Abstract
The genus Austraplectana Baker, 1981 is a poorly known group of cosmocercoid nematodes. In the present study, a new species of Austraplectana, A. extranes sp. n., was described using both light and scanning electron microscopy, based on specimens collected from the carbine barred frog Mixophyes carbinensis Mahony, Donnellan, Richards & McDonald (Anura: Myobatrachidae) and the cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae) in Australia. Austraplectana extranes sp. n. can be easily distinguished from its congener by the much longer and different morphology of tails in both sexes, the different number and arrangement of caudal papillae, the presence of single precloacal median heart-shaped papilla and large posterior protuberance with cuticular comb-like fringe in male.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baker (1981) established the genus Austraplectana, currently including only the type species A. kartanum (Johnston & Mawson, 1941), which parasitizes some native frogs in Australia, including the Jervis Bay tree frog Litoria jervisiensis (Duméril & Bibron) (Anura: Hylidae), the striped rocket frog L. nasuta (Gray) (Anura: Hylidae), the motorbike frog Ranoidea moorei (Copland) (Anura: Hylidae) and the moaning frog Heleioporus eyrei (Gray) (Anura: Limnodynastidae) (Johnston & Mawson, 1941; Inglis, 1968; Baker, 1981).
During a helminthological survey in Australian amphibians, some nematode parasites collected from the carbine barred frog Mixophyes carbinensis Mahony, Donnellan, Richards & McDonald (Anura: Myobatrachidae) and the cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae), were identified as a new species of Austraplectana. The detailed morphology of the new species was studied using light and scanning electron microscopy.
Materials and methods
Host collection
One M. carbinensis and 67 R. marina collected from Mt Lewis (-16.580997, 145.334851), Bloomfield (-15.921538, 145.351538), Cape Tribulation (-16.088319, 145.460714) and Paluma (-19.006527, 146.189245), northern Queensland, Australia, were dissected for parasites. All hosts were collected by hand at night and placed into either a collecting bucket or moist herpetological bag and transported to the laboratory. Hosts were euthanised by an overdose of pentobarbitone sodium solution (Lethabarb Euthanasia injection, Virbac, Australia).
Light and scanning electron microscopy
Nematodes were isolated from the intestine of hosts. Specimens were fixed and stored in room temperature 70% ethanol until study. For light microscopy studies, nematodes were cleared in lactophenol consisted of phenol, lactic acid and glycerol according to the volumic ratio 1: 1: 2. For scanning electron microscopy (SEM), specimens were re-fixed in 4% formaldehyde solution, post-fixed in 1% OsO4, dehydrated via an ethanol series and acetone, and then critical point dried. Samples were coated with gold and examined using a Hitachi S-4800 scanning electron microscope at an accelerating voltage of 20 kV. Measurements (the range, followed by the mean in parentheses) are given in micrometers (μm) unless otherwise stated. Type specimens were deposited in College of Life Sciences, Hebei Normal University, Hebei Province, China.
Results
Suborder Ascaridina Inglis, 1983
Superfamily Cosmocercoidea Railliet, 1916
Family Cosmocercidae Railliet, 1916
Subfamily Austraplectaninae Baker, 1981
Genus Austraplectana Baker, 1981
Austraplectana extranes sp. n. (Figs. 1–3)
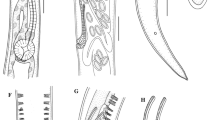
Scanning electron micrographs of Austraplectana extranes sp. n. collected from the cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae) in Australia. A, cephalic end of female (white arrows showing inner flanges, black arrows showing outer cephalic papillae), apical view; B, magnified image of cloacal region of male, ventral view; C, magnified image of somatic papillae and the beginning of lateral ala (white arrows showing lateral ala) of female; D, magnified image of tail tip of male, ventral view; E, posterior end of female (white arrows showing lateral ala), lateral view; F, magnified image of precloacal pedunculate papilla of male. Abbreviations: D, dorsal lip; S, subventral lip.
