Abstract
Blood flukes of the genus Cardicola Short, 1953 are considered the most potentially pathogenic parasites in bluefin tuna cultures. Morphological study and genetic analyses of the ribosomal internal transcribed spacer ITS-2 and the mitochondrial cytochrome c oxidase 1 (cox1) gene fragments revealed the occurrence of four aporocotylid species (C. forsteri Cribb, Daintith & Munday, 2000, C. orientalis Ogawa, Tanaka, Sugihara & Takami, 2010, C. opisthorchis Ogawa, Ishimaru, Shirakashi, Takami & Grabner, 2011 and Cardicola sp.) in 421 Thunnus thynnus (L.) from the Western Mediterranean (274 fished from the wild and 147 from sea-cages). Cardicola opisthorchis was the most abundant species, with higher prevalence in the cage-reared fish than in those fished in the wild (21 vs 6%, p < 0.05). Adults of three species were recovered: C. forsteri from both gills and heart, C. opisthorchis from heart and C. orientalis from gills. The secondary gill lamellae were profusely infected by eggs of C. orientalis. A fourth species was found in four tunas, based on the molecular analyses of eggs apparently indistinguishable in size and shape from the eggs of C. orientalis. The findings provided evidence that infections with Cardicola spp. differed in relation to locality, host origin (wild vs cage-reared) and site of infection. It is necessary to estimate the possible different pathogenic effects of each species of Cardicola in order to take appropriate control measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Aporocotylidae Odhner, 1912 (Platyhelminthes: Trematoda) includes a large number of blood flukes (37 genera described to date) infecting the circulatory system of fishes, both chondrichthyes and osteichthyes (Nolan et al., 2004). These parasites are often unnoticed as the adults are usually difficult to find along the intricate circulatory system, often being detected by the presence of eggs trapped in the gills or in the heart of the hosts (Paperna & Dzikwoski, 2006; Cribb et al., 2011).
Cardicola Short, 1953 is a species-rich aporocotylid genus comprising more than 30 species that parasitise fishes of several teleost families, some of them such as sparids and scombrids, with high importance in fisheries and aquaculture (Padrós et al., 2001; Holzer et al., 2008; Nolan et al., 2004). Five species have been found in tunas (Scombridae: Thunnini): Cardicola ahi Yamaguti, 1970 in Thunnus albacares (Bonnaterre) and T. obesus (Lowe); C. congruenta Lebedev & Mamaev, 1968 in Euthynnus affinis (Cantor); C. forsteri Cribb, Daintith & Munday, 2000 in T. maccoyii (Castelnau) and T. thynnus (L.) (see Bullard et al., 2004); C. opisthorchis Ogawa, Ishimaru, Shirakashi, Takami & Grabner, 2011 in T. orientalis (Temminck & Schlegel) and T. thynnus (see Ogawa et al., 2011); and C. orientalis Ogawa, Tanaka, Sugihara & Takami, 2010 in T. maccoyii and T. orientalis (see Shirakashi et al., 2013). All these species have been recorded in the Pacific Ocean, although C. forsteri was also collected in T. thynnus from the North Atlantic and the Mediterranean and C. opisthorchis in T. thynnus from the Mediterranean (Aiken et al., 2007).
Aporocotylids are one of the few trematodes associated with severe pathologies in fish cultures (Paperna & Dzikowski, 2006). These pathologies are related to the effect of parasite eggs in the gills, which accumulate and obstruct the correct blood circulation in gill vessels, and reduce the respiratory surface when the eggs hatch and break the gill epithelium (Cribb et al., 2011). Aporocotylids are considered to be the most potentially dangerous pathogens in tuna farms in Japan and Australia (Aiken et al., 2006; Hayward et al., 2010; Dennis et al., 2011; Shirakashi et al., 2012). Since 1996, the farming of the Atlantic bluefin tuna T. thynnus has increased considerably in the Mediterranean, in order to reduce the fishing pressure increased by the high demand in markets, mainly from Asia (Sibeni & Calderini, 2014). This culture is based on fattening of tunas captured in the wild and reared in sea-cages until they reach to marketable size. During the relative short time of history of this activity in Mediterranean farms, no significant pathologies have been described, except for parasitoses with apparent mild effects, including the presence of eggs of Cardicola spp. (Mladineo, 2006; Ruíz de Ybáñez et al., 2011).
During the course of the project “PARATUN” (AGL2010-20892) funded by the Spanish Government, numerous samples of wild and cage-reared bluefin tunas from the Atlantic and Spanish Mediterranean were analysed and more than one species of Cardicola were found in heart and gills. The aim of this study was to identify, based on morphological characteristics and molecular markers, the aporocotylid species infecting T. thynnus in the Western Mediterranean. Detailed morphological and molecular characteristics for their recognition are provided.
Materials and methods
Sample collection and processing
A total of 274 wild tunas caught in the Western Mediterranean (Balearic Sea, Sardinian Sea and the Strait of Gibraltar) during 2010–2012 was examined for the presence of aporocotylid digeneans (Table 1). Cage-reared tunas (n = 147) from a fattening farm off the Spanish Mediterranean coast (Balearic Sea, off the northeastern Iberian Peninsula coast) were also examined in 2011 and 2012. Immediately after landing, the gills and heart of all specimens were excised, stored individually in plastic bags and frozen at –20°C.
In the laboratory, the samples were thawed and examined by naked eye and under stereomicroscope (Leica MZ APO) under a magnification of up to ×40. The gills were dissected and the location of eggs and adult parasites was recorded according to Culurgioni et al. (2014). To detect the presence of eggs, two filaments (= primary lamellae) of opposite hemibranchs, from five areas along the longitudinal axis of each holobranch were separated (see Fig. 1). These filaments were immerged in glycerol and examined with a stereomicroscope under a magnification of ×40 with transmitted light; the addition of 5% KOH was found often helpful to clarify larger filaments. In order to distinguish the morphology of the eggs, infected secondary lamellae were examined under light microscope at magnifications of up to ×1,000. The heart was dissected in a Petri dish in slices c.0.5 cm thick and flushed with saline solution. Shredded tissues were shaken in saline solution and sieved in a 400 μm mesh sieve. Adult blood flukes were counted and stored in 70% ethanol for morphological examination, but from a selected specimen of each species, a small piece of the left side of the posterior body half was excised and fixed in 100% ethanol for DNA isolation. Samples of eggs with homogeneous morphological characteristics were isolated from the gill filaments and fixed in 100% ethanol for molecular identification.
