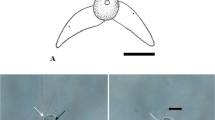
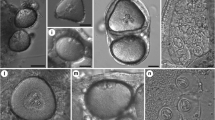
Abstract
Aurantiactinomyxon is one of the most diverse myxozoan collective groups, comprising types that mostly infect freshwater and marine oligochaetes belonging to the family Naididae Ehrenberg, 1828, but also Lumbriculidae Claus, 1872. In this study, a comprehensive revision of all known aurantiactinomyxon types is performed and highlights the fallibility of using the form and length of the valvular processes as main criterion for differentiating among style-less actinospore morphotypes. The demise of the guyenotia collective group is proposed based on the ambiguous features of several types that allow conformity with both the aurantiactinomyxon and guyenotia definitions. Nonetheless, the information presently available clearly shows that a general shift is needed in our approach to actinospore grouping, which should probably be based on actinospore functionality relative to environment and host ecology, rather than on morphology. Life cycle studies based on experimental transmission and molecular inferences of the 18S rDNA have linked aurantiactinomyxon (including former guyenotia) to myxozoans belonging to a diverse array of genera, including Chloromyxum, Henneguya, Hoferellus, Myxobolus, Paramyxidium, Thelohanellus and Zschokkella. This undoubtedly shows a high capacity of the aurantiactinomyxon morphotype to promote infection in intrinsically distinct vertebrate hosts and environmental habitats, consequently increasing interest in its study for attaining a better understanding of myxozoan-host interactions. The identification of novel and known types, however, is impeded by the lack of concise information allowing a comprehensive analysis of biological, morphological, and molecular criteria. In this sense, the compilation of data presented in this study will ultimately help researchers seeking to perform reliable identifications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections of aquatic oligochaetes by actinospores were first reported by Štolc (1899), who created the Actinomyxidia to encompass hexactinomyxon, synactinomyxon and triactinomyxon types that the author found infecting tubificids in Czechia. Over time, recognition of the homology between these organisms and myxozoans, led to the relocation of Actinomyxidia to the phylum Myxozoa Grassé, 1970, which became divided into the classes Myxosporea Bütschli 1881 (fish parasites producing myxospores) and Actinosporea Noble, 1980 (worm parasites producing actinospores). In 1984, the ground-breaking discovery that Myxobolus cerebralis Hofer, 1903 develops triactinomyxon actinospores in the gut epithelium of the oligochaete Tubifex tubifex (Müller) (Wolf & Markiw, 1984), showed that myxozoan life cycles comprise both myxospore and actinospore phases, with members of the classes Myxosporea and Actinosporea representing morphologically distinct phases of the same species. This led to a major taxonomic revision, with Kent et al. (1994) proposing the demise of the class Actinosporea, and the use of its generic names as vernacular designations for actinospore morphotypes established within distinct collective groups.
Currently, there are ca. 20 valid actinospore collective groups (Lom & Dyková, 2006; Rangel et al., 2011; Milanin et al., 2017; Atkinson et al., 2019; Rocha et al., 2019a, 2019b, 2020), with aurantiactinomyxon being one of the most diverse. This collective group was first described by Janiszewska (1957), who defined its actinospores having a style-less epispore, with three equal processes that curve downwards and embrace with their whole base the epispore cavity. Lom & Dyková (2006) updated the definition, and described aurantiactinomyxon as having three stout, semicircularly curved, leaf-like valvular processes attached to an ellipsoidal body with protruding polar capsules at the apex and containing a sporoplasm with many secondary cells. To date, 61 aurantiactinomyxon types have been reported based on these definitions (Table 1), with differentiation between types mostly relying on morphometric comparisons. Molecular data of the 18S rDNA is available for only 20 types. Another eight 18S rDNA sequences are available in GenBank but constitute unpublished submissions to the NCBI database; while the sequences with GenBank accession numbers MN294775 and MN294776 appear identified as aurantiactinomyxon in the database but have been published as belonging to the presently demised echinactinomyxon collective group (see Rocha et al., 2019a; Gao et al., 2021).
Assigning novel types to a specific collective group can be complicated given the overlapping definitions of several groups that share main morphological features, such as the formation of single spores versus multi-spore cages, and presence/absence of style or valvular processes. In fact, a boundary-less “continuum of form” has been suggested to exist between collective groups that differ based solely on the form of a specific morphological character, as is the case of aurantiactinomyxon, echinactinomyxon Janiszewska, 1957, guyenotia Naville, 1930, neoactinomyxum Granata, 1922 and raabeia Janiszewska, 1955 (see Hallett et al., 2006; Atkinson, 2011). Differentiation between these style-less morphotypes is based on the shape and length of the valvular processes, which are traditionally defined as being long and straight in echinactinomyxon, curved in raabeia, leaf-like and curved downwards in aurantiactinomyxon, digitiform in guyenotia, and short and spherical in neoactinomyxum (see review in Lom & Dyková, 2006). Although the original definition of aurantiactinomyxon also stated that the valvular processes embraced with their whole base the epispore cavity (Janiszewska, 1957), this criterion has been widely disregarded by researchers (see types in Burtle et al., 1991; Bellerud, 1993; Yokoyama et al., 1993; Yokoyama, 1997; Trouillier et al., 1996; Hallett et al., 1997; El-Mansy et al., 1998a, b; Székely et al., 1998; Özer et al., 2002a; Rosser et al., 2014; Milanin et al., 2018), not being included in the updated definition by Lom & Dyková (2006).
