Abstract
Using novel molecular and morphological data we elucidated the life-cycle of Gorgocephalus yaaji Bray & Cribb, 2005 from off Lizard Island, on the northern Great Barrier Reef, Australia. ITS2 rDNA sequences generated for larval trematodes from the infected snail species Echinolittorina austrotrochoides Reid (Littorinidae) were identical to those from adult G. yaaji from the fish Kyphosus cinerascens (Forsskål) (Kyphosidae). Cercariae develop in rediae in E. austrotrochoides, emerge from the snail, encyst on algae as metacercariae, and are inferred to then be consumed by the herbivorous definitive fish host, K. cinerascens. In addition, we generated the first ITS2 rDNA sequences for a gorgocephalid previously reported from the littorind gastropod Austrolittorina unifasciata Gray. Although infections previously reported from A. unifasciata were the first larval gorgocephalids characterised, this study is the first to connect an intramolluscan infection to a sexual adult. In light of the new life-cycle information, a review of mollusc associations for the digenean superfamily Lepocreadioidea was performed, highlighting gaps in the knowledge and revealing patterns of host-parasite association. We find that distinct patterns of first intermediate host association are discernible for three lepocreadioid lineages: the Aephnidiogenidae Yamaguti, 1934, Gorgocephalidae Manter, 1966, and the Lepocreadiidae Odhner, 1905. However, the evolutionary origin for these patterns of host association remains unclear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Gorgocephalidae Manter, 1966 is a small family of digeneans within the superfamily Lepocreadioidea Odhner, 1905 (see Bray & Cribb, 2012). Presently the family comprises just three species, all from fishes of the genus Kyphosus Lacépède (Perciformes: Kyphosidae) (Manter, 1966; Zhukov, 1983; Olson et al., 2003; Bray, 2005; Bray & Cribb, 2005). Gorgocephalus kyphosi Manter, 1966 was described from off South Australia, G. manteri Zhukov, 1983 from the Gulf of Mexico, and G. yaaji Bray & Cribb, 2005 from the Great Barrier Reef (Bray & Cribb, 2005). O’Dwyer et al. (2015) described the first gorgocephalid intramolluscan infection from the periwinkle snail Austrolittorina unifasciata Gray (Gastropoda: Littorinidae) from off New South Wales, Australia, characterising it as ‘Gorgocephalus sp. Aus.’. O’Dwyer et al. (2015) did not match this form to an adult, but showed that it represented a species distinct from the only other species previously characterised by rDNA, G. kyphosi (see Olson et al., 2003). O’Dwyer et al. (2015) did not observe the metacercaria of their Gorgocephalus species. However, it had previously been speculated that the metacercariae would be associated with algae, due to the herbivorous nature of the definitive host fishes (Bray & Cribb, 2012).
Here we report the complete life-cycle of G. yaaji from off Lizard Island, on the northern Great Barrier Reef, provide a description of the redia, cercaria and metacercaria, and compare molecular data generated from G. yaaji with that of samples of gorgocephalid cercariae collected from the snail A. unifasciata as part of this study and those reported in O’Dwyer et al. (2015). In addition, we review the mollusc associations for the superfamily Lepocreadioidea and discuss patterns of host association.
Materials and methods
Specimen collection
Specimens of Echinolittorina austrotrochoides Reid, were collected from intertidal rocks at Lizard Island, northern Great Barrier Reef (14°40′S, 145°27′E). Reid & Williams (2004) and Reid (2007) were used for identification of gastropods. Snails were isolated in 5 ml of seawater in individual 10 ml wells and left for 24–48 h in order to observe natural emergence of cercariae. Emerged cercariae were immediately fixed in near boiling saline and preserved in 70% ethanol for both morphological and molecular analyses. Some emerged cercariae were placed in wells with algae collected from beach rock, which had been rinsed repeatedly in clean seawater. Cercariae encysted on the algae within 24 h and were subsequently preserved in situ in 70% ethanol. All snails, including those from which cercariae had emerged, were dissected and larval trematodes were fixed in near boiling saline and preserved in 70% ethanol.
Specimens of Kyphosus cinerascens (Forsskål) were collected by spear off Lizard Island. The intestine and pyloric caeca were excised from the body and examined for adult gorgocephalids using the gut wash method as described by Cribb & Bray (2010). The one adult gorgocephalid recovered was fixed in near boiling saline and preserved in 70% ethanol for parallel morphological and molecular analyses.
Specimens of an additional snail species, A. unifasciata, were collected from intertidal rocks at Kioloa, New South Wales, Australia (35°32′S, 150°23′E), to provide material for a molecular comparison between the previously characterised gorgocephalid intramolluscan infection of O’Dwyer et al. (2015) and those we collected at Lizard Island. Snails were dissected and larval trematodes were preserved directly in 70% ethanol at room temperature.
Morphological and molecular analyses
The adult trematode, cercariae and rediae for morphological examination were washed in fresh water, overstained in Mayer’s haematoxylin, destained in a solution of 1.0% HCl and neutralised in 0.5% ammonium hydroxide solution. Specimens were then dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Metacercariae encysted on algae were examined using temporary wet mounts. Drawings were made of rediae, cercariae and metacercariae using an Olympus BH-2 compound microscope with an attached drawing tube, and illustrations were digitised in Adobe Illustrator. Measurements were made with a SPOT Insight™ digital camera (Diagnostic Instruments, INC) mounted on an Olympus BH-2 compound microscope, connected to a computer using SPOT™ imaging software. Measurements are provided in micrometres and given as the range followed by the mean in parentheses. Where length is followed by breadth, the two measurements are separated by ‘×’. All vouchers are lodged in the Queensland Museum (QM), Brisbane, Australia with registration numbers QM G235044–235054.