Adult parasites were stained in iron acetocarmine, differentiated in acid alcohol (100 ml of 70% ethanol and 1 ml concentrated hydrochlorhidric acid), washed in distilled water, dehydrated in an ethanol series, cleared in dimethyl phthalate and mounted in Canada balsam for morphological studies. Voucher material is deposited in the Natural History Museum, London, UK (NHMUK). Photographs were taken using a LeicaDF295 digital camera and processed with Adobe Photoshop CS 8.0 and CorelDRAW 11 Graphic suite. All measurements are in micrometres.
Infection parameters
Parasitological terms, prevalence (P in %) and mean intensity (MI), were calculated according to Bush et al. (1997). Confidence intervals of prevalence and mean intensity were calculated using the free software Quantitative Parasitology 3.0 (Reiczigel & Rózsa, 2005). Possible differences between mean fork lengths of tuna samples were verified with Student’s t-test. Possible correlations between abundance of infection and host size were evaluated using the Spearman’s rank correlation coefficient (Rs); their significance was tested using the R software (“spearman” method, “cor.test” function, “stats” library of the R-software; R Development Core Team, 2014). Differences between parasite populations were evaluated using Fisher’s exact test for prevalence and Welsh’s bootstrap t-test for mean intensity (Reiczigel & Rózsa, 2005). Analyses were carried out only on data from entire specimens of T. thynnus, which were analysed for both gill and heart microhabitat of the parasites.
DNA extraction and amplification
Total DNA was isolated from single adults (three species) and 10 egg samples (c.50–100 eggs per sample) using the DNeasy®Blood & tissue Kit (Quiagen, Venlo, The Netherlands).
Two DNA fragments were amplified via polymerase chain reaction (PCR): the internal transcribed spacer 2 of the nuclear ribosomal DNA (ITS-2 rDNA) and partial fragment of the cytochrome c oxidase subunit I mitochondrial gene (cox1). The ITS-2 was amplified with five different primer pairs: GA1 (forward; 5′-AGA ACA TCG ACA TCT TGA AC-3′) and ITS2.2 (reverse; 5′-CCT GGT TAG TTT CTT TTC CTC CGC-3′) (Anderson & Barker, 1998); specific primers for Cardicola spp. BF_F (forward; 5′-GGA AAT TGT GCY ACC TGG CA-3′) and BF_R (reverse; 5′-GCA CAA GCG CTA CCA-3′) (Shirakashi et al., 2012); and three species-specific primer pairs: (i) Cfor_F (forward; 5′-TGA TTG CTT GCT TTT TCT CGA T-3′) and Cfor_R (reverse; 5′-TAT CAA AAC ATC AAT CGA CAT C-3′) for C. forsteri; (ii) Cori_F (forward; 5′-TGC TTG CTA TTC CTA GAT GTT TAC-3′) and Cori_R (reverse; 5′-AAC AAC TAT ACT AAG CCA CAA-3′) for C. orientalis; and (iii) Copt_F (forward; 5′-TTC CTA AAT GTG TGT GCA-3′) and Copt_R (reverse; 5′-TCA AAA CAT CAA TCG ACA CT-3′) for C. opisthorchis (see Polinski et al., 2013). Sequences for Cardicola sp. were amplified with GA1-ITS2.2 and also with Cori_F - Cori_R.
The partial cox1 fragment was amplified with the primers JB3 (forward; 5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and JB4.5 (reverse; 5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′) (Morgan & Blair, 1998; Razo-Mendivil et al., 2008). The thermocycling profile of the PCR amplifications consisted in an initial DNA denaturation at 95°C for 3 min, followed by 40 cycles of amplification at 95°C for 50 s, annealing at 50°C for 50 s and extension at 72°C for 2 min, and final extension at 72°C for 7 min, with the exception of amplifications using GA1 - ITS2.2 primer pair when an annealing temperature of 55°C was used (modified from Olson et al., 2003).
DNA sequencing, alignment and analysis
The amplicons were purified with the NucleoSpin Gel and PCR Clean-up kit (Machery-Nagel, Düren, Germany), following the manufacturer’s instructions and sequenced by Macrogen Europe Inc. (Amsterdam, The Netherlands) on a 3730XL DNA analyzer (Applied Biosystems, Foster City, USA) using the primers used for PCR.
Consensus sequences were assembled using Bioedit 7.2.3 (Hall, 1999) and submitted to BLAST on GenBank. The new ITS2 rDNA sequences of the aporocotylids from T. thynnus were aligned with the Cardicola spp. sequences available in GenBank (Table 2) with Clustal W in Bioedit 7.2.3 using gap opening penalty of 15 and gap extension penalty of 6 and refined by eye (Hall, 1999). New cox1 mDNA sequences are also provided to be used in future studies.
Phylogenetic tree inference was carried out by a likelihood-based Bayesian tree sampling procedure (BI) using MrBayes v 3.2.3 (Ronquist & Huelsenbeck, 2003), and a maximum parsimony (MP) approach using Mega v 6.06 (Tamura et al., 2013). The general time-reversible model with estimates of invariant sites and gamma distributed among-site rate variation (GTR+I+G) was estimated as the best substitution model for the dataset using jModelTest 2.1 (Darriba et al., 2012). Thus, parameters corresponding to this model (nst = 6, rates = invgamma) were applied in the BI analysis. Posterior probability distributions were generated using the Markov Chain Monte Carlos (MCMC) method. The MCMC was run for 500,000 generations sampling every 100th tree and ‘burn-in’ was set at 3,500 generations. The trees were visualised with FigTree v 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/)
Family Aporocotylidae Odhner, 1912
Genus Cardicola Short, 1953
Cardicola forsteri Cribb, Daintith & Munday, 2000
Host: Bluefin tuna Thunnus thynnus (L.) (fork length 94–260 cm).
Site in host: Adults in gill arteries and heart.
Localities: Balearic Sea (farms) (42°28′N–38°54′N, 0°34′W–4°34′E); Sardinian Sea (39°10′N–41°40′N, 7°60′E–9°25′E); Strait of Gibraltar (35°90′N–36°17′N, 5°32′W–5°60′W).