Small-sized, whitish nematodes. Body cylindrical, maximum width at about region of mid-body. Oral aperture simple, somewhat triangular, surrounded by 3 small lips, each with remarkable inner flanges (Figs. 1A, 2A). Dorsal lip with 2 double labial papillae each possessing 1 pair of small aerial-like protrusions; subventral lips each with 1 double labial papilla also possessing 1 pair of small aerial-like protrusions and large amphid (Figs. 1A, 2A). Eight large outer cephalic papillae present at base of lips (Figs. 1A, 2A). Cuticle with fine transverse striations (Fig. 1C). Somatic papillae present in both sexes (Figs. 1C, 2F). Lateral alae well developed, extending from some distance posterior to base of lips to anterior of cloaca (Figs. 1C, 2C, 3C, D). Oesophagus divided into anterior short pharynx, cylindrical corpus, slightly narrow isthmus and terminating posterior bulb with valves (Fig. 3A). Nerve ring located at about 1/2 of oesophageal length (Fig. 3A). Excretory pore situated at about middle level between nerve ring and posterior oesophageal bulb (Fig. 3A). Tail of both sexes conical, with long filamentous tip (Figs. 1D, E, 2C, 3C-E).
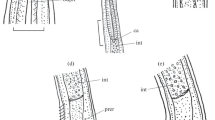
Scanning electron micrographs of Austraplectana extranes sp. n. collected from the cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae) in Australia. A, cephalic end of male (white arrows showing amphids), apical view; B, magnified image of cloacal region of male, ventral view; C, posterior end of male, ventral view; D, magnified image of paracloacal pedunculate papilla; E, magnified image of precloacal pedunculate papilla; F, somatic papillae and lateral alae (arrowed) of male. Abbreviations: cp, double labial papillae; D, dorsal lip; S, subventral lip.
Austraplectana extranes sp. n. collected from the cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae) in Australia. A, anterior part of male, lateral view; B, region of vulva, lateral view; C, posterior end of male, ventral view; D, posterior end of male, lateral view; E, posterior end of female, lateral view; F, spicules, ventral view; G, egg.
Male (based on 10 mature specimens): Body 1.90–3.90 (2.78) mm long; maximum width 140–440 (268). Oesophagus 386–546 (492) long in total length, representing 13.6–23.1 (18.3) % of body length; pharynx + corpus + isthmus 314–435 (398) long, size of posterior bulb 72–111 (93) × 82–121 (99). Nerve ring 163–275 (225) and excretory pore 290–386 (327) from cephalic extremity, respectively. Spicules short and robust with remarkable alae, equal in length, distal end pointed, 138–213 (178) long, representing 4.48–9.86 (6.69) % of body length (Fig. 3F). Expansion of cuticle in posterior end of body supported by 5 pairs of sub-lateral pedunculate papillae (2 pairs precloacal, 3 pairs postcloacal) (Fig. 3C); 8 pairs of additional precloacal ventral pedunculate papillae, 2 pairs of adcloacal ventral pedunculate papillae and 1 pair of postcloacal ventral pedunculate papillae present (postcloacal sessile papillae indistinguishable from somatic papillae) (Figs. 1B, D, F, 2B, D, E, 3C, D). Single median precloacal heart-shaped papilla and large posterior protuberance with cuticular comb-like fringe present (Figs. 1B, 2B, 3C). Tail 280–500 (381) long, representing 7.69–22.8 (14.5) % body length, long filamentous tip with numerous somatic papillae (Figs. 1D, 2C, 3C, D).
Female (based on 10 mature specimens): Body 2.93–4.15 (3.66) mm long; maximum width 300–520 (410). Oesophagus 507–628 (579) long in total length, representing 13.8–18.6 (16.0) % of body length; pharynx + corpus + isthmus 411–507 (465) long, size of posterior bulb 97–121 (114) ×116–135 (128). Nerve ring 213–350 (280) and excretory pore 338–449 (402) from anterior extremity, respectively. Vulva transverse slit, vulval lips not protruded, 1.10–1.80 (1.51) mm from anterior extremity, at 35.5–43.9 (41.0) % of body length (Fig. 3B). Vagina muscular, uteri didelphic, opistodelphic. Eggs oval, with smooth surface, 58–70 (65) × 40–53 (47) (n=20) (Fig. 3G). Tail 600–900 (757) long, representing 18.5–24.6 (20.8) % body length (Figs. 1E, 3E).