Total genomic DNA was extracted from trematodes using phenol/chloroform extraction techniques (Sambrook & Russell, 2001). DNA was extracted from individual naturally emerged cercariae of E. austrotrochoides, as well as from individual rediae and individual cercariae excised from infected E. austrotrochoides and A. unifasciata. As just a single adult G. yaaji was obtained, the worm was cut in half, with the anterior portion being stained and mounted for morphological identification for comparison with adult specimens described in Bray & Cribb (2005) and the posterior portion being processed for molecular analyses (hologenophore sensu Pleijel et al., 2008). The D1–D3 regions of 28S nuclear ribosomal DNA were amplified using the primers LSU5 (5′-TAG GTC GAC CCG CTG AAY TTA AGC-3′) (Littlewood et al., 2000) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′) (Snyder & Tkach, 2001) and the ITS2 region was amplified using the primers 3S (5′-GGTACC GGT GGATCA CGT GGC TAG TG-3′) (Bowles et al., 1993) and ITS2.2 (5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) (Cribb et al., 1998). PCR for both the 28S and ITS2 regions was performed with a total volume of 20 μl consisting of 5 µl of 5× MyTaq Reaction Buffer (Bioline), 0.75 µl of each primer (10 pmols), 0.25 µl Taq polymerase (Bioline MyTaq™ DNA Polymerase) and 2 µl of DNA template (approximately 10 ng), made up to 20 µl with Invitrogen™ ultraPURE™ distilled water. Amplification was carried out on a MJ Research PTC-150 thermocycler. The following profile was used to amplify the 28S region: an initial 95°C denaturation for 4 min, followed by 30 cycles of 95°C denaturation for 1 min, 56°C annealing for 1 min, 72°C extension for 2 min, followed by a single cycle of 95°C denaturation for 1 min, 55°C annealing for 45 s and a final 72°C extension for 4 min. The profile used to amplify the ITS2 region: an initial single cycle of 95°C denaturation for 3 min, 45°C annealing for 2 min, 72°C extension for 90 s, followed by 4 cycles of 95°C denaturation for 45 s, 50°C annealing for 45 s, 72°C extension for 90 s, followed by 30 cycles of 95°C denaturation for 20 s, 52°C annealing for 20 s, 72°C extension for 90 s, followed by a final 72°C extension for 5 min.
Amplified DNA was purified using Bioline ISOLATE II PCR and Gel Kit and QIAGEN® QIAquick™ PCR purification kit, per the manufacturer’s protocol. Cycle sequencing of purified DNA was carried out using ABI Big Dye™ v.3.1 chemistry following the manufacturer’s recommendations using the same primers used for PCR amplification as well as the additional 28S rDNA primers 300F (5′-CAA GTA CCG TGA GGG AAA GTT-3′) (Littlewood et al., 2000) and ECD2 (5′-CTT GGT CCG TGT TTC AAG ACG GG-3′) (Littlewood et al., 2000) and the ITS2 primer GA1 (5′-AGA ACA TCG ACA TCT TGA AC-3′) (Anderson & Barker, 1998). Cycle sequencing was carried out at the Australian Genome Research Facility using an AB3730x1 capillary sequencer. Sequencher™ version 4.5 (GeneCodes Corp.) was used to assemble and edit contiguous sequences. The start and end of the ITS2 rDNA region were determined by annotation using the ITS2 Database Metazoa model (Koetschan et al., 2012).
Lepocreadioidea life-cycle literature review
Life-cycle information for species of the superfamily Lepocreadioidea was acquired from the literature using Google Scholar and Web of Science searches using the keywords “cercariae” and “trematode genus”, or “trematode family” for each family and genus considered part of the superfamily Lepocreadioidea by Bray & Cribb (2012). Additional records were identified from references reported within these publications. Each reference was critically examined, with likely and potentially erroneous records excluded. Taxonomic standing was updated for the molluscs and trematodes within these references based on the classification recognised by the World Register of Marine Species (WoRMS, 2016).
Results
A single adult gorgocephalid was recovered from the intestine of one of two K. cinerascens examined at Lizard Island and was consistent with the description of G. yaaji by Bray & Cribb (2005) from the same locality. Intramolluscan gorgocephalid infections were found in three of 200 E. austrotrochoides collected at Lizard Island. Infections were localised in the digestive glands and gonads of the gastropods. The rediae were orange in life, numerous and largely immobile. The majority of cercariae free in the gastropod tissues were immature, consistent with completion of development outside the rediae. Cercariae emerged overnight from one infected E. austrotrochoides isolated in a tray with seawater. Naturally emerged cercariae swim vigorously in tight circles, with the tail held over the body. Cercariae placed with algae encysted to form metacercariae on the algae within 24 h.
Family Gorgocephalidae Manter, 1966
Genus Gorgocephalus Manter, 1966
Gorgocephalus yaaji Bray & Cribb, 2005
First intermediate host: Echinolittorina austrotrochoides Reid (Littorinimorpha: Littorinidae).
Locality: off Lizard Island, Great Barrier Reef, Australia (14°40′S, 145°27′E).
Voucher material: One adult hologenophore (QM G235044); rediae (QM G235045–235049); cercariae (QM 235050–235054).
Representative DNA sequences: Adult hologenophore 28S rDNA (KU951489); ITS2 rDNA (KU951490); larval trematode 28S rDNA, three identical replicates (one submitted to GenBank, KU951487); ITS2 rDNA, nine replicates (one submitted to GenBank, KU951488).
Description (Figs. 1A–C)
Redia
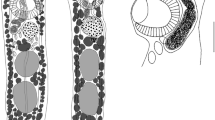
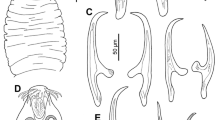
[Based on 20 mounted specimens; Fig. 1B.] Body small, blunted anteriorly, containing 5–16 (10) developing cercarial bodies and multiple germinal balls, tapering slightly to oval posterior, with distinctive, highly variable posterior protuberance of uncertain function in most specimens, 211–355 × 90–180 (265 × 111). Pharynx subglobular, 27–48 × 25–42 (34 × 35). Intestine small, cuplike, occupying maximum of one fourth of body length.