Prevalence: 3% (12 out of 421 fish examined, based on adult presence).
Voucher material: 2 specimens (NHMUK 2015.4.1.1-2).
Representative sequences: GenBank KP988303–KP988304 (ITS2); KP988302 (cox1).
Description (Figs. 2A, 3A)
[Based on 6 adults (means; see Table 3 for ranges).] Body wide, lanceolate, 4,620 × 849; body width to body length ratio 1:5.4. Tegumental spines very conspicuous, rectricted to ventro-lateral rows of 11–14 spines each. Oral sucker absent. Anterior nerve commissure well defined, close to mouth. Mouth ventral. Oesophagus long (1,395; n = 4), length representing 30% of body length, surrounded by gland-cells along its 2 medial quarters. Intestine H-shaped; anterior caeca of almost equal length, 360 (n = 3), distance from distal end to anterior extremity of body 1,121 (n = 3); posterior caeca of similar length, 2,061 (n = 4), distance from distal end to posterior extremity of body 1,040; anterior to posterior caeca length ratio 1:5.7. Testis with poorly defined contours, intra- and extra-caecal, overlapping posteriorly anterior part of ovary and extending to just anterior to caecal intersection, 2,138 × 472 (n = 4). Vas deferens wide, slightly sinuous, ventral to testis. Seminal vesicle curved, 344 × 62 (n = 4). Cirrus-sac absent. Ejaculatory duct not observed. Male genital pore dorsal, at 67 (n = 4) from sinistral body margin and 121 (n = 4) from posterior extremity of body. Ovary median, irregularly lobed, transversely oval, 358 × 519. Oviducal seminal receptacle elongate-oval, 529 × 98 (n = 4). Vitellarium follicular, follicles small, diffuse, extending between nerve commissure and ovary. Vitelline duct ventral to ovary. Uterus postovarian, sinistral, ascending sinuously from oötype to ovary, descending in coils at ovary. Female genital pore dorsal, at 140 (n = 2) from sinistral body margin and 403 (n = 2) from posterior extremity of body. Intrauterine eggs irregular in ascending (proximal) portion of uterus [19 × 12; area 125–260 (169) µm2; n = 20], becoming slightly larger and sub-oval in descending (distal) portion [21 × 15; area 205–352 (244) µm2; n = 23] (see Fig. 3A, inset a).
Schematic drawings of adult Cardciola spp. ex Thunnus thynnus from the Western Mediterranean. A, C. forsteri; B, C. opisthorchis; C, C.orientalis. C. opisthorchis has been reconstructed following the original description as no complete individuals were found; the reconstructed portion is indicated by the area with broken line contour. Scale-bars: 500 μm
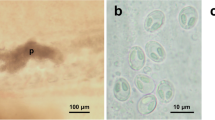
Cardicola spp. ex Thunnus thynnus from the Western Mediterranean. A–C, Posterior body extremities of whole mounts in Canada balsam of C. forsteri (A), C. opisthorchis (B) and C. orientalis (C) (insets a and b: intrauterine eggs of the distal portion of uterus); D, Light microscopy photograph of eggs of Cardicola sp. in a secondary lamella of T. thynnus; E, Stereomicroscope photograph of eggs of Cardicola spp. in secondary lamellae of T. thynnus cleared with glycerine. Scale-bars: A–C, 200 μm; insets a and b, 10 μm; D, 50 μm; E, 500 μm
Remarks
Findings of this species, supported by adult examination or molecular confirmation, have been reported in cage-reared T. maccoyii (type-host) from off South Australia (Cribb et al., 2000) and in T. thynnus from the Northwest Atlantic (Bullard et al., 2004) and the Mediterranean (Aiken et al., 2007). Adults of C. forsteri have not been described to date from T. thynnus. The present specimens are very similar to those from T. maccoyii used in the original description but are slightly larger 4,174–5,009 vs 2,512–3,688 µm (Cribb et al., 2000), with larger body length to width ratio (5.4 vs 4.3) and longer oesophagus and seminal vesicle (see Table 3).
Cardicola opisthorchis Ogawa, Ishimaru, Shirakashi, Takami & Grabner, 2011
Host: Bluefin tuna Thunnus thynnus (L.) (fork length 94–260 cm).
Site in host: Adults in heart.
Localities: Balearic Sea (farms) (42°28′N–38°54′N, 0°34′W–4°34′E); Sardinian Sea (39°10′N–41°40′N, 7°60′E–9°25′E); Strait of Gibraltar (35°90′N–36°17′N, 5°32′W–5°60′W).
Prevalence: 21% (50 out of 421 fish examined, based on adult presence).
Voucher material: 4 incomplete specimens (NHMUK 2015.4.1.3-6).
Representative sequences: GenBank KP988306–KP988307 (ITS2); KP988305 (cox1).
Description (Figs. 2B, 3B)
[Based on 6 incomplete adults as no complete worms were found (means; see Table 3 for ranges).] Body narrow, very elongated and slender; longest fragment 5,767 long, mean width 267. Tegumental spines inconspicuous, restricted to perpendicular ventro-lateral rows of 5–7 spines each, closer at posterior region. Oral sucker absent. Anterior nerve commissure and cords well defined, close to mouth. Oesophagus 1,428 long (n = 3) with inconspicuous gland-cells along its 2 medial quarters. Intestinal caeca length not measured (no fragments containing caecal intersection were available); posterior caeca extending to testis level, in some specimens up to first 1/4 of testis. Testis well defined, mostly postcaecal, overlapping ovary posteriorly, wide, almost reaching body margins, > 2,633 long, 248 wide (n = 3). Vas deferens ventral to testis, sinuous, slightly narrow. Seminal vesicle 101 × 28 (n = 3). Cirrus-sac absent. Ejaculatory duct not observed. Male genital pore dorsal, at 43 (n = 2) from sinistral body margin and 124 (n = 2) from posterior extremity of body. Ovary median, post-testicular, subtriangular, 429 × 249 (n = 3). Oviducal seminal receptacle elongate-oval, 240 × 48 (n = 2). Vitellarium follicular, follicles small, extending between nerve commissure and ovary. Vitelline duct ventral to ovary, widening at mid-level of ovary. Uterus postovarian, median to sinistral, ascending with coils from oötype to ovary, descending with soft coils, almost straight before genital pore. Female genital pore dorsal, at 59 (n = 2) from sinistral body margin and 192 (n = 2) from posterior extremity of body. Intrauterine eggs irregular in proximal portion of uterus [18 × 10; area 67–132 (88) µm2; n = 19], becoming elongated, ellipsoid to crescent shaped in distal portion [39 × 8; area 101–177 (139) µm2; n = 10] (see Fig. 3B, inset b).
