Abstract
A new clausidiid copepod was found associated with the ghost shrimp Neocallichirus jousseaumei (Nobili) in the Persian Gulf, on the Iranian coast. The new species shares the armature formula of swimming legs 2 to 4 with C. persiaensis, but can be easily distinguished from its congeners by unique characteristics of the females: the prominent spine on endopodal segment 1 of the antenna, the armature of the maxilliped, and the elongated basis of the swimming legs. Distinguishing features observed in males include the distinct projections on the maxilliped and the armature of legs 1 and 4. In addition to traditional light microscopy-based descriptions, confocal laser scanning microscopy (CLSM) was used to obtain high resolution images and 3-D reconstructions of entire copepods. Structures of taxonomic importance that exhibit complex shapes (male maxilliped and female urosome) were scanned to generate 3-D prints that gave valuable insights about female/male interlocking mechanisms. The taxonomic status and host specificity of Clausidium spp. are discussed and a key to valid species is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Copepods are one of the most abundant metazoans on Earth. Copepods of the family Clausidiidae Embleton, 1901 represent an early offshoot of the poecilostome lineage within the order Cyclopoida Burmeister, 1834 (see Khodami et al., 2017). Most clausidiids live in loose association with marine invertebrate hosts (Huys & Boxshall, 1991) and species of Clausidium Kossmann, 1874 are recorded exclusively living in association with ghost shrimps (Boxshall & Halsey, 2004). Although it has been suggested that members of Clausidium are parasitic on their host organisms (Wilson, 1935; Pillai, 1959), this has yet to be demonstrated quantitatively. There is very little available information on the behavior of these copepods or their interactions with their host, or with the environment. Although Clausidium spp. are relatively rarely recorded because of the cryptic lifestyle of their hosts, a total of 17 species of Clausidium has been described to date (Walter & Boxshall, 2018; Sepahvand et al., 2017a, b). In the present study, we describe a new species associated with the ghost shrimp Neocallichirus jousseaumei (Nobili) from Iranian coastal waters of the Persian Gulf and remarks are provided on microhabitat selection.
Materials and methods
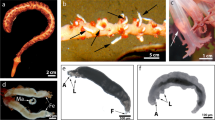
All specimens examined were sampled from a single location north of Ouli village (27°50′14.62′′N, 51°53′24.85′′E), in Bushehr Province, Iran. Hosts were collected by digging in the substrate during low tide. The copepods were relaxed with drops of 1.5% menthol added to the sea water and were separated from the host by filtration through a 63 µm mesh net. The specimens were transferred to 75% ethanol. Observations of living specimens showed that the copepods were found attached to carapace and chelipeds of the host Neocallichirus jousseaumei (Fig. 1A–C).
The habitus was drawn from whole specimens mounted temporarily on slides in glycerin, and adhesive plastic discs were used to support the coverslip (Kihara & Rocha, 2009). Specimens were dissected under a Leica MZ12 stereomicroscope (Leica, Wetzlar, Germany). Dissected parts were mounted on slides using glycerin as the mounting medium, and preparations were sealed with transparent nail varnish. The material was studied with a Leica DMR differential interference contrast microscope (Leica, Wetzlar, Germany) equipped with a drawing tube.
For confocal laser scanning microscopy (CLSM), selected material was stained overnight in a 1:1 solution of Congo Red and Acid Fuchsin. Whole specimens and dissected parts were mounted on slides in glycerin following the procedure described by Michels & Büntzow (2010). Certain parts of the body of special interest, such as the anal somite in both sexes and the male maxilliped, but which are difficult to position due to their 3-dimensional shape were mounted in Karo® light corn syrup on slides.
The material was scanned using a Leica TCS SP5 (Leica, Wetzlar, Germany) equipped with a Leica DM5000 B upright microscope (Leica, Wetzlar, Germany) and 3 visible-light lasers (DPSS 10 mW 561 nm; HeNe 10 mW 633 nm; Ar 100 mW 458 nm, 476 nm, 488 nm and 514 nm), combined with the software LAS AF 2.2.1. - Leica Application Suite Advanced Fluorescence (Leica, Wetzlar, Germany).