Cercaria
[Based on 20 naturally emerged specimens; Fig. 1A.] Oculate gymnocephalous cercariae with conspicuously thick tegument and bipartite tail composed of proximal section bearing series of lateral flaps and scaled distal section. Body elongate, oval, 165–210 × 47–93 (179 × 65); forebody 94–113 (100) long. Tegument 6–8 (6.4) thick at median level of ventral sucker. Eye-spots two, in anterior forebody, 10–14 × 9–14 (12 × 11), each with two small “lenses” visible only in live specimens. Oral sucker subterminal, 31–40 × 25–37 (36 × 31). Ventral sucker postequatorial, 31–40 × 25–37 (36 × 31). Oral sucker length to ventral sucker length ratio 1: 0.9–1.4 (1: 1.13); oral sucker width to ventral sucker width ratio 1: 0.80–1.20 (1: 0.99). Prepharynx indistinct, obscured in most specimens. Pharynx spherical, 14–18 × 14–18 (16 × 16). Caecum single, short, terminating slightly posterior to anterior margin of ventral sucker. Excretory vesicle Y-shaped, with arms extending to middle of ventral sucker. Main excretory collecting ducts not discerned; caudal excretory tubule visible only to first 2 or 3 annulations of distal part of tail. Tail longer than body [114–139 (124)% of body length], 191–268 (222) long, 17–26 (21) wide at base; proximal section 97–129 (116) long, bearing 14–22 flap-like projections along lateral margins; distal part 93–139 (106) long, with 18–22 scale-like annulations but lacking lateral projections. Flame-cell formula not discerned during live observations.
Metacercaria
[Based on 10 naturally emerged cercariae encysted on algae; Fig. 1C.] Small, elongate-oval, thick-walled, 98–110 × 80–97 (102 × 88).
Molecular results
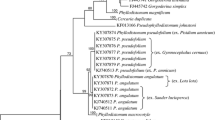
ITS2 and partial 28S rDNA sequences were generated in this study for the three infections from E. austrotrochoides and one adult G. yaaji from off Lizard Island (GenBank accession numbers: KU951487–951490). Three ITS2 sequences and one 28S sequence were generated from three infected A. unifasciata from Kioloa (KU951485–951486). All ITS2 sequences were 461 bp long comprising 123 bp of flanking 5.8S, 288 bp of ITS2 and 48 bp of flanking 28S rDNA. All ITS2 and 28S sequence replicates generated from infected E. austrotrochoides were identical, and matched 100% with those generated from the single adult G. yaaji. All ITS2 replicates generated from infected A. unifasciata were identical, and the 28S sequence was 100% identical to that of Gorgocephalus sp. “Aus” (see O’Dwyer et al., 2015). The 28S sequences of G. yaaji differed by a single base from Gorgocephalus sp. “Aus”, and from the sequence of G. kyphosi (see Olson et al., 2003) from the brassy drummer Kyphosus vaigiensis (Quoy & Gaimard) collected at Lizard Island, Great Barrier Reef, by 18 base pairs. The ITS2 sequences of G. yaaji and those generated from infected A. unifasciata differed by two base pairs.
Review of Lepocreadioidea host associations
The review of the literature identified 26 unique Lepocreadioidea species/mollusc combinations, three unique Lepocreadioidea genus/mollusc combinations, and ten otherwise unidentified unique Lepocreadioidea cercaria/mollusc combinations (Table 1). These host/parasite combination data are summarised graphically in Fig. 2.
Multiple records of additional cercarial descriptions (those assigned at family level only), which had been associated with the Lepocreadioidea, were excluded. This exclusion was deemed necessary because of the difficulties inherent in connecting morphological cercarial descriptions with the lepocreadioid families recognised presently. For example, Cable (1963) described three “Lepocreadiid” cercariae: Cercaria caribbea LXV, LXVI, and LXVII from Kingston Harbor, Jamaica. He noted that Cercaria caribbea LXVII resembled the cercariae of species of Microcreadium Simer, 1929 and Homalometron Stafford, 1904, both of which belong to the family Apocreadiidae Skrjabin, 1942, now assigned to the superfamily Apocreadioidea Skrjabin, 1942, not the Lepocreadioidea (see Bray & Cribb, 2012). Thus this record was excluded. Cercaria caribbea LXV and LXVI both have spiny cuticles and long setiferous tails, resembling the known cercariae of both the Aephnidiogenidae and Lepocreadiidae, which seemingly overlap in the morphology of the tail bristles, number of penetration/cephalic glands and flame-cell formulas. Presently, Cercaria caribbea LXV and LXVI, though both likely members of the Lepocreadioidea cannot be connected with certainty to one of the currently recognised families. Several other “lepocreadiid” cercariae described only on the basis of morphology cannot be reliably assigned to a family until molecular evidence become available. Therefore, records of likely Lepocreadioidea cercariae, which cannot be assigned to family at this time, were included in Table 1 and Fig. 2 as “uncertain” family status.
Discussion
Identification of species
Because the intramolluscan gorgocephalids we collected from A. unifasciata were from the same host, a similar locality and have identical 28S rDNA sequences, we conclude that they relate to the same species as described by O’Dwyer et al. (2015). The molecular data generated demonstrates that G. yaaji and Gorgocephalus sp. “Aus.” are closely related, differing by only 1 and 2 bases in the 28S and ITS2 rDNA regions, respectively. Used alone as evidence, this minor but consistent molecular difference might not be enough to consider Gorgocephalus sp. “Aus” distinct from G. yaaji. However, there are also noticeable morphological differences between the cercariae of Gorgocephalus sp. “Aus.” and those of G. yaaji. The cercariae of Gorgocephalus sp. “Aus.” are consistently larger-bodied than those of G. yaaji in both live and fixed specimens. O’Dwyer et al. (2015) reported measurements of both live cercariae and cercariae killed in hot seawater, though all of these cercariae were apparently recovered from dissected snails, whereas the cercariae measured in this study were all killed in hot saline after natural emergence. This could lead to inconsistencies in comparison, as measurements of cercariae from dissected snails often include immature specimens, which can skew measurement ranges and means. The eyespots are smaller in G. yaaji, and each bear two “lenses” clearly visible in live specimens which were not reported in the Gorgocephalus sp. “Aus.” description. O’Dwyer et al. (2015) based their examination largely on live specimens so it is unlikely that this distinct feature would have been missed. In addition, Gorgocephalus sp. “Aus.” and G. yaaji infect littorinids of separate genera. Given the host, molecular and morphological differences between these forms, it is best for Gorgocephalus sp. “Aus.” to be considered distinct from G. yaaji at present.