Recently, Rocha et al. (2019a) showed that the shape of the valvular processes is a morphological character too variable for distinguishing between raabeia and echinactinomyxon, based on the observation of a type producing actinospores with long valvular processes that were either curved or straight. Consequently, the demise of the echinactinomyxon collective group was proposed and the definition of raabeia was updated to encompass actinospores having straight valvular processes (Rocha et al., 2019a). Similarly, many of the aurantiactinomyxon types included in this synopsis display ambiguous features allowing conformity with the definitions of other collective groups. For instance, Aurantiactinomyxon janiszewskai and the Aurantiactinomyxon type of Xi et al., 2013 have long valvular processes that best resemble those of raabeia (Bellerud, 1993; Xi et al., 2013). Although the processes of the first curve downwards as traditionally described for aurantiactinomyxon, the second is depicted has having straight processes and would probably be better allocated to raabeia. The boundary between these collective groups is further blurred by the report of aberrant spores displaying unequal and different-shaped caudal processes, as is the case of the Raabeia type 4 of Özer et al., 2002 (see Özer & Wootten, 2002). The distinction between aurantiactinomyxon and neoactinomyxum is also tenuous, given that several aurantiactinomyxon types have short valvular processes, which only differ from neoactinomyxum by being triangular or rounded with slightly pointed ends rather than completely spherical (see types in Hallett et al., 1997; Negredo & Mulcahy, 2001; Oumona et al., 2003; Székely et al., 2000, 2003, 2004; Xi et al., 2015; Zhao et al., 2016). It should be noted that a few neoactinomyxum have been reported to have triangular valvular processes [see types described by Borkhanuddin et al. (2014) and Xi et al. (2015)].
However, it is with guyenotia that the lack of a distinctive boundary is most evident. Several aurantiactinomyxon types described in the literature have digitiform valvular processes that conform with the definition of guyenotia, including the Aurantiactinomyxon type of Burtle et al., 1991, Aurantiactinomyxon type of El-Matbouli et al., 1992, Aurantiactinomyxon type 1 of Yokoyama et al., 1993, Aurantiactinomyxon type 3 of Hallett et al., 1997, Aurantiactinomyxon types 2 and 5 of El-Mansy et al., 1989a, Aurantiactinomyxon type 3 of Özer et al., 2002, Aurantiactinomyxon type 1 of Oumouna et al., 2003, Aurantiactinomyxon type A of Eszterbauer et al., 2006, and the Aurantiactinomyxon of Hallett et al., 2006. The inclusion of types with digitiform processes within the aurantiactinomyxon collective group was previously noticed by Xiao & Desser (1998), who suggested they should be transferred to guyenotia. However, the fallibility of this morphological criterion has led authors to compare aurantiactinomyxon and guyenotia interchangeably (see Burtle et al., 1991; Eszterbauer et al., 2006; Xi et al., 2013). Moreover, Hallett et al. (2002) proved that a single aurantiactinomyxon type can produce actinospores with different process length and shape, having observed two distinct phenotypes associated with the same genotype: one displaying swollen, leaf-like processes with either pointed or rounded ends, and the other having elongated, digitiform-like processes. This clearly shows that there is no real boundary between aurantiactinomyxon and guyenotia. Consequently, the demise of the guyenotia collective group is here proposed, with the transference of its types to aurantiactinomyxon. Original names are retained so as not to increase confusion. The decision to invalidate the oldest group rather than the most recent one relates to the low number of guyenotia that have been reported in the literature; only 5 types of guyenotia have been described versus the 61 types of aurantiactinomyxon that are presently known (see Table 1). Accordingly, aurantiactinomyxon is tentatively defined as having a spherical, subspherical, cylindrical or triangular actinospore body with 3 polar capsules protruding from the apex. Three equally sized latero-posterior valvular processes arise from the actinospore body without a style, curving downwards and tapering to a rounded or pointed end, being leaf-like, propeller-like, digitiform or triangular. Nonetheless, this should be regarded as a temporary definition, given that the increase of our knowledge of actinospore biodiversity will undoubtedly blur even more the boundaries between aurantiactinomyxon, raabeia, and even neoactinomyxum. Overall, this “continuum of form” demonstrates that a general shift is needed in our approach to actinospore grouping (Atkinson, 2011), which should probably be based on actinospore functionality relative to environment and host ecology, rather than on morphology.