Series of stacks were obtained, collecting overlapping optical sections throughout the whole preparation; the imaging settings were according to the software. Final images were obtained by maximum projection, and CLSM illustrations were composed and adjusted for contrast and brightness using the software Adobe Photoshop CS4 (Adobe Systems, San José, USA).
Total body length was measured from the anterior margin of the rostrum to the posterior margin of the caudal rami. The descriptive terminology follows Huys et al. (1996). Abbreviations used in the text are: ae, aesthetasc; P1-P6, legs 1–6; exp, exopod; enp, endopod; exp (enp)-1 (-2, -3), proximal (middle, distal) segments of a ramus.
The type-material is deposited in the collection of the Forschungsinstitut Senckenberg, Frankfurt, Germany.
Order Cyclopoida Burmeister, 1834
Family Clausidiidae Embleton, 1901
Genus Clausidium Kossmann, 1874
Clausidium iranensis n. sp.
Type-host: Neocallichirus jousseaumei Nobili (Axiidea: Callianassidea).
Type-locality: North of Ouli village (27°50′14.62′′N, 51°53′24.85′′E), Bushehr Province, Iran.
Type-material: Holotype female dissected on 23 slides (reg. nos SMF 37191/1-23). Dissected paratypes consist of 2 females (reg. nos SMF 37192/1-20–37193/1-20), 3 males (reg. nos SMF 37194, SMF 37195 and SMF37196) and 2 couples (reg. nos SMF 37197 and SMF 37198). Undissected paratypes comprise 9 females, 3 males and 21 couples (reg. no. SMF 37199) deposited in the collection of the Forschungsinstitut Senckenberg, Frankfurt, Germany. All material was collected from the type-locality by V. Sepahvand.
Site on host: All specimens collected from the mud shrimps usually on the surface of the carapace and chelipeds, but a single specimen was found on an abdominal tergite.
Etymology: The species name iranensis refers to the provenance of the type-material.
Description (Figs. 1–12)
Female [Based on 5 specimens; Figs. 2–6,10A, B, 11, 12A–E).] Total length, excluding caudal setae, 1,192–1,487 μm (n = 20; mean 1,400 μm). Body broadly rounded (Figs. 2A,10A, B, 11A, B), dorsoventrally compressed. Prosome (Figs. 2A,10A, B, 11A, B) longer than urosome (2.6:1). Maximum width measured at posterior margin of dorsal cephalothoracic shield. First pedigerous somite fused with cephalosome. Prosomites ornamented with minute integumental pits, sensilla and numerous pores distributed as illustrated in Figs. 2A,10A, B,11A, B. Epimera of third and fourth pedigerous somites expanded posteriorly. Posterior margin of fourth pedigerous somite slightly concave medially. Urosome (Fig. 3B, C) 3-segmented, distinctly narrower than prosome; comprising fifth pedigerous somite, genital double-somite, and anal somite. Somite bearing P5 (Figs. 10B,11B) fused to genital complex ventrally but with distinct suture dorsally; twice as wide as long in ventral view and with P5 arising ventrolaterally. Genital double-somite (Fig. 3B, C) as long as wide, with dorsal and lateral cuticular sutures marking plane of fusion between genital and first abdominal somites. Genital apertures located dorsolaterally on each side (Fig. 3B). Egg-sacs originating at dorsolateral aperture on each side and extending to middle of anal somite. Anal somite (Figs. 3B, C, 10A, B,11B, C,12D–E) probably incorporating second to fourth abdominal somites; almost quadrate, incised medially, with intricate folds dorsally and along lateral margins, outer corners with convoluted 3-D shape as illustrated in Fig. 12D, E. Caudal ramus (Figs. 2B, C,3B, C) about 4.0 times longer than wide, and armed with 6 setae. Seta I absent, setae II and III pinnate; setae IV and V strongly developed and geniculate (seta V 1.6 times longer than seta IV); seta VI small; seta VII located at inner posterior corner, both naked. Caudal ramus with rounded lappet on posterior margin of ventral surface covering basal portion of setae II-VI (Fig. 2B). Rostrum (Figs. 10B,11B) rectangular incorporated into cephalothorax, demarcated by sclerotised areas laterally.