Remarks
Cardicola opisthorchis was originally described based on material from T. orientalis off Japan (Ogawa et al., 2011). This species has already been reported in T. thynnus from the Mediterranean (Aiken et al., 2007; Ogawa et al., 2011), but this material was not described. The specimens from T. thynnus examined here were narrower than those in T. orientalis (205–375 vs 390–670 µm) (Ogawa et al., 2011). They also had narrower testis and ovary, and a shorter and narrower oviducal seminal receptacle compared with the type-specimens from T. orientalis (Table 3).
Cardicola orientalis Ogawa, Tanaka, Sugihara & Takami, 2010
Host: Bluefin tuna Thunnus thynnus (L.) (fork length 94–260 cm).
Site in host: Adults and eggs in gill arteries.
Localities: Balearic Sea (farms) (42°28′N–38°54′N, 0°34′W–4°34′E); Sardinian Sea (39°10′N–41°40′N, 7°60′E–9°25′E); Strait of Gibraltar (35°90′N–36°17′N, 5°32′W–5°60′W).
Prevalence: 4% (15 out of 421 fish examined, based on adult presence).
Voucher material: 1 specimen (NHMUK 2015.4.1.7).
Representative sequences: GenBank KP988309–KP988310 (ITS2).
Description (Figs. 2C, 3C)
[Based on 5 adults (means; see Table 3 for ranges).] Body lanceolate, elongated, 3,041 × 365; body width to body length ratio 1:8.3. Tegumental spines restricted to perpendicular ventro-lateral rows of 19–23 spines each. Oral sucker absent. Anterior nerve commissure conspicuous, close to anterior extremity. Oesophagus 723 long, length representing 23.8% of body length, surrounded by gland-cells along its middle third. Intestine H-shaped; anterior caeca short, of equal length, 191 long, distance from distal end to anterior extremity of body 522 (n = 3); posterior caeca of equal length, 1,427 long, extending to level of ovary, distance from distal end to posterior extremity of body 1,015; anterior to posterior caeca length ratio 1:7.5. Testis inter-caecal, compact, with bundles of fibre-like structures and well-defined contour, extending from ovary to just anterior to caecal intersection, 1,300 × 113 (n = 5). Vas deferens narrow, straight. Seminal vesicle elongated, as wide as uterus, 254 × 71 (n = 2). Cirrus-sac absent. Ejaculatory duct not observed. Male genital pore dorsal, close to sinistral body margin (at 32), at 103 from posterior extremity of body. Ovary irregular to piriform, narrow, median, 127 × 172 (n = 3). Oviducal seminal receptacle tubular, narrow. Vitellarium follicular, diffuse, extending from nerve commissure to ovary. Vitelline duct ventral to ovary. Uterus postovarian, median, descending straight and narrow from oötype, widening and ascending at level of anterior portion of seminal vesicle, and descending from ovary to wide metraterm. Female genital pore dorsal, at 76 from sinistral body margin and 234 from posterior extremity of body. Intrauterine eggs irregular, observed only in proximal portion of uterus [11 × 7; area 57–79 (63) µm2; n = 15].
Extrauterine eggs (found in gill secondary lamellae and identified with molecular analysis) ellipsoidal to slightly oval, 25–54 × 20–49 (40 × 31) (n = 207); perimeter 80–152 (115); area 467–1,384 (928) µm2.
Remarks
To date, C. orientalis was only reported in bluefin tunas from the Pacific Ocean: T. orientalis (type-host) from off Japan and T. maccoyii from off Australia (Shirakashi et al., 2013). This is the first report of C. orientalis from T. thynnus and the first report in the Atlantic basin. The dimensions of the specimens of C. orientalis from T. thynnus were similar to those of the specimens collected from T. maccoyii (see Shirakashi et al., 2013) and from T. orientalis off Nagasaki Prefecture (Japan), and higher than those from T. orientalis off Mie Prefecture (Japan) (Ogawa et al., 2010). The present specimens exhibit longer oesophagus, anterior caeca and seminal vesicle compared with the specimens previously described from other Thunnus spp. (Ogawa et al., 2010; Shirakashi et al., 2013) (Table 3).
Cardicola sp.
Host: Bluefin tuna Thunnus thynnus (L.) (fork length 115–189 cm).
Site in host: Eggs in gill arteries.
Locality: Balearic Sea (farms) (42°28′N–38°54′N, 0°34′W–4°34′E).
Representative sequences: GenBank KP988311–KP988314 (ITS2).
Description (Fig. 3D)
No adults were found. Extrauterine eggs (n = 30) from the secondary lamellae of the gills were ellipsoidal, 30–52 × 23–42 (38 × 30); perimeter 89–150 (109); area 568–1,284 (891) µm2.
Remarks
The molecular analyses (see below) indicate that these eggs do not belong to any of the species described in T. thynnus. Taking into account the strict host-specificity of the species within the family Aporocotylidae in general and within the genus Cardicola in particular (Ogawa et al., 2010), the eggs sequenced in our study probably belong to a new species. Although the eggs of C. orientalis and Cardicola sp. appear to be morphologically undistinguishable, the information on egg shape may be helpful to differentiate them from other species in T. thynnus such as C. opisthorchis. Shirakashi et al. (2012) indicated that the extrauterine eggs of C. opisthorchis and C. orientalis can be distinguished by their shape (crescent and oval, respectively). However, caution is required when identifying eggs from gills analysed by digestion since the chemical products often break the shell and dissolve the embryos thus leading to abnormal and confusing egg shapes.
Prevalence and mean intensity of Cardicola spp.