The great majority of aurantiactinomyxon types reported in the literature infect freshwater oligochaetes belonging to the family Naididae Ehrenberg, 1828 [currently includes members of the former Tubificidae (Erséus et al., 2008)], with reports mainly from the species Branchiura sowerbyi Beddard, but also T. tubifex, Limnodrilus hoffmeisteri Claparède, and Dero digitata (Müller), and less frequently from Lophochaeta ignota Štolc, and members of the genera Nais Müller and Pristina Ehrenberg. A few types have their oligochaete hosts identified only up to the genus- or family-level (see Marques, 1984; Grossheider & Körting, 1992; Benajiba & Marques, 1993), while a few others lack host information (see El-Mansy et al., 1998a; Oumouna et al., 2003; Hallett et al., 2006). Only the three Aurantiactinomyxon types described by Hallett et al. (1997), and the Aurantiactinomyxon type of Rocha et al., 2019, are known to occur in the marine environment, parasitizing naidid oligochaetes belonging to the genera Limnodriloides Pierantoni, Pacifidrilus Erséus, and Tubificoides Lastočkin. The only exceptions to the usage of naidids as hosts are Aurantiactinomyxon pavinsis, widely reported from the freshwater lumbriculid Stylodrilus heringianus Claparède (see Marques, 1984; Oumouna et al., 2003; Holzer et al., 2004; Marcucci et al., 2009), the Aurantiactinomyxon of Freeman & Kristmundsson, 2018, and the Aurantiactinomyxon type of McGeorge et al., 1997 as reported from Lumbriculus variegatus (Müller) by Özer & Wootten (2001). The former Guyenotia type of Xiao & Desser, 1998 was also reported from L. variegatus (Xiao & Desser, 1998). A few types have been reported from more than a single host species: Aurantiactinomyxon raabei junioris, Aurantiactinomyxon minor, Aurantiactinomyxon of El-Matbouli et al., 1992, Aurantiactinomyxon of Benajiba & Marques, 1993, and Aurantiactinomyxon of Székely et al., 1998 supposedly infect more than a single naidid species (Table 1), while Aurantiactinomyxon pavinsis and the Aurantiactinomyxon type of McGeorge et al., 1997 have been reported from both naidids and lumbriculids (Table 1). Considering that these reports are not backed-up by molecular data, Rocha et al. (2019c) suggested that aurantiactinomyxon might be host specific, further proposing that actinospores of new isolates be identified through a comprehensive morphological and biological comparison to known types sharing the same annelid host.
Individual prevalence of infection of aurantiactinomyxon types is typically low, ranging from 0.01% to 1.5% in wild environments, and from 0.26% to 4.6% in surveys performed from fish farms, though there is evidence of significant spatial and temporal variations (see El-Mansy et al., 1998a,b; Özer et al., 2002b; Eszterbauer et al., 2006) that probably reflect host genetics, proximity, and habitat preferences, as well as abiotic factors (see Alexander et al., 2015 and references therein). Higher prevalence of infection has been reported when considering the number of infected individuals within only a specific host species, rather than in relation to the annelids population that was sampled (see Székely et al., 2000; Negredo & Mulcahy, 2001), or when pooling all aurantiactinomyxon types occurring in a single annelid species to determine the prevalence of infection of the collective group in a specific sampling site (see El-Mansy et al., 1998a,b). Experimental transmission studies have also reported higher values of prevalence of infection. For instance, Székely et al. (1998) reported 12.5% and 16.7% prevalence of infection of the aurantiactinomyxon counterparts of Thelohanellus nikolskii Achmerov, 1955 and Thelohanellus hovorkai Achmerov, 1964, respectively.
About 60 myxosporean life cycles have been elucidated to date (see Eszterbauer et al., 2015), with aurantiactinomyxon types being actinospore counterparts to Chloromyxum truttae (Léger, 1906), Henneguya exilis (Kudo, 1929), the PGD agent Henneguya ictaluri Pote, Hanson, & Shivaji, 2000, Henneguya mississippiensis Rosser et al., 2005, Hoferellus carassii Achmerov, 1960, Hoferellus cyprini (Doflein, 1898) Berg, 1898, Myxobolus intimus Zaika, 1965, Paramyxidium giardi (Cépède, 1906) Freeman & Kristmundsson, 2018, T. hovorkai, Thelohanellus kitauei Egusa & Nakajima, 1981, T. nikolskii, and Thelohanellus testudineus Liu et al., 2013 (Eszterbauer et al., 2015 and references therein; Zhao et al., 2016, 2017; Rocha et al., 2019c; Borzák et al., 2021). The former Guyenotia type of Eszterbauer et al., 2006 has also been linked to an unidentified Zschokkella sp. from Carassius auratus Linnaeus, 1758 (Eszterbauer et al., 2006; data in GenBank). Clarification of the life cycles of H. carassii and H. cyprini were based solely on experimental transmission studies, with all others established through molecular inference, based on DNA match between myxosporean and actinosporean counterparts (99.2% to 100% similarity reported in the literature). However, the 18S rDNA sequences of the actinospores reported to match H. ictaluri and H. exilis were not made available (see Lin et al., 1999; Rosser et al., 2014), so that molecular information can only be found for the myxosporean stage. In turn, no sequence is available for the myxosporean stage of T. hovorkai, which accounts for two distinct actinospore stage sequences in GenBank. Anderson et al. (2000) reported a single 710 bp 18S rDNA sequence (AJ133419) obtained from both myxosporean and actinosporean stages of T. hovorkai. Actinospores were retrieved from infections in B. sowerbyi and were identified by the authors as belonging to the Aurantiactinomyxon type 2 of Yokoyama et al., 1993, previously reported to be the life cycle counterpart of T. hovorkai based on experimental transmission (see Yokoyama et al., 1993; Yokoyama, 1997; Székely et al., 1998). Later, Eszterbauer et al. (2006) obtained two similarly sized sequences (DQ231153 with 817 bp and DQ231154 with 785 bp) from aurantiactinomyxon actinospores in B. sowerbyi that were reported to match unpublished sequences of T. hovorkai obtained by the authors during a previous experimental infection study, though being morphologically and genetically different from the Aurantiactinomyxon type 2 of Yokoyama et al., 1993 (see Yokoyama, 1997; Yokoyama et al., 1993; Székely et al., 1998). Presently, both these aurantiactinomyxon types remain identified as life cycle counterparts of T. hovorkai, being included as such in Table 1.
