated in [5]. Encouraging results were obtained for certain complex oxides. The spinel NiAl2O4 exhibited high activity and selectivity at 823 K; the crystal structure of NiAl2O4 remained practically unchanged under the reaction conditions whereas the spinels NiMn2O4 and NiFe2O4 were destroyed to some degree or other [6]. The spinels MAl2O4 (M = Cu, Zn, or Ni) [7] and the perovskites La2NiO4, LaFe y Ni1–y O3, and LaCo1–x Zn x O3 [8, 9] were highly effective in SRE. It was shown recently that a catalyst based on cobalt hydrotalcite is active in the steam reforming of ethanol [10]; it is significant that only traces of metallic cobalt were identified after the reaction, i.e., cobalt in the oxidized state is an active particle in the mixed oxide catalyst. As shown in our previous work, a hydrogen yield of about 95% is obtained on manganese ferrite MnFe2O4 [11], while copper ferrite CuFe2O4 exhibited high activity and selectivity in the conversion of ethanol into acetaldehyde under the conditions of SRE [12].
Complex oxides can therefore be regarded as promising materials for catalysts of SRE. It is expedient to seek further improvement of the catalytic systems by reducing the reforming temperature, increasing the stability of the phase composition and crystal structure of the complex oxides, and optimizing the process parameters in order to achieve high selectivity and high yields of hydrogen.
The present work is devoted to comparative investigation of the catalytic action of ferrites MFe2O4 (M = Mg, Mn, Fe, Zn) not previously studied in SRE and obtained by coprecipitation.
Experimental
Unlike [11], in which the manganese ferrite MnFe2O4 was obtained by decomposition of the heteronuclear complex [MnFe2O(CH3COO)6(H2O)3]·2H2O, in the present work the MnFe2O4 was synthesized by coprecipitation. To a solution of Mn(NO3)2·6H2O (25 mmol) in 50 mL of water and Fe(NO3)3·9H2O (50 mmol) in 100 mL of water, heated to 333 K, we added slowly drop by drop 40 mL of an aqueous solution of NH3 (1 : 1) to pH 11 with vigorous stirring. The obtained brown reaction mixture was heated at 363 K for 5 h while the vigorous stirring was continued. The obtained precipitate was washed with water, ethanol, and diethyl ether by magnetic decantation, dried in air at room temperature, and calcined at 673 K for 2 h. Commercial reagents (“pure” grade) were used without further purification.
The ferrites MFe2O4 (M = Fe, Mg, Zn) were prepared by similar methods to [13–15]. Aqueous solutions of Fe(III) and M(II) nitrates were used (chlorides for M= Fe). The synthesis was finished by heating the obtained precipitates for 2 h at 673K in air [in the case of M = Fe in a stream of nitrogen in order to avoid oxidation of the Fe(II)].
X-ray diffraction (XRD) was carried out on a Bruker D8 Advance diffractometer with a Cu anode, λ = 0.154 nm, step 2θ = 0.050°, exposure time 5 s/step. The crystalline phases were identified against the ICDD file in the PDF-2 Version 2.0602 (2006) database. The BET measurements of the surface of the samples were made on a Sorptomatic 1990 instrument with the adsorption of nitrogen at 77 K. The carbon content of the catalyst after reforming was determined with a Carlo Erba 1106 analyzer.
The SRE reaction was conducted in a quartz reactor (internal diameter 10 mm) with a stationary layer of catalyst at atmospheric pressure. The temperature along the layer was monitored by means of a thermocouple in a quartz capillary. A sample with a mass of about 1 g and a size of 1-2 mm was placed in the reactor between two layers of quartz granules of the same size, heated to 573 K in stream of nitrogen (30 mL·min–1), and kept at this temperature for 2 h. The stream of nitrogen was then replaced by a stream of reaction mixture containing 2.7 mole % of C2H5OH, 50 mole % of H2O, and 47.3 mole % of N2 (H2O/C2H5OH = 19) (overall flow rate of mixture 0.17 mol·h–1), and it was kept at 573 K for 2 h, after which the temperature was raised gradually to 823 K in steps (at intervals of 50 K and kept at each temperature until a stationary state was reached). Chromatographic analysis of the reagents and products was done by the method in [16]. When the experiments were complete the catalyst was cooled in a stream of N2 and kept for characterization. In some of the experiments we also used reaction mixtures with different compositions: 5.9 mole % C2H5OH, 47.3 mole % H2O, 46.8 mole % N2 (H2O/C2H5OH = 8); 12.0 mole % C2H5OH, 35.9 mole % H2O, 52.1 mole % N2 (H2O/C2H5OH = 3).