Current evidence suggests that there are at least four distinct species within the family Gorgocephalidae. Two species, Gorgocephalus sp. “Aus.” and G. yaaji, have been shown to utilise snails of the family Littorinidae as first intermediate hosts. Therefore, it seems likely that G. kyphosi and G. manteri exploit littorinid snails as first intermediate hosts as well.
Gorgocephalid life-cycles
The prospect that gorgocephalid cercariae may encyst on algae was previously suggested by Bray & Cribb (2012). This was confirmed by placing small pieces of algae with infected snails isolated in 10 mm plastic wells. The cercariae readily encysted on the algae, but not on the walls of the plastic wells in which they were isolated. We infer that the life-cycle is completed by ingestion of such algae by the kyphosid host fish, which feed almost exclusively on algae (Clements & Choat, 1997).
The first intermediate host usage of the Gorgocephalidae provides an indication of the manner in which adult Gorgocephalus are recruited as well as insights into distributional patterns for the family. There are now two known intermediate hosts for the Gorgocephalidae, both gastropods of the family Littorinidae. Both of the known infected snail species occupy rocky habitat in the higher levels of the littoral fringe (Reid & Williams, 2004; Reid, 2007). These snails are submerged only during peak high tides, presumably allowing only short periods for cercarial emergence and dispersal. Thus, it seems likely that the majority of Gorgocephalus spp. metacercariae are ingested by fishes grazing on algae and vegetation near rocky shorelines.
Concentration of first intermediate host exploitation in the Littorinidae suggests that gorgocephalids will be absent from marine environments lacking these gastropods. In this context it is noteworthy that between 1998 and 2015, we have examined 25 Kyphosus vaigiensis (Quoy & Gaimard) and six Kyphosus cinerascens (both known hosts of gorgocephalids) from off Heron Island, on the Southern Great Barrier Reef. Though numerous digeneans of the families Enenteridae Yamaguti, 1958, Hemiuridae Looss, 1899, and Lecithasteridae Odhner, 1905, were recovered (Bray & Cribb, 2000, 2001, 2002a, b), no gorgocephalids have been found. In light of our new understanding of gorgocephalid life-cycles, this now comes as no surprise; Heron Island has very few littorinids (Rohde, 1973). While there is a large amount of beachrock along the shoreline of Heron Island, it is low lying and apparently not suitable habitat for significant populations of these snails.
The colonisation of new geographic locations by gorgocephalid trematodes presumably requires a population of susceptible littorinid snails, long term survival of adult worms within the host fish, and successful migration of kyphosid fish across long distances. Kyphosids are found mostly in close association with rocky reefs (Nelson, 2006), but are known to be capable of long range dispersal (Sakihara et al., 2015). Although species of Kyphosidae may be relatively abundant and well distributed throughout global marine habitats, only those habitats which provide an intersection of kyphosid and littorinid distributions are likely to support gorgocephalids.
Lepocreadioidea life-cycle patterns
The Aephnidiogenidae Yamaguti, 1934, along with the Lepidapedidae Yamaguti, 1958, was split from the Lepocreadiidae Odhner, 1905, and recognised in modern literature as a family by Bray & Cribb (2012). Aephnidiogenids infect both marine and freshwater teleost fishes as definitive hosts. The reported definitive fish hosts of the Aephnidiogenidae are all carnivorous, consistent with a three host life-cycle for the majority of species. Four complete life-cycles are known from gastropods of the families Barleeiidae and Tateidae.
The family Deropristidae Cable & Hunnien, 1942, though classically placed as a member of the Lepocreadioidea, has not yet been evaluated molecularly (Bray & Cribb, 2012). Thus, its inclusion in this treatment represents an issue which cannot be fully resolved until molecular data for this family becomes available. Members of the family are primarily parasites of sturgeons, with a few species infecting the intestines of teleosts (Choudhury & Dick, 1998). Life-cycle information is available for two species, Deropristis inflata (Molin, 1859) and Skrjabinopsolus manteri (Cable, 1952) (Table 1), though the records for D. inflata likely represent a complex rather than a single species, with intramolluscan records from the gastropod families Cerithiidae, Hydrobiidae, Cochliopidae and Pleuroceridae.
Although the Enenteridae has been evaluated molecularly, and has been identified as a sister taxon to the Gyliauchenidae Fukui, 1929 (see Bray & Cribb, 2012), life-cycle information is completely lacking for the group. Ching (1991) reported cercariae of an enenterid from Alvania compacta Carpenter (Rissoidae). Unfortunately, we see no reason to consider this species as an enenterid, as the author draws attention to a close resemblance to Neophasis Stafford, 1904, a genus belonging to the Apocreadiidae.
Adults of the Gorgocephalidae are known only from fishes of the genus Kyphosus. Two first intermediate hosts have been identified for this family thus far (O’Dwyer et al., 2015; present study), both of which are gastropods of the family Littorinidae. Curiously, species of the Gorgocephalidae are alone among the Lepocreadioidea families in their use of littorinids as first intermediate hosts. The digenean fauna of littorinids has been intensively studied globally (e.g. Granovitch & Mikhailova, 2004; Thieltges et al., 2009; O’Dwyer et al., 2014, 2015), but no other lepocreadioids have been reported.