A more comprehensive and clear understanding of the diversity of this collective group is necessary to help clarify important interactions with annelid hosts and involvement in myxozoan life cycles. The description of novel types and re-description of known types that remain without molecular data, namely those comprising reports from several hosts, will surely contribute towards this aim. Thus far, molecular-based studies are limited by the paucity of available data but have shown that morphologically similar aurantiactinomyxon actinospores may be distantly related (Rocha et al., 2019c), in the same manner that morphologically different actinospores can share the same genotype (see Hallett et al., 2002; Eszterbauer et al., 2006; Zhao et al., 2016). Consequently, the combined analysis of biological, morphological, and molecular criteria is imperative for performing reliable type identification (Rocha et al., 2019c). This task is significantly hampered by the difficulty in obtaining earlier reports, and due to imprecision and confusion of information in the literature.
In this study, a comprehensive summary of the biological characters and morphometry of all types described within the aurantiactinomyxon group and former guyenotia is provided as an important tool for researchers working in this field. Sixty-six types were counted, with data from original descriptions and subsequent reports. Aurantiactinomyxon eiseniellae Ormières & Frézil, 1969 was not included in the count, as Marques (1984) transferred this type to the neoactinomyxum collective group. Morphometric characters include actinospore body length and width, length and width of valvular processes, length and width of polar capsules, and number of secondary cells. Number of coils of polar tubules was not included, given that this information is available only for the Aurantiactinomyxon of Székely et al., 1998 (3–4), Aurantiactinomyxon of Xiao & Desser, 1998 (3–4), Aurantiactinomyxon of Rocha et al., 2019c (4–5), and the Guyenotia of Xiao & Desser, 1998 (3–4) (Borzák et al., 2021; Rocha et al., 2019c; Székely et al., 1998; Xiao & Desser, 1998). Information on host, locality and availability of molecular data is also provided.
References
Alexander, J. D., Kerans, B. L., El-Matbouli, M., Hallett, S. L. & Stevens, L. (2015). Annelid-Myxosporean Interactions. In B. Okamura, A. Gruhl, & J. L. Bartholomew (Eds). Myxozoan Evolution, Ecology and Development. Switzerland: Springer International Publishing, pp. 217–234. https://doi.org/https://doi.org/10.1007/978-3-319-14753-6_12
Anderson, C., Canning, E. U., Schäfer, S., Yokoyama, H., & Okamura, B. (2000). Molecular confirmation of the life cycle of Thelohanellus hovorkai Achmerov, 1960 (Myxozoa: Myxosporea). Bulletin of the European Association of Fish Pathologists, 20, 111–115.
Atkinson, S. D. (2011). Diversity, life cycles and population genetics of freshwater Myxozoa from the Pacific Northwest of North America. PhD thesis, The University of Queensland, Australia.
Atkinson, S. D., Hallett, S. L., Díaz-Morales, D., Bartholomew, J. L., & de Buron, I. (2019). First myxozoan infection (Cnidaria: Myxosporea) in a marine polychaete from North America and erection of actinospore collective group Saccimyxon. Journal of Parasitology, 105(2), 252–262. https://doi.org/https://doi.org/10.1645/18-183
Bartholomew, J. Rohivec, J. S., & Fryer, J. L. (1992). Ceratomyxa shasta infections of salmonid fish. In T. Kimura (Ed.), Proc OJI Int Symp Salmonid Diseases. Hokkaido University Press, Sapporo, p. 267–275.
Bellerud, B. L. (1993). Etiological and epidemiological factors effecting outbreaks of Proliferative Gill Disease on Mississippi channel catfish farms. PhD thesis, Mississippi State University, Starkville, Mississippi State, USA.
Bellerud, B. L., Pote, L. M., Chenney, E. M., & Hackthorn, J. A. (1992). Actinomyxid parasites present in aquatic oligochaete populations of PGD infected and uninfected catfish ponds. In Proceedings of the Eastern Fish Health and American Fisheries Society, Fish Health Section Workshop, Auburn, USA.
Bellerud, B. L., Pote, L. M., Lin, T. L., Johnson, M. J., & Boyle, C. R. (1995). Etiological and epizootological factors associated with outbreaks of Proliferative Gill Disease in channel catfish. Journal of Aquatic Animal Health, 7(2), 124–131. https://doi.org/https://doi.org/10.1577/1548-8667(1995)007<0124:EAEFAW>2.3.CO;2
Benajiba, M. H., & Marques, A. (1993). The alternation of actinomyxidian and myxosporidian sporal forms in the development of Myxidium giardi (parasite of Anguilla anguilla) through oligochaetes. Bulletin of the European Association of Fish Pathologists, 13(3), 100–103. https://eurekamag.com/research/002/709/002709762.php
Borkhanuddin, M. H (2013). Studies of fish parasitic myxozoans in Lake Balaton, Hungary and in freshwater and marine biotopes in Malaysia. PhD thesis, University of Pannonia, Hungary.