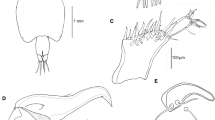
Clausidium iranensis n. sp. Confocal laser scanning microscopy maximum projections. Female: A, Antenna and oral region, ventral view; B, P1, anterior; C, P2-P3, anterior; D, Anal somite, dorsal; E, Detail of outer distal corner of anal somite, dorsal. Male: F, Antenna and oral region, ventral view; G, P1, anterior; H, P2-P3, anterior; I, Maxilliped, ventral view. Scale-bars: A–D, F, 25 µm; E, 7.5 µm; G–I, 10 µm
Antennule (Fig. 3A) 7-segmented; segment 2 longest, with long seta inserted on posterior distal corner and reaching tip of segment 5 (arrowed in Fig. 3A). Aesthetascs inconspicuous, very similar to other setae but with blunt tip. Segments 5 and 6 with aesthetasc fused basally to seta. Segment 7 with apical acrothek consisting of aesthetasc and 2 setae. Armature formula: 1-[5], 2-[15], 3-[6], 4-[4], 5-[6 + ae], 6-[2 + ae], 7-[7 + ae]. Antenna (Figs. 4B, 12A) uniramous, 4-segmented. Coxobasis elongate, ornamented with irregular row of spinules along inner margin and armed with single seta naked at inner distal corner. Endopod 3-segmented; segment 1 swollen, with stout pectinate spine on inner margin; segment 2 with row of long denticles along inner margin, and armed with 2 pectinate spines and 2 setae (1 pinnate and 1 naked); segment 3 with spinules on outer margin, and armed with 6 apical setae (2 pectinate, 3 pinnate and 1 naked). Labrum (Fig. 4A) twice as wide as long; free posterior margin and lateral projections ornamented with row of long spinules.
Mandible (Fig. 4C) well developed, armed with 3 distal elements; 1 toothed process, 1 spinulose seta, and 1 flat structure ornamented with long spinules. Maxillule (Fig. 4D) weakly bilobed at apex; proximal margin with large pore, and row of spinules, plus 1 lateral seta; outer lobe with 4 setae (3 pinnate and 1 naked); inner lobe with row of minute spinules along inner margin and 3 pinnate setae. Maxilla (Fig. 4E) 2-segmented: syncoxa twice as long as wide, with 2 pinnate setae and 1 pinnate spine; basis with large pectinate process, bearing 3 setae (2 pinnate and 1 naked) and 1 pinnate spine. Maxilliped (Fig. 4F) 4-segmented; syncoxa with 2 pinnate setae on inner margin. Basis with 1 naked and 1 pinnate inner setae. Endopod 2-segmented; first segment unarmed; second armed with 2 naked lateral setae, 1 naked distal seta and 3 stout distal spines with long spinules and projections (Fig. 4F).
P1 (Figs. 5A, 12B) biramous, both rami 3-segmented and highly modified as prehensile limb. Coxa and basis fused, forming well-developed segment with naked seta on outer corner near exopod origin; large blade-like seta on inner distal corner, about 3 times longer than wide, with indented apex and ornamented with concentric striations. Exopod slender; exp-1 with 1 outer seta; exp-2 with 1 reduced outer seta; exp-3 with 3 outer setae (proximal and distal ones reduced), 2 apical setae and 2 inner pinnate setae: rows of minute denticles present around insertion of outer elements. Endopod highly modified, tapering distally: enp-1 with stout curved process with corrugated areas along distal margin (marked with square in Fig. 5A); enp-2 with a minute seta (marked with asterisk in Fig. 5A); enp-3 elongated, irregular segment armed with large proximal and small distal sucking discs, and terminating in lobe with 2 setae (1 pinnate and 1 naked); proximal sucking disc 1.6 times larger than distal.
P2-P4 (Figs. 5C, D, 6A, 12C) biramous, with 3-segmented rami. Coxa of P2-4 with pinnate inner seta; basis of P2-P4 extremely elongate (about as long as P2 or longer than P3-P4 exopod); each with pinnate seta on outer distal corner and row of slender setules along inner margin. Exp-1 and exp-2 with setules on inner margin and spinules (exp-1) or denticles (exp-2) on outer margin; exp-3 with denticles along outer margin; outer spines on exopod serrate and each with terminal flagellum; apical spine with serrate outer margin and pinnate inner margin. Enp-1 and enp-2 with setules along outer margin; enp-2 with acute projection at outer distal corner and transverse row of minute spinules near distal margin: outer and apical spines serrate and with terminal flagellum; small, weakly developed sucking discs (Fig. 12C) present at outer distal corner of enp-1 and proximal and distal outer corners of enp-3.