The Gyliauchenidae is a distinctive family, with sexual adults overwhelmingly concentrated in herbivorous fishes (Hall & Cribb, 2005). The molecular phylogeny of the Lepocreadioidea by Bray & Cribb (2012) identified the Gyliauchenidae as the sister taxon to the Enenteridae. Just one life-cycle has been elucidated thus far, that of Gyliauchen volubilis Nagaty, 1956. The first intermediate host is Clypeomorus bifasciata (G.B. Sowerby II). Al-Jahdali & Hassanine (2012) found that the cercariae of G. volubilis emerged and encysted on aquatic vegetation and that the metacercariae were ingested by grazing rabbitfish, Siganus rivulatus Forsskål & Niebuhr (Siganidae). The sister taxon relationships between the Enenteridae and Gyliauchenidae could suggest that enenterids might also infect cerithiid gastropods as first intermediate hosts. However, the digenean fauna of the gastropod family Cerithiidae has been well studied (e.g. Holliman, 1961; Cable, 1956, 1963; Cannon, 1978; Abdul-Salam & Sreelatha, 1998; Bartoli & Gibson, 2007), and other than the Deropristidae, no other lepocreadioid families have been reported infecting these gastropods.
When split from the Lepocreadiidae and Aephnidiogenidae based on molecular evidence (Bray & Cribb, 2012), the Lepidapedidae was identified as sister to the Enenteridae + Gyliauchenidae. The reported definitive hosts for the Lepidapedidae are all carnivorous fishes, consistent with a three host life-cycle, as opposed to putative and demonstrated two host life-cycles for the Enenteridae and Gyliauchenidae. However, life-cycle information for this family is available for only one species, Lepidapedon elongatum (Lebour, 1908), which utilises a gastropod of the family Rissoidae (see Køie, 1985).
The Lepocreadiidae is the largest and most diverse family of the Lepocreadioidea. Species of Lepocreadiidae utilise carnivorous, herbivorous, and omnivorous fishes as definitive hosts. However, based on the records examined in this study, the Lepocreadiidae are relatively specific in their first intermediate hosts. With one exception (see below), first intermediate hosts for the family are all from two closely related superfamilies of the Hypsogastropoda, the Buccinoidea and Conoidea. These superfamilies form part of a traditional morphological clade known as the Neogastropoda. Molecular-based phylogenies of some workers have not supported the monophyly of the Neogastropoda (i.e. Harasewych et al., 1997; Colgan et al., 2007; Cunha et al., 2009). Conversely, an increase in sampling effort and a reexamination of the Neogastropoda by Zou et al. (2011) strongly supported the monophyly of the clade. Three families of the Neogastropoda have been reported as hosts for lepocreadiids: Columbellidae and Nassariidae of the superfamily Buccinoidea and Conidae of the superfamily Conoidea. These three families are closely related according to the phylogeny of Zou et al. (2011). Several cercarial species of uncertain status are reported from buccinoids, and these may well prove to be lepocreadiids.
A dramatic exception to the narrow range of lepocreadiid first intermediate host usage was reported by Hassanine (2006) who showed that Diploproctodaeum arothroni Bray & Nahhas, 1998 infects the bivalve Saccostrea cuccullata (Born) (Ostreidae). This record can only be considered evidence of isolated host switching, common at various levels among the Digenea (see Cribb et al., 2001), rather than the typical state for the family. It is noteworthy that D. arothroni belongs to a strongly-supported clade of genera (Bianium Stunkard, 1930, Diplocreadium Park, 1939, Diploproctodaeum La Rue, 1926 and Lobatocreadium Madhavi, 1972) which are overwhelmingly parasites of tetraodontiforms. It will be interesting to determine if this clade as a whole infects bivalves as first intermediate hosts. There is one additional report of putative lepocreadioid cercariae from a bivalve. While the focus of their study was pathology, Hanafy et al. (1997) provided a description of a lepocreadioid cercaria from Lajonkairia lajonkairii (Payraudeau, 1826) (Veneridae). The sexual adult of this cercaria remains unknown.
Kostadinova & Gibson (2005) currently recognise the family Liliatrematidae Gubanov, 1953 as a member of the superfamily Lepocreadioidea, but this placement has yet to be confirmed genetically (Bray & Cribb, 2012). Although the metacercariae of this family are known to develop in fish and the adult forms are found in birds (Kostadinova & Gibson, 2005), we were unable to find any records of first intermediate hosts for the family. The Liliatrematidae requires molecular evaluation in order to inform its placement within the greater digenean phylogeny.
Life-cycle overview
Our knowledge of the relationships between molluscs and digeneans has advanced greatly in recent years as molecular techniques revolutionise our understanding of the phylogenies of both hosts and parasites (Blair et al., 2001; Cribb et al., 2001; Lockyer et al., 2004). However, we continue to lack clarity in our understanding of these complex relationships across deep time. The apparently numerous host switching events over the course of digenean evolution leaves unraveling mollusc-trematode relationships a complex task. The immensity of this task is exemplified by the asymmetry of mollusc and lepocreadioid species richness data; whereas there are fewer than 500 species of lepocreadioids, there are tens of thousands of potential mollusc hosts.
This analysis indicates that three lepocreadioid families (the Aephnidiogenidae, Gorgocephalidae and Lepocreadiidae) have a relatively narrow first intermediate host range. Although only a single first intermediate host is known for the Gyliauchenidae and the Lepidapedidae, we think it likely that these families will have a narrow first intermediate host range as well, given the range observed in the Aephnidiogenidae, Gorgocephalidae and Lepocreadiidae. Overall, these host distributions suggest that the phylogenetic distinctions detected between these lepocreadioid families are also reflected in their life-cycles. Apart from infrequent host switching events, the evidence is consistent with a paradigm in which each family has radiated in association with a limited range of closely related gastropod hosts. Conversely, first intermediate host usage for the Deropristidae is spread across distantly related gastropod clades. This may be an indication of repeated host switching events, lower host specificity, or perhaps, that the Deropristidae does not truly belong to the Lepocreadioidea.