Adult Cardicola spp. were detected in 37% of the fish examined. Based on the morphology of the adults detected, blood flukes of three species of Cardicola were found, C. forsteri, C. orientalis and C. opisthorchis. The prevalence and mean intensity of adult Cardicola spp. according to the geographical area are shown in Fig. 4. Cardicola opisthorchis was the most prevalent and abundant species in both farmed (P = 23%; MI = 2. 7) and wild tunas (P = 6%; MI = 4.5) (Fig. 4). The prevalence of C. opisthorchis was higher in the farmed than in the wild tunas (p = 0.004). The prevalence and mean intensity of C. forsteri and C. orientalis showed similar values in farmed (P = 4%; MI = 1.0 and P = 5%; MI = 1.0, respectively) and wild tunas (P = 2%; MI = 1.0 and P = 3%; MI = 1.5, respectively). Host length differed between localities (t-values ranging from 5 to 36, p < 0.0001). No correlation was found between host length and parasite abundance in any of the samples (range for Rs = 0.05–0.10, p > 0.05). No differences in prevalence and mean intensity were found between wild tunas from different seas. No aporocotylid parasites were detected in wild fish sampled in the Balearic Sea (Fig. 4).
Prevalence (squares) and mean intensity (diamonds, only for adult worms) with 95% confidence intervals (lines) for Cardicola spp. in Thunnus thynnus from the Western Mediterranean. Data correspond to the analyses of gills and hearts from farmed (F, n = 39) and wild tunas (W, n = 133). Data for wild tunas are provided as a total (W) and separately, as Balearic Sea (B, n = 20), Strait of Gibraltar (G, n = 75) and Sardinian Sea (S, n = 38). Information on eggs refers to the presence of ovoid C. orientalis- and Cardicola sp.-like eggs. Significant differences (p ≤ 0.05) are indicated by stars
Only C. forsteri was found in both gills and heart; C. opisthorchis was collected from the heart only, and C. orientalis from the gills only. No statistically significant differences were found between the prevalence of C. forsteri in the heart and gills. Co-infections of C. forsteri and C. opisthorchis occurred in the heart of one cage-reared fish and in another from the wild.
Eggs found in the secondary lamellae of the gills were ellipsoidal to ovoid. No interspecific differences were found in relation to egg size and shape; however, the molecular study revealed that the eggs found belong to C. orientalis and to a fourth, apparently unknown species of Cardicola. The prevalence of blood flukes calculated based on egg detection was significantly higher in farmed than in wild tunas (67 vs 2%, p <0.0001) (Fig. 4).
Molecular analysis
ITS2 rDNA sequences were obtained for adult worms belonging to three species of Cardicola (C. forsteri: GenBank KP988303–KP988304; C. opisthorchis: KP988306–KP988307; C. orientalis: KP988309–KP988310). Comparison of the ITS2 sequences revealed that the new sequence for C. forsteri is identical with the sequences for isolates of C. forsteri from Spain (AB742428) and Australia (EF661575) (Aiken et al., 2007). In both the BI and MP phylogenetic analyses, this species clustered together with C. opisthorchis (6% sequence difference between the new sequence for C. forsteri and isolates EF653397, HQ324228, and KP988307 of C. opisthorchis; Aiken et al., 2007; Ogawa et al., 2011). The new ITS2 sequences for Cardicola forsteri had p-distance of 14% compared with C. orientalis (HQ324226 and KP988310; Ogawa et al., 2011), the third known species infecting bluefin tunas. The new ITS2 sequences for C. opisthorchis were identical with those for C. opisthorchis based on isolates from Spain (EF653397; Aiken et al., 2007) and Japan (HQ324228; Ogawa et al., 2011); they exhibited p-distance of 15% from C. orientalis (HQ324226 and KP988310; Ogawa et al., 2011). Finally, the new ITS2 sequence for C. orientalis was identical with C. orientalis isolated from Japan (HQ324226; Ogawa et al., 2011).
ITS2 rDNA sequences from eggs isolates from the gills of the fish were obtained only in amplifications with the primer pairs ITS2.2-GA1 and Cori_F-Cori_R (specific for C. orientalis). Sequences from six of these isolates were identical; a representative sequence (KP988308) clustered together with and was identical with sequences for C. orientalis obtained from adult worms (Fig. 5). A representative sequence for four isolates of Cardicola sp. (KP988311) appeared most similar to sequences for C. orientalis (p-distance of 13%), and rather distant from sequences for C. forsteri (p-distance of 20%) and C. opistorchis (p-distance of 19%), thus suggesting the presence of a fourth species of Cardicola in the samples of T. thynnus examined in the present study.
Phylogenetic tree based on Bayesian Inference analysis of ITS2 rDNA sequences of Cardicola spp., showing the position of C. forsteri, C. orientalis, C opistorchis from T. thynnus in the Western Mediterranean. Pearsonellum corventum was used as an outgroup. Host species is indicated for isolates from Thunnus spp. Asterisks indicate the newly-generated sequences in the present study. The scale-bar indicates the expected number of substitutions per site
Both BI (Fig. 5) and MP (data not shown) phylogenetic trees exhibited similar topologies. Cardicola aurata appeared separated from the remaining species of Cardicola spp. which were grouped in four well-supported clades from a basal polytomy. Sequences for C. forsteri and C. opisthorchis formed two strongly supported sister lineages within one of the clades and those for C. orientalis and Cardicola sp. exhibited a strongy suported sister-group relationship in another clade (Fig. 5).
Sequences of cox1 mitochondrial gene (446 nt) were only obtained from specimens of C. forsteri (KP988302) and C. opisthorchis (KP988305); these exhibited interspecific divergence of 14.6%. As no other cox1 sequences for Cardicola spp. are available in GenBank, these sequences are provided for future studies.
Discussion
This is the first record of four species of Cardicola infecting the same fish host, Thunnus thynnus. The closest precedent is the report of three congeneric species in Siganus fuscescens Houttuyn (Siganidae) (Nolan & Cribb, 2006a). This is also the first study to report C. orientalis in T. thynnus and to provide morphological characterisation of adult and eggs of Cardicola spp. from T. thynnus in the Mediterranean. Moreover, molecular evidence for the existence of a fourth species Cardicola is also provided. Although no adults of Cardicola sp. were found, this is probably a new species as no other species of this genus have been described from Thunnus spp. Adults must be found in order to provide a formal description of this species. Our observations show that all of the Cardicola spp. infecting bluefin tunas in the Pacific Ocean are also present in some areas of the Western Mediterranean, plus the possible new Mediterranean species. The fact that T. orientalis and T. maccoyii share an aporocotylid species (C. orientalis) is not surprising as their distributions overlap (Shirakashi et al., 2013). However, it is more surprising that T. thynnus, an Atlantic species, shares all Cardicola spp. with the two Pacific species of bluefin tuna (Nowak et al., 2006).