Armature formula of P2-P4 (Figs. 5C, D,6A, 12C) as follows (Roman numerals representing spines, Arabic numerals representing setae):
Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
P2 | 0-1 | 1-0 | I-0; I-1; III, I, 4 | 0-1; 0-2; I, II, 3 |
P3 | 0-1 | 1-0 | I-0; I-1; III, I, 4 | 0-1; 0-2; I, II, 3 |
P4 | 0-1 | 1-0 | I-0; I-1; III, I, 5 | 0-1; 0-2; I, II, 2 |
P5 (Fig. 6B) uniramous, 2-segmented and located laterally on somite. Protopod with 1 outer seta; free exopodal segment elongate with 3 serrate spines along outer margin and 1 serrate spine apically, spine I (arrowed in figure 6B) inserted near spine II, on distal third of segment. P6 (Fig. 3B) consisting of 2 setae.
Male [Based on 5 specimens; Figs. 7–9, 10C, D, 11, 12F–I.] Total length, excluding caudal setae, 500–666 μm (n = 15; mean 606 μm). Body cyclopiform (Figs. 7A, 10C, D, 11). Prosome (Figs. 7A, 10C, D, 11A, B) 2.7 times longer than urosome (maximum width measured at midlength of dorsal cephalothoracic shield). First pedigerous somite fused with cephalosome. Prosomites ornamented with minute integumental pits, sensilla and numerous pores distributed as illustrated in Figs. 7A, 10C, D, 11A, B. Cephalosome and 3 free prosomites with smooth posterior borders; somites bearing P2-P3 subequal; somite bearing P4 with distal margin concave medially. Urosome (Fig. 8A, B) 6-segmented, distinctly narrower than prosome. Somite bearing P5 about 1.9 times wider than long in ventral view and carrying P5 ventrolaterally. Ornamentation of pores and sensilla as illustrated in Fig. 8A, B. Third abdominal somite medially incised dorsally. Anal somite (Fig. 8A, B) extremely reduced and deeply incised medially. Caudal ramus (Fig. 7A, B) as in female.
Antennule, mandible, maxillule and maxilla resembling those of female. Antenna (Figs. 8C,12F) uniramous, 4-segmented. Coxobasis elongate, with spinules along inner margin, and with pinnate seta at inner distal corner. Endopod 3-segmented; segment 1 with pinnate spine inserted near middle of inner margin; segment 2 with row of denticles plus 2 naked setae, 1 pinnate spine and 1 pectinate spine; segment 3 offset, armed with 5 pinnate setae and 1 pinnate spine.
Maxilliped (Figs. 8D, 11C, D, 12F, I) well developed, strongly sexually dimorphic, modified as chelate grasping limb. Syncoxa with 2 pinnate setae. Basis with 1 naked seta, membranous projection with seta (marked with asterisk in Fig. 8D), plus unequal, curved, irregularly denticulate projections opposing tip of chela. Endopod 1-segmented forming chela comprising irregularly serrate claw armed with small seta proximally (marked with square in Fig. 8D).
P1 (Figs. 9A, B, 12G) similar to female. Coxa and basis fused with row of long spinules along proximal margin, 1 naked seta on outer corner and 1 pinnate seta on inner distal edge. Exopod with row of minute denticles around insertion of each outer element: exp-1 with outer seta; exp-2 with 1 reduced outer seta; exp-3 with 3 setae (proximal one reduced) on outer margin, 1 serrate recurved spine and 1 pinnate seta apically, and 2 pinnate setae on inner margin. Enp-1 with corrugated fringe along distal margin plus stout curved process and 1 long pinnate seta at inner distal corner; enp-2 and enp-3 armed with sucking discs as in female.