The overall pattern of host distribution for the Lepocreadioidea may have some predictive power in that it seems likely that the multiple incompletely identified lepocreadioid taxa infecting buccinoid gastropods will prove to be species of the family Lepocreadiidae. However, the data do not allow confident prediction of the intermediate hosts of enenterids or liliatrematids, or indeed, whether the Deropristidae will prove to belong elsewhere in the greater digenean system. The evolutionary basis of this host distribution pattern, either arising from cospeciation, or from host switching, remains uncertain.
References
Abdul-Salam, J., & Sreelatha, B. (1998). A list of larval digenetic trematodes parasitizing some marine invertebrates of Kuwait Bay. Kuwait Journal of Science & Engineering, 25, 409–434.
Al-Jahdali, M., & El-Said Hassanine, R. (2012). The life cycle of Gyliauchen volubilis Nagaty, 1956 (Digenea: Gyliauchenidae) from the Red Sea. Journal of Helminthology, 86, 165–172.
Anderson, G. R., & Barker, S. C. (1998). Inference of phylogeny and taxonomy within the Didymozoidae (Digenea) from the second internal transcribed spacer (ITS2) of ribosomal DNA. Systematic Parasitology, 41, 87–94.
Averbuj, A., & Cremonte, F. (2010). Parasitic castration of Buccinanops cochlidium (Gastropoda: Nassariidae) caused by a lepocreadiid digenean in San José Gulf, Argentina. Journal of Helminthology, 84, 381–389.
Bartoli, P. (1967). Etude du cycle évolutif d’un Trématode peu connu: Lepocreadium pegorchis (M. Stossich, 1900) (Trematoda, Digenea). Annales de Parasitologie Humaine et Comparée, 42, 605–619.
Bartoli, P., & Gibson, D. I. (2007). Synopsis of the life cycles of Digenea (Platyhelminthes) from lagoons of the northern coast of the western Mediterranean. Journal of Natural History, 41, 1553–1570.
Bartoli, P., & Prévôt, G. (1978). Le cycle biologique de Holorchis pycnoporus M. Stossich, 1901 (Trematoda, Lepocreadiidae). Zeitschrift für Parasitenkunde, 58, 73–90.
Bartoli, P., & Holmes, J. C. (1997). A transmission study of two sympatric digeneans: Spatial constraints and solutions. Journal of the Helminthological Society of Washington, 64, 169–175.
Bayssade-Dufour, C., & Maillard, C. (1974). Chetotaxie de quatre cercaires d’ Allocreadioidea. Annales de Parasitologie Humaine et Comparée, 49, 521–554.
Blair, D., Davis, G., & Wu, B. (2001). Evolutionary relationships between trematodes and snails emphasizing schistosomes and paragonimids. Parasitology, 123, 229–243.
Bowles, J., Hope, M., Tiu, W. U., Liu, X., & McManus, D. P. (1993). Nuclear and mitochondrial genetic markers highly conserved between Chinese and Philippine Schistosoma japonicum. Acta Tropica, 55, 217–229.
Bray, R. A. (2005). Family Gorgocephalidae Manter, 1966. In: Jones, A., Bray, R. A., & Gibson, D. I. (Eds.), Keys to the Trematoda. Volume 2. Wallingford: CABI International, pp. 657–661.
Bray, R. A., & Cribb, T. H. (2000). The status of the genera Hysterolecithoides Yamaguti, 1934, Neotheletrum Gibson & Bray, 1979 and Machidatrema León-Règagnon, 1998 (Digenea: Hemiuroidea), including a description of M. leonae n. sp. from Australian waters. Systematic Parasitology, 46, 1–22.
Bray, R. A., & Cribb, T. H. (2001). A review of the family Enenteridae Yamaguti, 1958 (Digenea), with descriptions of species from Australian waters, including Koseiria huxleyi n. sp. Systematic Parasitology, 48, 1–29.
Bray, R. A., & Cribb, T. H. (2002a). Further observations on the Enenteridae Yamaguti, 1958 (Digenea, Lepocreadioidea) of the Indo-West Pacific region, including a new species from Western Australia. Acta Parasitologica, 47, 208–223.
Bray, R. A., & Cribb, T. H. (2002b). Observations on the phylogeny of Opisthadena Linton, 1910 and related genera (Hemiuridae: Opisthadeninae) from Australian and French Polynesian waters. Folia Parasitologica, 49, 279–290.
Bray, R. A., & Cribb, T. H. (2005). Gorgocephalus yaaji n. sp. (Digenea: Gorgocephalidae) from the brassy chub Kyphosus vaigiensis (Perciformes: Kyphosidae) off Lizard Island, northern Great Barrier Reef and further records of G. kyphosi. Zootaxa, 1068, 39–46.
Bray, R. A., & Cribb, T. H. (2012). Reorganisation of the superfamily Lepocreadioidea Odhner, 1905 based on an inferred molecular phylogeny. Systematic Parasitology, 83, 169–177.
Cable, R. M. (1956). Marine cercariae of Puerto Rico. Scientific Survey of Porto Rico and the Virgin Islands, 16, 491–577.
Cable, R. M. (1963). Marine cercariae from Curaçao and Jamaica. Zeitschrift für Parasitenkunde, 23, 429–469.
Cable, R. M., & Hunninen, A. (1942). Studies on Deropristis inflata (Molin), its life history and affinities to trematodes of the family Acanthocolpidae. Biological Bulletin, 82, 292–312.
Cannon, L. (1978). Marine cercariae from the gastropod Cerithium moniliferum Kiener at Heron Island, Great Barrier Reef. Proceedings of the Royal Society of Queensland, 89, 45–57.
Ching, H. L. (1991). Lists of larval worms from marine invertebrates of the Pacific Coast of North America. Journal of the Helminthological Society of Washington, 58, 57–68.
Choudhury, A., & Dick, T. A. (1998). Systematics of the Deropristiidae Cable & Hunninen, 1942 (Trematoda) and biogeographical associations with sturgeons (Osteichthyes: Acipenseridae). Systematic Parasitology, 41, 21–39.