Borkhanuddin, M. H, Cech, G., Molnár, K., Németh, S., & Székely, C. (2014). Description of raabeia, synactinomyxon and neoactinomyxum developing stages of myxosporeans (Myxozoa) infecting Isochaetides michaelseni Lastočkin (Tubificidae) in Lake Balaton and Kis-Balaton Water Reservoir, Hungary. Systematic Parasitology, 88, 245–259. https://doi.org/https://doi.org/10.1007/s11230-014-9496-1
Borzák, R., Borkhanuddin, M. H., Cech, G., Molnár, K., Hallett, S. L., & Székely, C. (2021). New data on Thelohanellus nikolskii Achmerov, 1955 (Myxosporea, Myxobolidae) a parasite of the common carp (Cyprinus carpio, L.): The actinospore stage, intrapiscine tissue preference and molecular sequence. International Journal for Parasitology – Parasites and Wildlife, 15, 112–119. https://doi.org/https://doi.org/10.1016/j.ijppaw.2021.04.004
Burtle, G. J., Harrison, L. R., & Styer, E. L. (1991). Detection of a triactinomyxid myxozoan in an oligochaete from ponds with Proliferative Gill Disease in channel catfish. Journal of Aquatic Animal Health, 3(4), 281–287. https://doi.org/https://doi.org/10.1577/1548-8667(1991)003<0281:DOATMI>2.3.CO;2
El-Mansy, A., Székely, C., & Molnár, K. (1998a). Studies on the occurrence of actinosporean stages of fish myxosporeans in a fish farm of Hungary, with the description of triactinomyxon, raabeia, aurantiactinomyxon and neoactinomyxon types. Acta Veterinaria Hungarica, 46(2), 259–284.
El-Mansy, A., Székely, C., & Molnár, K. (1998b). Studies on the occurrence of actinosporean stages of myxosporeans in Lake Balaton, Hungary, with the description of triactinomyxon, raabeia and aurantiactinomyxon types. Acta Veterinaria Hungarica, 46(4), 437–450.
El-Matbouli, M., Fischer-Scherl, T., & Hoffmann, R. W. (1992). Transmission of Hoferellus carassii Achmerov, 1960 to goldfish Carassius auratus via an aquatic oligochaete. Bulletin of the European Association of Fish Pathologists, 12(2), 54–56.
Erséus, C., Wetzel, M. J., & Gustavsson, L. (2008). ICZN rules – a farewell to Tubificidae (Annelida, Clitellata). Zootaxa, 1744, 66–68. https://doi.org/https://doi.org/10.11646/zootaxa.1744.1.7
Eszterbauer, E., Atkinson, S., Diamant, A., Morris, D., El-Matbouli, M., & Hartikainen, H. (2015). Myxozoan life cycles: practical approaches and insights. In B. Okamura, A. Gruhl, & J. L. Bartholomew (Eds). Myxozoan Evolution, Ecology and Development. Switzerland: Springer International Publishing, pp. 175–198. https://doi.org/https://doi.org/10.1007/978-3-319-14753-6_10
Eszterbauer, E., Marton, S., Rácz, O. Z., Letenyei, M., & Molnár, K. (2006). Morphological and genetic differences among actinosporean stages of fish-parasitic myxosporeans (Myxozoa): difficulties of species identification. Systematic Parasitology, 65(2), 97–114. https://doi.org/https://doi.org/10.1007/s11230-006-9041-y
Freeman, M.A., & Kristmundsson, A. (2018). Studies of Myxidium giardi Cépède, 1906 infections in Icelandic eels identifies a genetically diverse clade of myxosporeans that represents the Paramyxidium n. g. (Myxosporea: Myxidiidae). Parasites & Vectors, 11, 551. https://doi.org/10.1186/s13071-018-3087-y
Gao, Z. -P., Yang, K., Chen, K., Xi, B. -W., & Xie, J. (2021). Morphological characters and DNA identification of several actinosporean collected from oligochaete Branchiura sowerbyi. Acta Hydrobiologica Sinica, 45(2), 446–454. https://doi.org/https://doi.org/10.7541/2021.2019.264
Grossheider, G., & Körting, W. (1992). First evidence that Hoferellus cyprini (Doflein, 1898) is transmitted by Nais sp. Bulletin of the European Association of Fish Pathologists, 12(1), 17–20. https://www.cabdirect.org/cabdirect/abstract/19940807289
Hallett, S. L., Atkinson, S. D., & El-Matbouli, M. (2002). Molecular characterisation of two aurantiactinomyxon (Myxozoa) phenotypes reveals one genotype. Journal of Fish Diseases, 25(10), 627–631. https://doi.org/https://doi.org/10.1046/j.1365-2761.2002.00405.x
Hallett, S. L., Atkinson, S. D., Erséus, C., & El-Matbouli, M. (2006). Myxozoan parasites disseminated via oligochaete worms as live food for aquarium fishes: descriptions of aurantiactinomyxon and raabeia actinospore types. Diseases of Aquatic Organisms, 69, 213–225. https://doi.org/https://doi.org/10.3354/dao069213
Hallett, S. L., Erséus, C., & Lester, R. J. G. (1997). Actinosporea from Hong Kong marine oligochaeta. In B. Morton (Ed.), Proceedings of the Eight International Marine Biological Workshop: the Marine Flora and Fauna of Hong Kong and Southern China. Hong Kong University Press, Hong Kong, 1–7.
Hanson, L. A., Lin, D., Pote, L. M. W., & Shivaji, R. (2001). Small subunit rRNA gene comparisons of four actinosporean species to establish a polymerase chain reaction test for the causative agent of Proliferative Gill Disease in channel catfish. Journal of Aquatic Animal Health, 13(2), 117–123. https://doi.org/https://doi.org/10.1577/1548-8667(2001)013<0117:SSRGCO>2.0.CO;2
Holzer, A. S., Sommerville, C., & Wootten, R. (2004). Molecular relationships and phylogeny in a community of myxosporeans and actinosporeans based on their 18S rDNA sequences. International Journal of Parasitology, 34, 1099-1111. https://doi.org/https://doi.org/10.1016/j.ijpara.2004.06.002
Janiszewska, J. (1957). Actinomyxidia II: new systematics, sexual cycle, description of new genera and species. Zoologica Poloniae, 8, 3–34.