The conversion of the ethanol X and the selectivity for the carbon-containing reaction products were calculated from the following equations:
where n is the number of C atoms in the product C n ; F Et, in is the initial flow rate of ethanol, mol·h–1, \( {F}_{{\mathrm{C}}_n} \) is the flow rate of the respective products.
The selectivity for hydrogen was taken as 100%, when 6 mol of H2 is formed from 1 mol of reacted C2H5OH. Then,
where \( {F}_{{\mathrm{H}}_2} \) is the flow rate of hydrogen at the outlet from the reactor, mol·h–1.
The yield of hydrogen \( {Y}_{{\mathrm{H}}_2} \) was calculated from the equation
Results and Discussion
According to the data from XRD, reflections were not observed on the diffractograms of the initial samples of complex oxides calcined at 673 K, and this can be explained by the small size of the nanoparticles of the catalysts. This suggestion is confirmed by the presence of ring reflections on the electron-diffraction patterns of the initial samples, from which the crystalline phases of ferrites with a lattice of the cubic spinel type FeFe2O4, MnFe2O4, MgFe2O4, and ZnFe2O4 were identified.
When the temperature was raised to 873 K after treatment in air the MnFe2O4 sample remained X-ray amorphous, and after 10 h it was oxidized with the formation of oxide phases Mn2O3 (ICDD N 01-089-2809) and Fe2O3 (ICDD N 01-085-0599) (Fig. 1a). On the diffractograms of all the samples after steam reforming of ethanol at 823 K there were reflections assigned to ferrite phases with spinel structures (Fig. 1b): FeFe2O4 (ICDD N 00-019-0629), MnFe2O4 (ICDD N 01-074-2403), MgFe2O4 (ICDD N 01-088-1942), ZnFe2O4 (ICDD N 00-022-1012).
Crystallization of the ferrites under the catalysis conditions was accompanied by a significant decrease of their specific surface area (Table 1). We also note that when the magnesium ferrite was calcined at 823Kin air for 2 h its specific surface area only decreased to 115 m2·g–1, i.e., decreased little, whereas the value after catalysis amounted to 14 m2·g–1.
The reason for such an effect of the reaction on the catalyst may be as follows. During steam reforming of ethanol there are alternating reduction and reoxidation steps in the oxide catalyst. The oxygen defects formed during reduction increase the rate of migration of the ions in the lattice of the catalyst and thereby promote its crystallization under the influence of the reaction mixture.
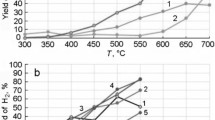
The conversion on the ferrites FeFe2O4, MnFe2O4, and MgFe2O4 is close to 100%, while the value of X for ZnFe2O4 under the same conditions is not greater than 70% (Fig. 2a).
To some degree this is due to the fact that zinc ferrite has a comparatively low specific surface area (Table 1). However, the following fact, connected with the difference in the crystal structure of the catalysts, cannot be disregarded: zinc ferrite represents a direct spinel, whereas the other ferrites have the structure of an inverse spinel. Accordingly, the ratio of the M2+ and FeS ions on their surface must differ, and this can also affect the catalytic activity.
The main products from catalytic conversion of ethanol at 823 K were H2 and CO2 on all the investigated ferrites (Fig. 2a), indicating that steam reforming predominantly takes place according to the reaction
Acetaldehyde, acetone, methane, and C2-C4 hydrocarbons were identified among the other reaction products. It is important to note that carbon monoxide CO (a typical product for SRE on any catalysts) is not formed in any significant amounts on the Fe, Mn, Mg, and Zn ferrites studied in the present work.