Clements, K., & Choat, J. (1997). Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Marine Biology, 127, 579–586.
Colgan, D., Ponder, W., Beacham, E., & Macaranas, J. (2007). Molecular phylogenetics of Caenogastropoda (Gastropoda: Mollusca). Molecular Phylogenetics and Evolution, 42, 717–737.
Cribb, T. H., Adlard, R. D., & Bray, R. A. (1998). A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). International Journal for Parasitology, 28, 1791–1795.
Cribb, T. H., Bray, R. A., & Littlewood, D. T. J. (2001). The nature and evolution of the association among digeneans, molluscs and fishes. International Journal for Parasitology, 31, 997–1011.
Cribb, T. H., & Bray, R. A. (2010). Gut wash, body soak, blender and heat-fixation: Approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7.
Criscione, F., & Ponder, W. F. (2013). A phylogenetic analysis of rissooidean and cingulopsoidean families (Gastropoda: Caenogastropoda). Molecular Phylogenetics and Evolution, 66, 1075–1082.
Cunha, R. L., Grande, C., & Zardoya, R. (2009). Neogastropod phylogenetic relationships based on entire mitochondrial genomes. BMC Evolutionary Biology, 9, 210.
Deblock, S. (1980). Inventaire des trématodes larvaires parasites des mollusques Hydrobia (Prosobranches) des côtes de France. Parassitologia, 22, 1–105.
Granovitch, A. I., & Mikhailova, N. A. (2004). Rocky shore trematodes of the west coast of Sweden: Distribution and life cycle strategies. Acta Parasitologica, 49, 228–236.
Hall, K. A., & Cribb, T. H. (2005). Family Gyliauchenidae Fukui, 1929. In: Jones, A., Bray, R. A., & Gibson, D. I. (Eds.) Keys to the Trematoda. Volume 2. Wallingford, CABI International, pp. 665–678.
Hanafy, M., Gab-Alla, A., & Hassanine, R. (1997). Larval trematode (Digenea: Lepocreadiidae) infection in the gonads of the commercial bivalve Venerupis decussata from Lake Timsah, Suez Canal. Journal of the Egyptian-German Society of Zoology, 24, 167–182.
Harasewych, M., Adamkewicz, S. L., Blake, J. A., Saudek, D., Spriggs, T., & Bult, C. J. (1997). Neogastropod phylogeny: A molecular perspective. Journal of Molluscan Studies, 63, 327–351.
Hassanine, R. M. E.-S. (2006). The life-cycle of Diploproctodaeum arothroni Bray and Nahhas, 1998 (Digenea: Lepocreadiidae), with a comment on the parasitic castration of its molluscan intermediate host. Journal of Natural History, 40, 1211–1222.
Holliman, R. B. (1961). Larval trematodes from the Apalachee Bay area, Florida, with a checklist of known marine cercariae arranged in a key to their superfamilies. Tulane Studies in Zoology, 9, 1–74.
Ito, J., & Shimura, S. (1980). On a new lepocreadiid cercaria, Cercaria isoninae n. sp. (Trematoda) from a littoral gastropod, Japeuthria ferrea from Kanagawa and Chiba Prefectures, Japan. Japanese Journal of Parasitology, 29, 181–187.
Kim, Y.-G., Kim, J.-Y., & Chun, S.-K. (1984). The trematode parasitized on the marine gastropod I. On the Cercaria yamagutii, Cercaria isoninae and Cercaria pseudogranifera. Korean Journal of Fisheries and Aquatic Sciences, 17, 543–548.
Koetschan, C., Hackl, T., Müller, T., Wolf, M., & Förster, F. (2012). ITS2 database IV: Interactive taxon sampling for internal transcribed spacer 2 based phylogenies. Molecular Phylogenentics and Evolution, 63, 585–588.
Køie, M. (1974). On the morphology and life-history of Opechona bacillaris (Molin, 1859) Looss, 1907 (Trematoda, Lepocreadiidae). Ophelia, 13, 63–86.
Køie, M. (1985). On the morphology and life-history of Lepidapedon elongatum (Lebour, 1908) Nicoll, 1910 (Trematoda, Lepocreadiidae). Ophelia, 24, 135–153.
Kostadinova, A., & Gibson, D. I. (2005). Family Liliatrematidae Gubanov, 1953. In: Jones, A., Bray, R. A., & Gibson, D. I. (Eds.) Keys to the Trematoda. Volume 2. Wallingford, CABI International, pp. 679–682.
Littlewood, D. T. J., Curini-Galletti, M., & Herniou, E. A. (2000). The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Molecular Phylogenetics and Evolution, 16, 449–466.
Lockyer, A. E., Jones, C. S., Noble, L. R., & Rollinson, D. (2004). Trematodes and snails: An intimate association. Canadian Journal of Zoology, 82, 251–269.
Macfarlane, W. (1951). The life cycle of Stegodexamene anguillae n.g., n. sp., an allocreadiid trematode from New Zealand. Parasitology, 41, 1–10.
Manter, H. W. (1966). A peculiar trematode, Gorgocephalus kyphosi gen. et sp. n. (Lepocreadiidae: Gorgocephalinae subfam. n.), from a marine fish of South Australia. Journal of Parasitology, 52, 347–350.
Martin, W. E. (1938). Studies on trematodes of Woods Hole: The life cycle of Lepocreadium setiferoides (Miller and Northup), Allocreadiidae, and the description of Cercaria cumingiae n. sp. Biological Bulletin, 75, 463–474.
Martorelli, S. (1991). Primera cita de una cercaria tricocerca parásita de Dorsanum moniliferum (Mollusca: Buccinidae) para el Atlántico sud-occidental. Aportes al conocimiento de su ciclo de vida. Neotropica, 37, 57–65.
Nelson, J. S. (2006). Fishes of the World (4th ed.). Hoboken: John Wiley, 600 pp.
O’Dwyer, K., Blasco-Costa, I., Poulin, R., & Faltýnková, A. (2014). Four marine digenean parasites of Austrolittorina spp. (Gastropoda: Littorinidae) in New Zealand: Morphological and molecular data. Systematic Parasitology, 89, 133–152.