Kent, M. L., Andree, K. B., Bartholomew, J. L., El-Matbouli, M., Desser, S. S., Devlin, R. H., Feist, S. W., Hedrick, R. P., Hoffmann, R. W., Khattra, J., Hallett, S. L., Lester, R. J., Longshaw, M., Palenzeula, O., Siddall, M. E., & Xiao, C. (2001). Recent advances in our knowledge of the Myxozoa. Journal of Eukaryotic Microbiology, 48(4), 395–413. https://doi.org/https://doi.org/10.1111/j.1550-7408.2001.tb00173.x
Kent, M. L., Margolis, L., & Corliss, J. O. (1994). The demise of a class of protists - taxonomic and nomenclatural revisions proposed for the protist phylum Myxozoa Grasse, 1970. Canadian Journal of Zoology, 72(5), 932–937.
Lin, D., Hanson, L. A., & Pote, L. M. (1999). Small subunit ribosomal RNA sequence of Henneguya exilis (class Myxosporea) identifies the actinosporean stage from an oligochaete host. Journal of Eukaryotic Microbiology, 46(1), 66–68. https://doi.org/https://doi.org/10.1111/j.1550-7408.1999.tb04585.x
Lom, J., & Dyková, I. (2006). Myxozoan genera: definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitologica, 53(1), 1–36. https://doi.org/https://doi.org/10.14411/fp.2006.001
Marcucci, C., Caffara, M., & Goretti, E. (2009). Occurrence of actinosporean stages (Myxozoa) in the Nera River system (Umbria, central Italy). Parasitology Research, 105(6), 1517–1530. https://doi.org/https://doi.org/10.1007/s00436-009-1586-7
Marques, A. (1984). Contribution à la connaissance des Actinomyxidies: ultrastructure, cycle biologique, systématique. PhD thesis, Université des Sciences et Techniques de Languedoc, Montepellier, France.
McGeorge, J., Sommerville, C., & Wootten, R. (1997). Studies of actinosporean myxozoan stages parasitic in oligochaetes from the sediments of a hatchery where Atlantic salmon harbour Sphaerospora truttae infection. Diseases of Aquatic Organisms, 30(2), 107–119. https://doi.org/https://doi.org/10.3354/dao030107
Milanin, T., Atkinson, S. D., Silva, M. R. M., Alves, R. G., Maia, A. A. M., & Adriano, E. A. (2017). Occurrence of two novel actinospore types (Cnidaria: Myxosporea) in Brazilian fish farms, and the creation of a novel actinospore collective group, Seisactinomyxon. Acta Parasitologica, 62(1), 121–128. https://doi.org/https://doi.org/10.1515/ap-2017-0014
Milanin, T., Atkinson, S. D., Silva, M. R. M., Alves, R. G., Tavares, L. E. R., Ribeiro, A. M., & Maia, A. A. M. (2018). Occurrence of two novel actinospore types (Cnidaria: Myxozoa) in fish farms in Mato Grosso do Sul state, Brazil. Parasitology Research, 117(6), 1757–1764. https://doi.org/https://doi.org/10.1007/s00436-018-5856-0
Morris, D. J., & Freeman, M. A. (2010). Hyperparasitism has wide-ranging implications for studies on the invertebrate phase of myxosporean (Myxozoa) life cycles. International Journal for Parasitology, 40(3), 357–369. https://doi.org/https://doi.org/10.1016/j.ijpara.2009.08.014
Naville, A. (1930). Le cycle chromosomique d'une nouvelle Actinomyxidie: Guyenotia sphaerulosa n. gen.; n.sp. Quarterly Journal of Microscopical Science, 73, 545–575.
Negredo, C., Dillane, E., & Mulcahy, M. F. (2003). Small subunit ribosomal DNA characterization of an unidentified aurantiactinomyxon form and its oligochaete host Tubifex ignotus. Diseases of Aquatic Organisms, 54(3), 229–241. https://doi.org/https://doi.org/10.3354/dao054229
Negredo, C., & Mulcahy, M. F. (2001). Actinosporean infections in oligochaetes in a river system in southwest Ireland with descriptions of three new forms. Diseases of Aquatic Organisms, 46(1), 67–77. https://doi.org/https://doi.org/10.3354/dao046067
Oumouna, M., Hallett, S., Hoffmann, R., & El-Matbouli, M. (2003). Seasonal occurrence of actinosporeans (Myxozoa) and oligochaetes (Annelida) at a trout hatchery in Bavaria, Germany. Parasitology Research, 89(3), 170–184. https://doi.org/https://doi.org/10.1007/s00436-002-0683-7
Ormières, R. (1968). A propos de deux parasites d’oligochètes de Besse: Diaspora (Coccidiomorpha Doflein, 1901) et Aurantiactinomyxon (Actinomyxidia Štolc, 1899). Annales de la Station Biologique de Besse-en-Chandesse, 3, 185–191.