P2-P4 (Fig. 9C, D) with coxae larger and basis less elongate compared with female. Armature formula of P2-P4 as follows:
Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
P2 | 0-1 | 1-0 | I-0; I-1; III, I, 4 | 0-1; 0-1; I, II, 3 |
P3 | 0-1 | 1-0 | I-0; I-1; III, I, 4 | 0-1; 0-1; I, II, 3 |
P4 | 0-1 | 1-0 | I-0; I-1; III, I, 4 | 0-1; 0-1; I, II, 2 |
P5 (Fig. 8A) not as curved as in female. Protopodal segment with long outer seta; free exopodal segment elongate with 2 serrate spines and naked seta on outer margin and 1 serrate spine apically: spine I inserted near spine II, on distal third of segment. P6 (Fig. 8A, B) represented by membranous flaps and armed with pinnate seta.
Remarks
The genus Clausidium was established by Kossmann in 1874 although the first two species currently classified within this genus were initially placed in other genera: Clausidium caudatum (Say, 1818), was originally placed in the genus Binoculus Geoffroy St. Hilaire, 1762 which was suppressed by the ICZN and placed on the Official List of Rejected Names (Opinion 502), and C. apodiforme (Philippi, 1839) which was initially placed in a new genus named Hersilia by Philippi (1839). However, Hersilia Philippi, 1839 was doubly preoccupied, by Hersilia Savigny, 1826 (Arachnida) and by Hersilia Dejean, 1835 (Coleoptera). Clausidium is the oldest available replacement name. There are 17 nominal species in Clausidium (see Walter & Boxshall, 2018) but, several of these are inadequately described by modern standards. The original description of C. apodiforme (as Hersilia apodiformis) was extremely basic and lacking in detail essential for modern systematics. Say’s (1818) description of C. caudatum was similarly inadequate and even Pearse’s redescription (1947) of this species provided no information on the mouthparts and swimming legs 2-4. Wilson (1932) ignored the mouthparts and legs 1-5 in his brief description of C. dissimile based on a specimen from the branchial chamber of Gilvossius setimanus Dekay (as Callianassa stimpsoni).
Clausidium testudo Kossmann, 1874 and C. apodiforme both occur in Italian waters and, although there is a lack of detail in the original description (Philippi, 1839) of C. apodiforme, we can detect no significant difference that is sufficiently robust to justify maintaining both of these species as valid. Claus (1875) had already suggested that Hersilia of Philippi and Clausidium of Kossmann were synonymous. Therefore, we propose here to recognise C. testudo as a junior subjective synonym of C. apodiforme. Light & Hartman (1937) reviewed Clausidium and recognised C. californiense Wilson, 1935 as a synonym of C. vancouverense (Haddon, 1912). Humes (1949) compared the important characters of the five species assigned to the genus at the time, as well as describing a new species (C. tenax Humes, 1949). Subsequently new species were described from the South Atlantic (C. senegalense Humes, 1957), from South Africa (C. saldanhae Kensley, 1974), from the Peruvian coast (C. searsi Wilson, 1937) and from India (C. chelatum Pillai, 1959 and C. travancorense Pillai, 1959). Kihara & Rocha (2013) established a new species from Brazil and provided an identification key to species, together with a list of known hosts. Recent taxonomic studies by Sepahvand et al. (2017a, b) and by Hwang et al. (2016) established three further new species from the Persian Gulf and Gulf of Oman, and one species from the Yellow Sea, respectively.