The acetaldehyde is formed as a result of dehydrogenation of the ethanol:
Under the conditions of the experiment the content of CH3 CHO in the reaction products was small (Fig. 2, a and b) on account, clearly, of its conversion into other products, including acetone:
Reaction (III) may occur by a mechanism involving aldol condensation of acetaldehyde followed by dehydrogenation and decarboxylation of the formed surface intermediate product [17–19]: 2CH3CHO → CH3CH(OH)CH2CHO; CH3CH(OH)CH2CHO + Os → CH3CH(OH)CH2COOs+ Hs; CH3CH(OH)CH2COOs + Hs → CH3COCH3 + CO2 + H2. The surface oxygen is formed as a result of decomposition of water H2O → Os + H2. Indirect evidence for the aldol condensation mechanism can be obtained from the fact that crotonaldehyde (CH3 CHCHCHO), which can be formed by dehydration of the aldol, and also 1-butanol as product of the hydrogenation of the surface C4 intermediate were identified in the products of SRE at 673 K with H2O/C2H5OH = 3.
From comparison of the data obtained for MnFe2O4 at 823 K (Fig. 2a) and 673 K for the same ratio H2O/C2H5OH = 19 (Fig. 2b) it is clear that the content of acetone in the reaction products is much lower at the higher temperature, whereas the content of the final products of SRE (CO2 and H2) is higher. This is clearly a consequence of steam reforming of the acetone:
The transformation of ethanol and the intermediate organic products into CO2 and H2 takes place on the surface of the complex oxides through a series of consecutive oxidation–reduction reactions, which may include the intermediate formation of adsorbed COs. The absence of CO in the gas phase detected in the present work can be attributed to the fact that adsorbed CO is oxidized to CO2(COs + Os → CO2) much faster than it is desorbed. It is clear also that all the investigated catalysts do not accelerate the water gas shift reaction otherwise the appearance of CO on account of the reaction CO2 +H2 ↔ CO + H2O would be observed. (According to thermodynamic calculations, for the conditions adopted in the present work [4] the equilibrium content of CO in the products of SRE is about 2%.)
Calculation of the yield of the targeted product (hydrogen) from the data presented in Fig. 2a showed that fairly high values, amounting to 70.2%, 83.4%, and 82.3% respectively, are obtained for FeFe2O4, MnFe2O4, and MgFe2O4 at 823 K. The yield of hydrogen on ZnFe2O4 was significantly lower (44.5%) on account of the lower values for the conversion of ethanol and the selectivity for hydrogen.
In the composition of the studied ferrites with general formula MFe2O4 there are metals with variable (M = Fe, Mn) and constant (M = Mg, Zn) valence. At the same time they all accelerate the SRE reaction. Moreover, magnesium ferrite is not significantly inferior to manganese ferrite in the yield of hydrogen (and accordingly of CO2). This indicates that Fe3+ ions, capable of redox transitions in oxidation–reduction pairs Fe3+ ↔ Fe2+, have a determining effect on the catalytic action of the ferrites. Iron ions are localized at least partly at octahedral positions in the structure of the ferrites, and according to [20–22] the surface of oxides with spinel structure consist mainly of sites of octahedral coordination, and their catalytic activity is indeed determined by the octahedral cations.
The experiments on the effect of the H2O/C2H5OH ratio in the case of MnFe2O4, most effective with respect to the hydrogen yield, showed that the ferrite catalyst is active in the SRE reaction even at elevated ethanol content in the reaction mixture right up to the stoichiometric H2O/C2H5OH ratio (Fig. 2b); the qualitative composition of the reaction products is identical for all the mixtures. It is also important to note that after reaction on MnFe2O4 for 14 h at 823 K there are no signs of deactivation of the catalyst; moreover, according to elemental analysis carbon was not detected in the sample after catalysis.
Catalytic steam reforming of ethanol includes a series of elementary reactions that take place through the formation of various intermediate compounds with the participation not only of oxidation–reduction but also of acid–base active centers of the catalyst. In order to achieve high activity and selectivity in the SRE process it is necessary to have a specific balance of oxidation–reduction and acid–base characteristics in the oxide catalyst. Quantitative determination of these characteristics will form the subject of our further investigations.