Özer, A., & Wootten, R. (2001). Release of actinosporean and myxosporean spores from their hosts, with special reference to both stages of Sphaerospora truttae (Myxozoa, Myxosporea). Acta Parasitologica, 46(2), 103–112. <Go to ISI>://WOS:000169005100004
Özer, A., & Wootten, R. (2002). Biological characteristics of some actinosporeans. Journal of Natural History, 36(18), 2199–2209. https://doi.org/https://doi.org/10.1080/00222930110089175
Özer, A., Wootten, R., & Shinn, A. P. (2002a). Survey of actinosporean types (Myxozoa) belonging to seven collective groups found in a freshwater salmon farm in Northern Scotland. Folia Parasitologica, 49(3), 189–210. https://doi.org/https://doi.org/10.14411/fp.2002.036
Özer, A., Wootten, R., & Shinn, A. P. (2002b). Infection prevalence, seasonality and host specificity of actinosporean types (Myxozoa) in an Atlantic salmon fish farm located in Northern Scotland. Folia Parasitologica, 49(4), 263–268. https://doi.org/https://doi.org/10.14411/fp.2002.050
Pote, L. M., Chenney, E. F., Lin, T. L., & Hackathorn, J. A. (1992). Experimental transmission of Proliferative Gill Disease (PGD) in channel catfish after exposure to actinosporea released by Dero sp. isolated from a pond during an outbreak of PGD. Proceedings of the 8th Southern Conference on Animal Parasites, Mississippi State, USA.
Pote, L. M., Hanson, L. A., & Shivaji, R. (2000). Small subunit ribosomal RNA sequences link the cause of Proliferative Gill Disease in channel catfish to Henneguya n. sp. (Myxozoa: Myxosporea). Journal of Aquatic Animal Health, 12(3), 230–240. https://doi.org/https://doi.org/10.1577/1548-8667(2000)012<0230:SSRRSL>2.0.CO;2
Pote, L. M., & Waterstrat, P. (1993). Communications: motile stage of Aurantiactinomyxon sp. (Actinosporea: Triactinomyxidae) isolated from Dero digitata found in channel catfish ponds during outbreaks of Proliferative Gill Disease. Journal of Aquatic Animal Health, 5(3), 213–218. https://doi.org/https://doi.org/10.1577/1548-8667(1993)005<0213:CMSOAS>2.3.CO;2
Rangel, L. F., Cech, G., Székely, C., & Santos, M. J. (2011). A new actinospore type Unicapsulactinomyxon (Myxozoa), infecting the marine polychaete, Diopatra neapolitana (Polychaeta: Onuphidae) in the Aveiro Estuary (Portugal). Parasitology, 138(6), 698–712. https://doi.org/https://doi.org/10.1017/s0031182011000163
Rocha, S., Alves, Â., Fernandes, P., Antunes, C., Azevedo, C., & Casal, G. (2019a). New actinosporean description prompts union of the raabeia and echinactinomyxon collective groups (Cnidaria, Myxozoa). Diseases of Aquatic Organisms, 135(3), 175–191. https://doi.org/https://doi.org/10.3354/dao03389
Rocha, S., Rangel, L. F., Castro, R., Severino, R., Azevedo, C., Santos, M. J., & Casal, G. (2019b). The potential role of the sphaeractinomyxon collective group (Cnidaria, Myxozoa) in the life cycle of mugiliform-infecting myxobolids, with the morphological and molecular description of three new types from the oligochaete Tubificoides insularis. Journal of Invertebrate Pathology, 160, 33–42. https://doi.org/10.1016/j.jip.2018.12.001
Rocha, S., Alves, Â., Antunes, C., Azevedo, C., & Casal, G. (2019c). Molecular data infers the involvement of a marine aurantiactinomyxon in the life cycle of the myxosporean parasite Paramyxidium giardi (Cnidaria, Myxozoa). Parasitology, 146(12), 1555–1563. https://doi.org/https://doi.org/10.1017/s0031182019000866
Rocha, S., Alves, Â., Antunes, C., Fernandes, P., Azevedo, C., & Casal, G. (2020). Characterisation of sphaeractinomyxon types (Cnidaria: Myxozoa) from marine and freshwater oligochaetes in a Portuguese estuary, with the demise of the endocapsa collective group. Folia Parasitologica, 67, 2020.002.https://doi.org/10.14411/fp.2020.002
Rosser, T. G., Griffin, M. J., Quiniou, S. M. A., Greenway, T. E., Khoo, L. H., Wise, D. J., & Pote, L. M. (2014). Molecular and morphological characterization of myxozoan actinospore types from a commercial catfish pond in the Mississippi delta. Journal of Parasitology, 100(6), 828–839. https://doi.org/https://doi.org/10.1645/13-446.1
Štolc, A. (1899). Actinomyxidies, nouveau groupe de Mesozoaires parent des Myxosporidies. Bulletin International de l’Académie des Sciences de Bohème, 12, 1–12.
Styer, E. L., Harrison, L. R., & Burtle, G. J. (1991). Communications: experimental production of Proliferative Gill Disease in channel catfish exposed to a myxozoan-infected oligochaete, Dero digitata. Journal of Aquatic Animal Health, 3, 288–291. https://doi.org/https://doi.org/10.1577/1548-8667(1991)003<0288:CEPOPG>2.3.CO;2
Styer, E. L., Harrison, L. R., & Burtle, G. J. (1992). Six new species of actinomyxids from Dero digitata. International Workshop on Myxosporea, October 6–8, 1992, České Budějovice, Czech Republic (abstract only).