O’Dwyer, K., Faltýnková, A., Georgieva, S., & Kostadinova, A. (2015). An integrative taxonomic investigation of the diversity of digenean parasites infecting the intertidal snail Austrolittorina unifasciata Gray, 1826 (Gastropoda: Littorinidae) in Australia. Parasitology Research, 114, 2381–2397.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Palombi, A. (1934). Gli stadi larvali dei Trematodi del Golfo di Napoli. Pubblicazioni Stazione Zoologica Napoli, 14, 51–94.
Palombi, A. (1937). Il ciclo biologico di Lepocreadium album Stossich sperimentalmente realizzato. Rivista di Parassitologia, 1, 1–12.
Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., et al. (2008). Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371.
Ponder, W. F., Colgan, D. J., Healy, J. M., Nützel, A., Simone, L. R. L., & Strong, E. E. (2008). Caenogastropoda. In: Ponder, W. F. (Ed.), Phylogeney and Evolution of the Mollusca. Berkeley: University of California Press, pp. 331–383.
Reid, D. G. (2007). The genus Echinolittorina Habe, 1956 (Gastropoda: Littorinidae) in the Indo-West Pacific Ocean. Zootaxa, 1420, 1–161.
Reid, D. G., & Williams, S. T. (2004). The subfamily Littorininae (Gastropoda: Littorinidae) in the temperate southern hemisphere: The genera Nodilittorina, Austrolittorina and Afrolittorina. Records of the Australian Museum, 56, 75–122.
Rohde, K. (1973). Structure and development of Lobatostoma manteri sp. nov. (Trematoda: Aspidogastrea) from the Great Barrier Reef, Australia. Parasitology, 66, 63–83.
Russell-Pinto, F., & Bartoli, P. (2002). Cercaria sevillana n. sp., a new cercaria (Digenea: Microphallidae) from Nassarius reticulatus (L.) (Mollusca: Prosobranchia) in Portugal. Systematic Parasitology, 53, 175–182.
Sakihara, T., Nishiura, L., Shimoda, T., Shindo, T., & Nishimoto, R. (2015). Brassy chubs Kyphosus vaigiensis display unexpected trans-island movement along inshore habitats. Environmental Biology of Fishes, 98, 155–163.
Sambrook J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 234 pp.
Seitner, P. G. (1951). The life history of Allocreadium ictaluri Pearse, 1924 (Trematoda: Digenea). Journal of Parasitology, 37, 223–244.
Snyder, S. D., & Tkach, V. V. (2001). Phylogenetic and biogeographical relationships among some holarctic frog lung flukes (Digenea: Haematoloechidae). Journal of Parasitology, 87, 1433–1440.
Stunkard, H. W. (1969). The morphology and life-history of Neopechona pyriforme (Linton, 1900) n. gen., n. comb. (Trematoda: Lepocreadiidae). Biological Bulletin, 136, 96–113.
Stunkard, H. W. (1972). Observations on the morphology and life-history of the digenetic trematode, Lepocreadium setiferoides (Miller and Northup, 1926) Martin, 1938. Biological Bulletin, 142, 326–334.
Stunkard, H. W. (1980a). The morphology, life-history, and taxonomic relations of Lepocreadium areolatum (Linton, 1900) Stunkard, 1969 (Trematoda: Digenea). Biological Bulletin, 158, 154–163.
Stunkard, H. W. (1980b). Successive hosts and developmental stages in the life history of Neopechona cablei sp. n. (Trematoda: Lepocreadiidae). Journal of Parasitology, 66, 636–641.
Stunkard, H. W. (1983). The marine cercariae of the Woods Hole, Massachusetts region, a review and a revision. Biological Bulletin, 164, 143–162.
Thieltges, D. W., Ferguson, M. A., Jones, C. S., Noble, L. R., & Poulin, R. (2009). Biogeographical patterns of marine larval trematode parasites in two intermediate snail hosts in Europe. Journal of Biogeography, 36, 1493–1501.
Vaes, F. (1978). Notes on the life-cycle of Deropristis inflata (Molin, 1958) (Trematoda Digenea: Acanthocolpidae): Transmission between first and second intermediate host. Biologisch Jaarboek Dodonaea, 46, 202–209.
Watson, R. (1984). The life cycle and morphology of Tetracerasta blepta, gen. et. sp. nov., and Stegodexamene callista, sp. nov. (Trematoda: Lepocreadiidae) from the long-finned eel, Anguilla reinhardtii Steindachner. Australian Journal of Zoology, 32, 177–204.
WoRMS Editorial Board (2016). World Register of Marine Species. Available from http://marinespecies.org at VLIZ. Accessed (01/2016).
Yamaguti, S. (1975). Synoptical review of life histories of digenetic trematodes of vertebrates with special reference to the morphology of their larval forms. Tokyo: Keigaku Publishing Company, 590 pp. + 219 plates.
Zhukov, E. (1983). New representatives of the fauna of trematodes from the fishes of the Gulf of Mexico. Parazitologiya, 17, 112–117.
Zou, S., Li, Q., & Kong, L. (2011). Additional gene data and increased sampling give new insights into the phylogenetic relationships of Neogastropoda, within the caenogastropod phylogenetic framework. Molecular Phylogenetics and Evolution, 61, 425–435.
Acknowledgements
The authors thank the staff of the Lizard Island Research Station (Australian Museum) for their ongoing support of our field expeditions.
Funding
T H. Cribb is supported by the Australian Biological Resources Study and the Australian Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Huston, D.C., Cutmore, S.C. & Cribb, T.H. The life-cycle of Gorgocephalus yaaji Bray & Cribb, 2005 (Digenea: Gorgocephalidae) with a review of the first intermediate hosts for the superfamily Lepocreadioidea Odhner, 1905. Syst Parasitol 93, 653–665 (2016). https://doi.org/10.1007/s11230-016-9655-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-016-9655-7