Given that C. californiense is a synonym of C. vancouverense and C. testudo is a synonym of C. apodiforme, the genus now comprises a total of 16 valid species, including the new species described here. Clausidium iranensis n. sp. can be distinguished from its congeners using the following key to adult females:
1a Antenna 3-segmented …………… 2
1b Antenna 4-segmented …………… 3
2a Distal segment of antenna with 5 setae …………. C. caudatum (Say, 1818)
2b Distal segment of antenna with 7 setae …………. C. searsi Wilson, 1937
3a Endopod segment 2 of legs 2 and 3 with 1 inner seta ……………………….… 4
3b Endopod segment 2 of legs 2 and 3 with 2 inner setae ………….. 5
4a Exopod segment 3 of leg 1 with 5 elements …………..…. C. saldanhae Kensley, 1974
4b Exopod segment 3 of leg 1 with 7 elements …………. C. tenax Humes, 1949
5a Endopod segment 2 of leg 4 with 1 inner seta ……………………….… 6
5b Endopod segment 2 of leg 4 with 2 inner setae ……………………… 7
6a Endopod segment 2 of maxilliped with total of 5 elements; endopod segment 2 of antenna with 3 setae ………………………….. C. vancouverense (Haddon, 1912)
6b Endopod segment 2 of maxilliped with total of 6 elements; endopod segment 2 of antenna with 4 setae ……………………. C. rodriguesi Kihara & Rocha, 2013
7a Endopod segment 3 of leg 3 with 5 elements ………………… C. apodiforme (Philippi, 1839)
7b Endopod segment 3 of leg 3 with 6 elements ……………………. 8
8a Exopod segment 3 of leg 4 with 8 elements …..…….……… 9
8b Exopod segment 3 of leg 4 with 9 elements ………………….. 12
9a Exopod segment 3 of leg 1 with 7 setae ……………….….. 10
9b Exopod segment 3 of leg 1 with 4 or 5 setae …….…….. 11
10a Anal somite wider than long; most proximal setal element on exopodal segment of leg 5 located in proximal half of segment ………….
……………. C. persiaensis Sepahvand, Puyani, Kihara & Momtazi, 2017
10b Anal somite as long as wide; all 4 setal elements on exopodal segment of leg 5 located in distal third of segment …………… C. senegalense Humes, 1957
11a Exopod segment 3 of leg 1 with 4 setae ………….. C. dissimile Wilson, 1921
11b Exopod segment 3 of leg 1 with 5 setae ……….…… C. chelatum Pilai, 1959
12a Basis of leg 4 elongate (about 6× longer than wide); all setal elements on leg 5 short (longest less than half length of segment) ……………….. C. iranensis n. sp.
12b Basis of leg 4 at most about 3× longer than wide; 1 or 2 setal elements on leg 5 more than half length of segment …………………. 13
13a Apical element on leg 5 shorter than lateral seta and markedly shorter than segment …………… C. makranensis Sepahvand & Kihara, 2017
13b Apical element on leg 5 longer than lateral seta and as long as or longer than segment ……… 14
14a Inner blade-like element on leg 1 as long as endopod and tapering to fine point ……… C. maximus Hwang, Lee & Kim, 2016
14b Inner bladelike element on leg 1 shorter than endopod and with bluntly pointed apex ….. 15
15a All 4 setal elements on exopodal segment of leg 5 well developed (shortest element about half length of segment) ………. C. travancorense Pillai, 1959
15b Exopodal segment of leg 5 bearing 3 well developed and 1 vestigial setal elements ………… C. sarii Sepahvand, Puyani, Kihara & Momtazi, 2017
Discussion
Female-male interlocking mechanism
The paired genital apertures are located dorsolaterally on the genital double somite of the female, but ventrally on the genital somite of the male. During the mating process males of many associated copepods exhibit mate guarding behavior (Boxshall, 1990). Males clasp onto late copepodid females using their maxillipeds in the case of members of the poecilostome lineage, and wait until the final molt when the female becomes sexually receptive. In our material the males are clasping onto adult females (e.g. Fig. 11A–D) after the final molt and are ready to transfer the paired spermatophores. The claw of the male maxilliped has a particular 3-D shape which interlocks precisely with the modified genital region of the adult female urosome. This mechanism is probably an important component of the specific mate recognition system in clausidiids and is revealed in detail here using CLSM (Fig. 11C, D).
Host preference and microhabitat selection
Symbiotic interactions typically exhibit some degree of specificity (Burns, 1993). However, both partner species involved in any symbiosis can often exhibit some degree of flexibility in the species with which they associate (Begon et al., 1996). Clausidium species have only been reported as living on the body surface of callianassid shrimps (Boxshall & Halsey, 2004). Based on previous studies it seems that many species are restricted to a single host species. However, two species of Clausidium (C. dissimile and C. vancouverense) have been reported from multiple hosts (Table 1). Corsetti & Strasser (2003) examined host usage in C. dissimile and demonstrated that it prefers Sergio trilobata (Biffar) over Lepidophthalmus louisianensis (Schmitt).