This situation could be explained by two hypotheses: (i) the three species of Cardicola already existed in the ancestor of the three bluefin tuna species; and (ii) the three parasite species may have been transmitted in overlapping areas of the distribution of bluefin tuna species. The first hypothesis has been proposed to explain the presence of similar aporocotylid species (Paradeontacylix spp.) in very distant populations (off Japan and in the Mediterranean) of Seriola dumerili (Risso), another pelagic migratory fish (Repullés-Albelda et al., 2008). The second hypothesis is based on the wide distribution of Thunnus spp., long-distance migratory species. Thunnus maccoyii is the only species whose distribution overlaps with T. orientalis and T. thynnus (FAO, 1994; Collette et al., 2011); however, to date C. opisthorchis has not been reported in this fish. The present results indicate the possibility that all species of Cardicola infecting bluefin tunas may infect any species of Thunnus. In this way, even other species of Thunnus with circumglobal distributions, i.e. T. alalunga (Bonnaterre), T. albacares (Bonnaterre) and T. obesus (Lowe), may contribute spreading the parasite. Aiken et al. (2007) proposed a third hypothesis to explain the circumglobal distribution of Cardicola spp. in bluefin tunas, indicating that the larval stages may be transported within intermediate hosts as biofouling on ships’ hulls.
Adult blood flukes were found in cage-reared and in wild fish, although values of infection were very low and only C. opisthorchis exhibited significantly higher prevalence in the cage-reared sample than in the wild. Eggs are much easier to detect and consequently values of infection based on egg presence were much higher than those based on detection of adults. Our data show that eggs were more prevalent in cage-reared than in wild tunas indicating that probably there are more suitable conditions for completion of the life-cycle of Cardicola spp. in the aquaculture facilities than in the wild. Although the present results may have been biased by the limited number of host specimens examined from the Balearic and Sardinian Seas, it is worth noting the lack of infection in the wild fish from the Balearic Sea. This can be attributed to the smaller size of the fish examined from this area compared with those from the remaining basins, and to differences in oceanic conditions between the basins. However, it was demonstrated that Cardicola spp. can infect bluefin tunas of different size-classes, including young-of-the-year (Shirakashi et al., 2011). Taking into account that tunas represent the real reservoir for the Cardicola spp. (they have a larger lifespan than that of the intermediate hosts and are less influenced by environment conditions than intermediate hosts) the health management of cage-reared bluefin tunas captured in the Balearic Sea can be favoured by the low levels of infections with Cardicola spp. in wild and resident tunas in this basin. Moreover this fact suggests that Cardicola spp. was imported to the fattening facilities with tunas caught outside the Balearic Sea, and that the control of intermediate host infections may have avoided the increase of the infection in farmed hosts.
No important morphological differences between the three species of Cardicola infecting T. thynnus and their original descriptions were observed (see Cribb et al., 2000; Ogawa et al., 2010, 2011) apart from the dimensions of the body and some internal organs (i.e. seminal vesicle). These differences may be due to the adaptation to different host species and may represent characteristic features of distinct parasite populations. However, they may be attributed to other factors such as host size, different degree of maturation, or to different types of collection and fixation or preservation techniques used. For example, the type-specimens of C. opisthorchis and C. orientalis were collected from juvenile fish, and the Mediterranean parasites described herein were mostly found in adult fish.
Molecular analyses of ITS2 rDNA demonstrated that three of the Cardicola spp. in Mediterranean bluefin tunas are genetically identical to the species present in bluefin tunas from the Pacific Ocean (Fig. 5). Investigation of other DNA markers such as the mitochondrial cox1 gene may help elucidate intraspecific differences at a finer scale as demonstrated for other aporocotylids (Repullés-Albelda et al., 2008). The scarce number of sequences deposited (none of them from Cardicola spp.) hampers the comparison of the results obtained with the cox1 marker; however, the sequences generated in our study may be used in future exploration of the infraspecific diversity of Cardicola spp.
Detection of eggs in the gills of the fish still remains an optimal tool to diagnose infections with Cardicola spp.; however, the co-occurrence of more than one species hampers identification to the species level. According to Shirakashi et al. (2012) the eggs of C. opisthorchis in the gills are identifiable based on their peculiar crescent shape, whereas those of C. orientalis are known to be oval. Cribb et al. (2000) described only intrauterine eggs of C. forsteri; there are no data for eggs of this species in fish gills. Descriptions of intrauterine eggs of the blood flukes are usually not a good source to identify eggs released in the circulatory system since they are usually rather irregular, taking their definitive size and shape upon release (Repullés-Albelda et al., 2008). However the distal intrauterine eggs of C. opisthorchis described here already show the crescent shape typical of the species, although still smaller and slightly creased (Fig. 3B, inset b). The extrauterine eggs from the gills described by us were all oval (Fig. 3D, E); although morphologically undistinguishable, these were molecularly assigned to C. orientalis and Cardicola sp. using the ITS2 marker. These results are in contrast with those of Mladineo & Tudor (2004), Mladineo et al. (2006, 2008) and Ruíz de Ybáñez et al. (2010) who reported the presence of eggs of C. forsteri in Mediterranean T. thynnus; however, their identification was not supported by molecular evidence. According to Shirakashi et al. (2012), the eggs of the species of Cardicola infecting T. orientalis may show different patterns of dispersion in the blood stream, those of C. opisthorchis being predominantly in the afferent filament artery and not in the secondary lamellae. This may explain the fact that these species were not found in our study since the afferent side of the filament is too thick to be cleared with glycerine in the large tunas examined.