In conclusion we note that the catalytically active phase in the investigated complex oxides MFe2O4 (M = Mg, Mn, Fe, Zn) in steam reforming of ethanol is a phase containing ferrites with spinel structures; on manganese and magnesium ferrites the yield of the targeted product of SRE (hydrogen) is more than 80%; the products of the reactions on the investigated ferrites do not contain CO, which is an important indicator for the use of hydrogen in low-temperature fuel cells.
References
A. Haryanto, S. Fernando, N. Murali, et al., Energy Fuels, 19, 2098-2106 (2005).
P. D. Vaidya and A. E. Rodrigues, Chem. Eng. J., 117, 39-49 (2006).
M. Ni, D. Y. C. Leung, and M. K. H. Leung, Int. J. Hydrogen Energy, 32, 3238-3247 (2007).
Y. I. Pyatnitsky, L. Yu. Dolgykh, I. L. Stolyarchuk, and P. E. Strizhak, Teor. Éksp. Khim., 49, No. 5, 265-283 (2013). [Theor. Exp. Chem., 49, No. 5, 277-297 (2013) (English translation).]
J. Llorca, P. R. Piscina, J. Sales, et al., Chem. Commun., 641-642 (2001).
H. Muroyama, R. Nakase, T. Matsui, et al., Int. J. Hydrogen Energy, 35, 1575-1581 (2010).
M. N. Barroso, M. F. Gomez, L. A. Arrua, et al., Catal. Lett., 109, 13-19 (2006).
Z. Li, W. Yi, and H. Qun, Trans. Nonferrous Met. Soc. China, 19, 1444-1449 (2009).
S. Q. Chen and Y. Liu, Int. J. Hydrogen Energy, 34, 4735-4746 (2009).
R. Espinala, E. Taboadaa, E. Molinsb, et al., Appl. Catal. B, 127, 59-67 (2012).
I. L. Stolyarchuk, L. Yu. Dolgikh, I. V. Vasilenko, et al., Teor. Éksp. Khim., 48, No. 2, 119-123 (2012). [Theor. Exp. Chem., 48, No. 2, 129-134 (2012) (English translation).]
L. Yu. Dolgykh, I. L. Stolyarchuk, L. A. Staraya, et al., Teor. Éksp. Khim., 51, No. 4, 225-229 (2015). [Theor. Exp. Chem., 51, No. 4, 230-235 (2015) (English translation).]
D. K. Kim, M. Mikhaylova, Yu. Zhang, et al., Chem. Mater., 15, 1617-1627 (2003).
C.-P. Liu, M.-W. Li, Z. Cui, et al., J. Mater. Sci., 42, 6133-6138 (2007).
J. Philip, G. Gnanaprakash, G. M. P. Panneerselvam, et al., J. Appl. Phys., 102, 054305 (2007).
L. Dolgykh, I. Stolyarchuk, I. Deynega, and P. Strizhak, Int. J. Hydrogen Energy, 31, 1607-1610 (2006).
T. Nishiguchi, T. Matsumoto, H. Kanai, et al., Appl. Catal. A, 279, 273-277 (2005).
K. Inui, T. Kurabayashi, and S. Sato, J. Catal., 212, 207-215 (2002).
I. Charkendorff and W. Niemantsverdriet, Concepts of Modern Catalysis and Kinetics, Wiley-VCH, Weinheim (2003).
J. P. Jacobs, A. Maltha, J. G. H. Reitjes, et al., J. Catal., 47, 294-300 (1994).
C. G. Ramankutty and S. Sugunan, Appl. Catal. A, 218, 39-51 (2001).
C. G. Ramankutty, S. Sugunan, B. Thomas, et al., J. Mol. Catal. A, 187, 105-117 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 52, No. 4, pp. 244-248, July-August, 2016.
Rights and permissions
About this article
Cite this article
Stolyarchuk, I.L., Dolgykh, L.Y., Vasylenko, I.V. et al. Ferrites MFe2O4 (M = Mg, Mn, Fe, Zn) as Catalysts for Steam Reforming of Ethanol. Theor Exp Chem 52, 246–251 (2016). https://doi.org/10.1007/s11237-016-9475-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-016-9475-5