The exhibition of host preference by Clausidium species may in influenced by a number of aspects of host biology. Ghost shrimps are diverse in their behavior patterns, for example in the degree of burrowing (Sepahvand et al., 2013) and in their grooming behavior. In addition, it is likely that feeding mechanisms differ between different ghost shrimps. During this study it was noted that C. iranensis n. sp. typically inhabits the external surface of the carapace and chelipeds of their hosts, with the greatest density observed over the large chelipeds (Fig. 1B). A single specimen was found on the abdomen. In contrast, C. persiaensis inhabits the carapace and the branchial chamber of its host, Callianidea typa (V. Sepahvand, unpublished data). The choice of microhabitat may reflect differences in host biology.
References
Begon, M., Harper, J. L., & Townsend, C. R. (1996). Ecology: Individuals, populations and communities (3rd ed.). Oxford: Blackwell Sciences.
Boxshall, G. A. (1990). Precopulatory mate guarding in copepods. Bijdragen to de Dierkunde, 60, 209–213.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. London: The Ray Society, 966 pp.
Burns, T. P. (1993). Discussion: mutualism as pattern and process in ecosystem organization. In: Kawanabe, H., Cohen, J. E. & Iwaski K. (Eds) Mutualism and community organization. Oxford: Oxford University Press, pp. 239–251.
Campos, E., Campos, A. R., & Manriquez, I. (2009). Intertidal Thalassinidean shrimps (Thalassinidea: Callianassidae and Upogebidae) of the West Coast of Baja California, Mexico: Annotated checklist, keys for identification and symbionts. Crustaceana, 82, 1249–1263.
Claus, C. (1875). Neue Beträge zur kenntniss parasitischer Copepoden nebst Bemerkungen über des System derselben. Zeitschrift für Wissenschaftliche Zoologie 25, 327–360, pls. 22–26.
Corsetti, J. L., & Strasser, K. M. (2003). Host selection of the symbiotic copepod Clausidium dissimile in two sympatric populations of ghost shrimp. Marine Environmental Research, 256, 151–159.
Humes, A. G. (1949). A new copepod (Cyclopoida: Clausidiidae) parasitic on mud shrimps in Louisiana. Transactions of the American Microscopical Society, 68, 93–103.
Humes, A. G. (1957). Une nouvelle espèce de Clausidium (Copepoda, Cyclopoida) parasite d’une Callianassa au Senegal. Bulletin de l’Institute français d’Afrique noire (A), 19, 485–490.
Huys, R., & Boxshall, G. A. (1991). Copepod Evolution. London: The Ray Society, 468 pp.
Huys, R., Gee, J. M., Moore, C. G., & Hamond, R. (1996). Marine and brackish water harpacticoid copepods. Part 1. In: Kermack, D. M., Barnes, R. S. K. & Crothers, J. H. (Eds) Synopses of the British Fauna (New Series) No. 51. London: The Linnean Society of London and The Estuarine and Coastal Sciences Association, 352 pp.
Hwang, H., Lee, J., & Kim, I.-H. (2016). Two new species of Clausidiidae (Copepoda, Poecilostomatoida) from Korea. Animal Systematics, Evolution and Diversity, 32, 93–104.
Iannacone, I., Alvariño, L., & Alayo, M. (2008). Aspectos ecologicos de los metazoos parasitos de Callichirus seilacheri (Bott 1955) (Decapoda: Callianassidae) en Lima, Peru. Neotropical Helminthology, 2, 9–17.
Kensley, B. (1974). A new species of Clausidium from South Africa (Copepoda, Cyclopoida, Clausidiidae). Crustaceana, 27, 154–158.
Khodami, S., McArthur, J., Blanco-Bercial, L., & Martinez Arbizu, P. (2017). Molecular phylogeny and revision of copepod orders (Crustacea: Copepoda). Scientific Reports, 7, 9164.
Kihara, T. C., & Rocha, C. (2009). Técnicas para estudo taxonômico de copépodes harpacticóides da meiofauna marinha. Editora Asterisco.
Kihara, T. C., & Rocha, C. E. F. (2013). First record of Clausidium (Copepoda, Clausidiidae) from Brazil: a new species associated with ghost shrimps Neocallichirus grandimana (Gibbes, 1850) (Decapoda, Callianassidae). ZooKeys, 335, 47–67.