The virulence of each species of Cardicola seems apparently different and some factors such as host species or age, may also be related. Ishimaru et al. (2013) reported mortalities higher than 50% associated with the presence of Cardicola spp. in young-of-the-year T. orientalis. In contrast, Nowak (2004) indicated that C. forsteri represented a low risk for the culture of T. maccoyii. In the case of T. thynnus cultures, very little is known. Mladineo et al. (2008) and Ruíz de Ybáñez et al. (2011) described some mild pathologies related to Cardicola sp. in T. thynnus (slight inflammatory and granulomatous reaction to eggs). As each parasite species seems to be associated to different degrees of damage, the knowledge of the specific identity of the species infecting cultured fish becomes particularly crucial for prophylaxis and therapeutic strategies. In fact, anthelmintics such as praziquantel have been seen to show different efficacy depending on the Cardicola species (Sun et al., 2011; Hardy-Smith et al., 2012; Ishimaru et al., 2013). Moreover, co-infections with other pathogens in culture conditions must also be taken into account, as well as the synergic effects of other parasites, such as caligid sea lice (Hayward et al., 2010). Prophylactic measures, e.g. removing the intermediate hosts, will also depend on the knowledge of the invertebrates harbouring the larvae of each species in the Mediterranean. Current findings indicate that the specific identity of the blood fluke eggs must be taken with caution, especially when the virulence of the species may differ thus highlighting the need of further studies to characterise the eggs of C. forsteri and C. opisthorchis and their location in T. thynnus.
References
Aiken, H. M., Bott, N. J., Mladineo, I., Montero, F. E., Nowak, B. F., & Hayward, C. J. (2007). Molecular evidence for cosmopolitan distribution of platyhelminth parasites of tunas (Thunnus spp.). Fish and Fisheries, 8, 167–180.
Aiken, H. M., Hayward, C. J., & Nowak, B. F. (2006). An epizootic and its decline of a blood fluke Cardicola forsteri in farmed southern bluefin tuna, Thunnus maccoyii. Aquaculture, 254, 40–45.
Anderson, G. R., & Barker, S. C. (1998). Inference of phylogeny and taxonomy within the Didymozoidae (Digenea) from the second internal transcribed spacer (ITS2) ribosomal DNA. Systematic Parasitology, 41, 87–94.
Bullard, S. A., Goldstein, R. J., Goodwin, R. H, I. I. I., & Overstreet, R. M. (2004). Cardicola forsteri (Digenea: Sanguinicolidae) from the heart of a northern bluefin tuna, Thunnus thynnus (Scombridae), in the Northwest Atlantic Ocean. Comparative Parasitology, 71, 245–246.
Bush A. O., Lafferty K. D., Lotz J. M., & Shostak A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revised. Journal of Parasitology, 83, 575–583.
Collette, B., Acero, A., Boustany, A., Canales Ramirez, C., Cardenas, G., Carpenter, K. E., Chang, S. K., Chiang, W., Di Natale, A., Die, D., Fox, W., Graves, J., Hinton, M., Juan Jorda, M., Minte Vera, C., Miyabe, N., Montano Cruz, R., Nelson, R., Restrepo, V., Schaefer, K., Schratwieser, J., Serra, R., Sun, C., Uozumi, Y. & Yanez, E. (2011). Thunnus orientalis. In: IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
Cribb, T. H., Adlard, R. D., Hayward, C. J., Bott, N. J., Ellis, D., Evans, D., & Nowak, B. F. (2011). The life cycle of Cardicola forsteri (Trematoda: Aporocotylidae), a pathogen of ranched southern bluefin tuna, Thunnus maccoyi. International Journal for Parasitology, 41, 861–870.
Cribb, T. H., Daintith, M., & Munday, B. (2000). A new blood-fluke, Cardicola forsteri,(Digenea: Sanguinicolidae) of southern blue-fin tuna (Thunnus maccoyii) in aquaculture. Transactions of the Royal Society of South Australia Incorporated, 124, 117–120.
Culurgioni, J., Mele, S., Merella, P., Addis, P., Figus, V., Cau, A., Karakulak, F. S., Garippa, G. (2014). Metazoan gill parasites of the Atlantic bluefin tuna Thunnus thynnus (Linnaeus) (Osteichthyes: Scombridae) from the Mediterranean and their possible use as biological tags. Folia Parasitologica, 61, 148–156.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772–772.
Dennis, M. M., Landos, M., & D’Antignana, T. (2011). Case - control study of epidemic mortality and Cardicola forsteri-associated disease in farmed southern bluefin tuna (Thunnus maccoyii) of South Australia. Veterinary Pathology Online, 48, 846–855.
FAO (1994). FAO Fisheries Technical Paper No. 337. World review of highly migratory species and straddling stocks. Rome: FAO, 70 pp.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hardy-Smith, P., Ellis, D., Humphrey, J., Evans, M., Evans, D., Rough, K., Valdenegro, V., Nowak, B. (2012). In vitro and in vivo efficacy of anthelmintic compounds against blood fluke Cardicola forsteri. Aquaculture, 334, 39–44.
Hayward, C. J., Ellis, D., Foote, D., Wilkinson, R. J., Crosbie, P. B., Bott, N. J., & Nowak, B. F. (2010). Concurrent epizootic hyperinfections of sea lice (predominantly Caligus chiastos) and blood flukes (Cardicola forsteri) in ranched southern bluefin tuna. Veterinary Parasitology, 173, 107–115.
Holzer, A. S., Montero, F. E., Repullés, A., Nolan, M. J., Sitja-Bobadilla, A., Alvarez-Pellitero, P., Zarza, C., Raga, J. A., (2008). Cardicola aurata sp. n. (Digenea: Sanguinicolidae) from Mediterranean Sparus aurata L. (Teleostei: Sparidae) and its unexpected phylogenetic relationship with Paradeontacylix McIntosh, 1934. Parasitology International, 57, 472–482.
Ishimaru, K., Mine, R., Shirakashi, S., Kaneko, E., Kubono, K., Okada, T., Sawada, Y., Ogawa, K., (2013). Praziquantel treatment against Cardicola blood flukes: Determination of the minimal effective dose and pharmacokinetics in juvenile Pacific bluefin tuna. Aquaculture, 402, 24–27.
Mladineo, I. (2006). Parasites of Adriatic cage reared fish. Acta Adriatica, 47, 23–28.
Mladineo, I., & Tudor, M. (2004). Digenea of Adriatic cage-reared Northern bluefin tuna (Thunnus thynnus thynnus). Bulletin of the European Association of Fish Pathologists, 24, 144–152.