Sun, H.-W., Xi, B.-W., & Xie, J. (2014). Morphological characters and DNA identification of a new actinosporean type Guyenotia CZ collected from oligochaete Branchiura sowerbyi. Acta Hydrobiologica Sinica, 38(6), 1179–1184. https://doi.org/https://doi.org/10.7541/2014.171
Székely, C., Avenant-Oldewage, A., & Molnár, K. (2004). Description of a new actinosporean type from South African freshwaters. Dieases of Aquatic Organisms, 61, 95–102. https://doi.org/https://doi.org/10.3354/dao061095
Székely, C., El-Mansy, A., Molnár, K., & Baska, F. (1998). Development of Thelohanellus hovorkai and Thelohanellus nikolskii (Myxosporea : Myxozoa) in oligochaete alternate hosts. Fish Pathology, 33(3), 107–114. https://doi.org/https://doi.org/10.3147/jsfp.33.107
Székely, C., Sitjà-Bobadilla, A., & Álvarez-Pellitero, P. (2000). First report on the occurrence of an actinosporean stage (Myxozoa) in oligochaetes from Spanish freshwaters. Acta Veterinaria Hungarica, 48(4), 433–441. https://doi.org/https://doi.org/10.1556/004.48.2000.4.6
Székely, C., Yokoyama, H., Urawa, S., Timm, T., & Ogawa, K. (2003). Description of two new actinosporean types from a brook of Fuji Mountain, Honshu, and from Chitose River, Hokkaido, Japan. Diseases of Aquatic Organisms, 53(2), 127–132. https://doi.org/https://doi.org/10.3354/dao053127
Trouillier, A., El-Matbouli, M., & Hoffmann, R. W. (1996). A new look at the life-cycle of Hoferellus carassii in the goldfish (Carassius auratus auratus) and its relation to "Kidney Enlargement Disease" (KED). Folia Parasitologica, 43, 173–187.
Wolf, K., & Markiw, M. E. (1984). Biology contravenes taxonomy in the Myxozoa: new discoveries show alternation of invertebrate and vertebrate hosts. Science, 225, 1449–1452.
Xi, B. W., Zhang, J. Y., Xie, J., Pan, L. K., Xu, P., & Ge, X. P. (2013). Three actinosporean types (Myxozoa) from the oligochaete Branchiura sowerbyi in China. Parasitology Research, 112(4), 1575–1582. https://doi.org/https://doi.org/10.1007/s00436-013-3306-6
Xi, B. W., Zhou, Z. G., Xie, J., Pan, L. K., Yang, Y. L., & Ge, X. P. (2015). Morphological and molecular characterization of actinosporeans infecting oligochaete Branchiura sowerbyi from Chinese carp ponds. Diseases of Aquatic Organisms, 114(3), 217–228. https://doi.org/https://doi.org/10.3354/dao02859
Xiao, C. X., & Desser, S. S. (1998). Actinosporean stages of myxozoan parasites of oligochaetes from Lake Sasajewun, Algonquin Park, Ontario: New forms of echinactinomyxon, neoactinomyxum, aurantiactinomyxon, guyenotia, synactinomyxon and antonactinomyxon. Journal of Parasitology, 84(5), 1010–1019. https://doi.org/https://doi.org/10.2307/3284635
Yokoyama, H. (1997). Transmission of Thelohanellus hovorkai Achmerov, 1960 (Myxosporea: Myxozoa) to common carp Cyprinus carpio through the alternate oligochaete host. Systematic Parasitology, 36(2), 79–84. https://doi.org/https://doi.org/10.1023/a:1005752913780
Yokoyama, H., Ogawa, K., & Wakabayashi, H. (1993). Involvement of Branchiura sowerbyi (Oligochaeta, Annelida) in the transmission of Hoferellus carassii (Myxosporea, Myxozoa), the causative agent of kidney enlargement disease (KED) of goldfish Carassius auratus. Fish Pathology, 28, 135–139. https://doi.org/https://doi.org/10.3147/jsfp.28.135
Zhao, D., Borkhanuddin, M. H., Wang, W., Liu, Y., Cech, G., Zhai, Y., & Székely, C. (2016). The life cycle of Thelohanellus kitauei (Myxozoa: Myxosporea) infecting common carp (Cyprinus carpio) involves aurantiactinomyxon in Branchiura sowerbyi. Parasitology Research, 115(11), 4317–4325. https://doi.org/https://doi.org/10.1007/s00436-016-5215-y
Zhao, D. D., Zhai, Y. H., Liu, Y., Wang, S. J., & Gu, Z. M. (2017). Involvement of aurantiactinomyxon in the life cycle of Thelohanellus testudineus (Cnidaria: Myxosporea) from allogynogenetic gibel carp Carassius auratus gibelio, with morphological, ultrastructural, and molecular analysis. Parasitology Research, 116(9), 2449–2456. https://doi.org/https://doi.org/10.1007/s00436-017-5547-2
Acknowledgments
The author wishes to acknowledge Doctor Stephen Atkinson for his suggestions. This research was funded by national funds through Foundation for Science and Technology (FCT), within the scope of the project PTDC/BIA-BMA/6363/2020, and the FCT employment contract 2022.06670.CEECIND.
Funding
Open access funding provided by FCT|FCCN (b-on).
Author information
Authors and Affiliations
Contributions
SR performed all literature review and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rocha, S. Synopsis of the aurantiactinomyxon collective group (Cnidaria, Myxozoa), with a discussion on the validity of morphotype definition and demise of guyenotia. Syst Parasitol 100, 307–323 (2023). https://doi.org/10.1007/s11230-023-10089-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10089-1