Taxonomic summary
Type host: Carbine barred frog Mixophyes carbinensis Mahony, Donnellan, Richards & McDonald (Anura: Myobatrachidae).
Type locality: Mt Lewis (-16.580997, 145.334851), northern Queensland, Australia.
Other host and localities: Cane toad Rhinella marina (Linnaeus) (Anura: Bufonidae), collected from Bloomfield (-15.921538, 145.351538), Cape Tribulation (-16.088319, 145.460714), Paluma (-19.006527, 146.189245), all in northern Queensland, Australia.
Site of infection. Rectum.
Prevalence and intensity of infections: Single Mixophyes carbinensis examined and infected with 26 nematodes; 43.3% (29 out of 67 Rhinella marina) infected with intensity of 3–100 (mean 27.1) nematodes.
Type specimens. Holotype, male (HBNU–N-2022A0830-1L-N); allotype, female (HBNU–N-2022A0830-2L-N); paratypes: 30 males, 50 females (HBNU–N-2022A0830-3L-N).
Etymology: The specific epithet is derived from the Latin word extrane-, referring to the strange characteristics of the new species.
Discussion
With remarkable lateral alae and somatic papillae in both sexes, well developed pedunculate papillae in the male and the didelphic uteri containing eggs of normal size (less than 100 μm in diameter) in the female, the morphological characters of our present specimens agreed well with the monotypic genus Austraplectana Baker, 1981. Austraplectana extranes sp. n. can be easily distinguished from A. kartanum by the size and morphology of tails in both sexes (male tail 0.28–0.50 mm, female tail 0.60–0.90 mm, tail with long filamentous tip in the new species vs male tail 0.13–0.17 mm, female tail 0.27–0.33 mm, tail without long filamentous tip in the latter) and the presence of single median precloacal heart-shaped papilla and large posterior protuberance with cuticular comb-like fringe in male (vs precloacal papilla and large posterior protuberance absent in A. kartanum).
The genus Austraplectana is a poorly known group of cosmocercoid nematodes, which has received little attention since its inception. The cephalic structures are very important characters for species identification and delimitation of genera in zooparasitic nematodes. However, the detailed morphology of cephalic structures of most of cosmocercoid species have not been adequately described (Ni et al., 2022). The present study clearly showed the particular cephalic structures of A. extranes sp. n. using SEM, including each double cephalic papillae with 1 pair of aerial-like protrusions and the presence of large outer papillae at base of each lip. Moreover, the new species shared some characters (i.e., tail with long filamentous tip, cloacal region of male with single median precloacal papilla and large posterior protuberance with cuticular comblike fringe) with some species of the genera Cosmocera, Oxysomatium and Raillietnema in the family Cosmocercidae, for example, the tail of Cosmocera spp. commonly possessing long filamentous tip (Sou & Nandi, 2015; Chen et al., 2020), the cloacal region of male of Oxysomatium spp. and Raillietnema spp. usually with single median precloacal papilla (ornament) and large posterior protuberance with cuticular comb-like fringe (Baker, 1980, 1982; Felix-Nascimento et al., 2020). However, the morphology of caudal papillae is the very important character for differentiating different genera in the Cosmocercidae, in which only the genus Austraplectana has well developed pedunculate papillae supported the expansion of cuticle in posterior end of male. Consequently, we considered that it is more reasonable to assign the present species in Austraplectana. A molecular phylogenetic analysis is needed to further clarify the systematic position of A. extranes sp. n. in the future.