Kossmann, R. (1874). Ueber Clausidium testudo, einen neuen Copepoden, nebst Bemerkungen uber das System der halbparasitischen Copepoden. Verhandlungen der Physikalischen Gesellschaft zu Wurzburg, 7, 280–294.
Light, S. F., & Hartman, O. (1937). A review of the genera Clausidium Kossmann and Hemicyclops Boeck (Copepoda, Cyclopoida), with the description of a new species from the northeast Pacific. University of California Publications in Zoology, 41, 173–188.
Manning, R. B., & Stevčić, Z. (1982). Decapod fauna of the Piran Gulf. Quaderni dei Laboratorio di Technologia della Pesca, 3, 285–304.
Michels, J., & Buntzow, M. (2010). Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. Journal of Microscopy, 238, 95–101.
Pearse, A. S. (1947). Parasitic copepods from Beaufort, North Carolina. Journal of the Elisha Mitchell Scientific Society, 63, 1–16.
Philippi, A. (1839). Einige zoologische Notizen. Archiv für Naturgeschichte, 5, 113–134.
Pillai, N. K. (1959). On two new species of Clausidium (Copepoda: Cyclopoida) parasitic on the shrimp Callianassa. Journal of the Marine Biological Association of India, 1, 57–65.
Pohl, M. E. (1946). Ecological observations on Callianassa major Say at Beaufort, North Carolina. Ecology, 27, 71–80.
Say, T. (1818). An account of the Crustacea of the United States. Journal of the Academy of Natural Sciences, Philadelphia, 1, 57–441.
Sepahvand, V., Sari, A., Salehi, H., Nabavi, S. M. B., & Ghorbanzadeh, S. G. (2013). Littoral mud shrimps (Decapoda: Gebiidea & Axiidea) of the Persian Gulf and Gulf of Oman, Iran. Journal of the Marine Biological Association of the United Kingdom, 95, 999–1008.
Sepahvand, V., Rastegar-Pouyani, N., & Kihara, T. C. (2017a). Two new species of Clausidium copepods (Crustacea, Poecilostomatoida) associated with ghost shrimps from Iran. Journal of the Marine Biological Association of the United Kingdom, 6, 1401–1409.
Sepahvand, V., Rastegar-Pouyani, N., Kihara, T. C., & Momtazi, F. (2017b). A new species of Clausidium Kossmann, 1874 (Crustacea, Copepoda, Cyclopoida, Clausidiidae) associated with ghost shrimps from Iran. Nauplius, 25, 1–16.
Walter, T. C., & Boxshall, G. (2018). World of Copepods database. Accessed through: World Register of Marine Species. http://www.marinespecies.org/aphia.php?p=taxdetails&id=346981. Accessed on 29 January 2018.
Wilson, C. B. (1921). The North American semiparasitic copepods of the genus Clausidium. Proceedings of the United States National Museum, 59, 425–431.
Wilson, C. B. (1932). The copepods of the Woods Hole region, Massachusetts. Bulletin of the United States National Museum, 158, 1–635.
Wilson, C. B. (1935). Parasitic Copepods from the Pacific Coast. American Midland Naturalist, 16, 776–797.
Wilson, C. B. (1937). Two new semi-parasitic copepods from the Peruvian Coast. Parasitology, 29, 206–211.
Acknowledgements
We are very grateful to Professor Dr Pedro Martinez Arbizu from Senckenberg am Meer, German Center for Marine Biodiversity Research, Wilhelmshaven for help and encouragement during this study. The authors express their gratitude to Dr Abdolvahab Maghsoudlou from Iranian National Institute for Oceanography and Atmospheric Science for his cooperation in this study. This is publication number 42 based on data from the Senckenberg am Meer Confocal Laser scanning Microscope Facility
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as C767274E-BA3B-4EBA-A295-9679C90A9C6C. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Arthropoda.
Rights and permissions
About this article
Cite this article
Sepahvand, V., Kihara, T.C. & Boxshall, G.A. A new species of Clausidium Kossmann, 1874 (Copepoda: Cyclopoida) associated with ghost shrimps from the Persian Gulf, including female-male interlocking mechanisms and remarks on host specificity. Syst Parasitol 96, 171–189 (2019). https://doi.org/10.1007/s11230-019-09839-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-019-09839-x