According to the previous studies (Johnston & Mawson, 1941; Inglis, 1968; Baker, 1981), the genus Austraplectana seems to be endemic to Australia. In the present study, the new species was found in both Australian native frog M. carbinensis and the exotic cane toad R. marina in northern Queensland, Australia. The life cycle of Austraplectana species has not been studied. Anderson (1992) indicated that the amphibian host becomes infected the third-stage larvae of cosmocercoid nematodes by either orally ingestion or by skin penetration. We speculated that the cane toad became infected with A. extranes sp. n. since R. marina has been introduced to Australia by sharing the same habitat or similarity in diet with the native frog M. carbinensis.
References
Anderson, R. C. (1992). Nematode Parasites of Vertebrates. Their Development and Transmission. CAB International, Wallingford, Oxon. pp. 578.
Baker, M.R. (1980). A revision of the genus Oxysomatium Railliet & Henry, 1916 (Nematoda, Cosmocercidae). Bulletin du Muséum National d’Histoire Naturelle, 2, 707–718.
Baker, M.R. (1981). Austraplectana n. gen. (Cosmocercidae, Austraplectaninae n. sous-fam.), Nematode parasite d’Amphibiens australiens. Bulletin du Muséum National d’Histoire Naturelle, 3, 111–116.
Baker, M.R. (1982). Systematic relationships of the Atractidae and Cosmocercidae (Nematoda: Cosmocercoidea): two new atractids parasitic in amphibians and fish. Canadian Journal of Zoology, 60, 2395–2402.
Chen, H.-X., Zhang, L.-P., Feng, Y.-Y., & Li, L. (2020). Integrated evidence reveals a new species of Cosmocerca (Ascaridomorpha: Cosmocercoidea) from the Asiatic toad Bufo gargarizans Cantor (Amphibia: Anura). Parasitology Research, 119, 1795–1802.
Felix-Nascimento, G., Vieira, F. M., Muniz-Pereira, L.C., Moura, G.J.B.D., Ribeiro, L.B., & Oliveira, J.B. (2020). Two new species of Cosmocercidae (Nematoda: Cosmocercoidea) of Leptodactylus macrosternum Miranda-Ribeiro (Anura: Leptodactylidae) from Caatinga Biome, Brazil. Zootaxa, 4877, 274–290.
Inglis, W.G. (1968). Nematodes parasitic in western Australian frogs. Bulletin of the British Museum (National History), Zoology 16, 161–183.
Johnston, T.H., & Mawson, P.M. (1941). Some nematodes from Kangaroo Island, South Australia. Records of the South Australian Museum, 7, 146–148.
Ni, X.-F., Chen, H.-X., Xu, Z., Gu, X.-H., & Li L. (2022). Morphology, genetic characterization and molecular phylogeny of the poorly known nematode parasite Cissophyllus leytensis Tubangui & Villaamil, 1933 (Nematoda: Ascaridida) from the Philippine sailfin lizard Hydrosaurus pustulatus (Eschscholtz, 1829) (Reptilia: Squamata). Parasites & Vectors, 15, e116.
Sou, S.K., & Nandi, A.P. (2015). On a new species of Cosmocerca (Nematoda; Cosmocercidae) from Microhyla rubra (Anura: Microhylidae) from West Bengal, India. Acta Parasitologica, 60, 261–265.
Acknowledgements
The authors are grateful to many people who helped to collect amphibians for this study, especially Dr. D. Blair (James Cook University, Townsville, Australia), Dr. S. Richards (James Cook University, Townsville, Australia) and Dr. H. Spencer (Cape Tribulation).
Funding
This study was supported by the National Natural Science Foundation of China (No. 32170442), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB26000000). This study was also supported by the Australian Postgraduate Research Award during the time of collection of the parasite specimens for Dr. Diane P. Barton.
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ni, XF., Barton, D.P. & Li, L. Austraplectana extranes sp. n. (Nematoda: Cosmocercoidea) from Australian amphibians Mixophyes carbinensis Mahony, Donnellan, Richards & McDonald (Anura: Myobatrachidae) and Rhinella marina (Linnaeus) (Anura: Bufonidae). Syst Parasitol 100, 183–188 (2023). https://doi.org/10.1007/s11230-022-10080-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-022-10080-2