Mladineo, I., Zilic, J., & Cankovic, M. (2008). Health survey of Atlantic bluefin tuna, Thunnus thynnus (Linnaeus, 1758), reared in Adriatic cages from 2003 to 2006. Journal of the World Aquaculture Society, 39, 281–289.
Morgan, J. A. T., & Blair, D. (1998). Relative merits of nuclear ribosomal internal transcribed spacers and mitochondrial CO1 and ND1 genes for distinguishing among Echinostoma species (Trematoda). Parasitology, 116, 289–297.
Nolan, M. J., & Cribb, T. H. (2004). Two new blood flukes (Digenea: Sanguinicolidae) from Epinephelinae (Perciformes: Serranidae) of the Pacific Ocean. Parasitology International, 53, 327–335.
Nolan, M. J., & Cribb, T. H. (2006a). An exceptionally rich complex of Sanguinicolidae von Graff, 1907 (Platyhelminthes: Trematoda) from Siganidae, Labridae and Mullidae (Teleostei: Perciformes) from the Indo-west Pacific Region. Zootaxa, 1218, 3–80.
Nolan, M. J., & Cribb, T. H. (2006b). Cardicola Short 1953 and Braya n. gen. (Digenea: Sanginicolidae) from five families of tropical Indo-Pacific fishes. Zootaxa, 1265, 1–80.
Nowak, B. F. (2004). Assessment of health risks to southern bluefin tuna under current culture conditions. Bulletin of the European Association of Fish Pathologists, 24, 45–51.
Nowak, B., Mladineo, I., Aiken, H., Bott, N., & Hayward, C. (2006). Results of health surveys of two species of farmed tuna: Southern bluefin tuna (Thunnus maccoyii) in Australia and northern bluefin tuna (Thunnus thynnus) in the Mediterranean. Bulletin of the European Association of Fish Pathologists, 26, 38–42.
Ogawa, K., Ishimaru, K., Shirakashi, S., Takami, I., & Grabner, D. (2011). Cardicola opisthorchis n. sp. (Trematoda: Aporocotylidae) from the Pacific bluefin tuna, Thunnus orientalis (Temminck & Schlegel, 1844), cultured in Japan. Parasitology International, 60, 307–312.
Ogawa, K., Tanaka, S., Sugihara, Y., & Takami, I. (2010). A new blood fluke of the genus Cardicola (Trematoda: Sanguinicolidae) from Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel, 1844) cultured in Japan. Parasitology International, 59, 44–48.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Padrós, F., Zarza, C., & Crespo, S. (2001). Histopathology of cultured sea bream Sparus aurata infected with sanguinicolid trematodes. Diseases of Aquatic Organisms, 44, 47–52.
Paperna, I., & Dzikowski, R. (2006). Digenea (Phylum Platyhelminthes). In: Woo P. T. K. (Ed.) Fish diseases and disorders, Volume 1, 2nd edn. Wallingford, UK: CAB International.
Polinski, M., Hamilton, D. B., Nowak, B., & Bridle, A. (2013). SYBR, TaqMan, or both: Highly sensitive, non-invasive detection of Cardicola blood fluke species in Southern Bluefin Tuna (Thunnus maccoyii). Molecular and Biochemical Parasitology, 191, 7–15.
Razo-Mendivil, U., Rosas-Valdez, R., & Pérez-Ponce de León, G. (2008). A new cryptogonimid (Digenea) from the Mayan cichlid, Cichlasoma urophthalmus (Osteichthyes: Cichlidae), in several localities of the Yucatán Peninsula, Mexico. Journal of Parasitology, 94, 1371–1378.
Reiczigel, J., & Rózsa, L. (2005). Quantitative Parasitology 3.0. Budapest. Distributed by the authors.
Repullés-Albelda, A., Montero, F. E., Holzer, A. S., Ogawa, K., Hutson, K. S., & Raga, J. A. (2008). Speciation of the Paradeontacylix spp. (Sanguinicolidae) of Seriola dumerili two new species of the genus Paradeontacylix from the Mediterranean. Parasitology International, 57, 405–414.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Ruíz de Ybáñez, R. R., Peñalver, J., Martínez-Carrasco, C., Del Rio, L., Mª Dolores, E., Berriatua, E., & Muñoz, P. (2011). Blood fluke infection of cage reared Atlantic bluefin tuna, Thunnus thynnus in West Mediterranean. Fish Pathology, 46, 87–90.
Shirakashi, S., Kishimoto, Y., Kinami, R., Katano, H., Ishimaru, K., Murata, O., Itoh, N., Ogawa, K., (2012). Morphology and distribution of blood fluke eggs and associated pathology in the gills of cultured pacific bluefin tuna, Thunnus orientalis. Parasitology International, 61, 242–249.
Shirakashi, S., Tsunemoto, K., Webber, C., Rough, K., Ellis, D., & Ogawa, K. (2013). Two species of Cardicola (Trematoda: Aporocotylidae) found in southern bluefin tuna Thunnus maccoyii ranched in South Australia. Fish Pathology, 48, 1–4.
Sibeni, F., & Calderini, F. (2014). FishStatJ: Universal software for fishery statistical time series. FAO Fisheries and Aquaculture Department, Statistics and Information Service.
Sun, X., Yoshinaga, T., Doi, H., Shirakashi, S., Ishimaru, K., & Ogawa K. (2011). In vitro efficacy of praziquantel against fish blood flukes. Annual meeting of the Japanese Society of Fish Pathology, Tokyo: Japan, p. 30.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis Version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Acknowledgements
The authors are indebted to Grup Balfegó for their support and collaboration. We are grateful to P. Addis, J. Culurgioni (University of Cagliari), D. Macías and E. Rodríguez-Marín (Spanish Institute of Oceanography) for their support and collaboration during sampling. We also thank N. Fraija (University of Valencia) for her help in the laboratory analyses. Thanks are also due to F. Barraclough for revising the English. JP-A benefits from a “Atracció de talent” PhD student grant from the University of Valencia and JR-L benefits from a PhD fellowship from the Spanish Ministry of Education and Science (ESM).
Funding
This study was funded by the Ministry of Science and Innovation, Spanish Government (project AGL 2010-20892) and by the Valencian Local Government (projects PROMETEO 2015-018 and ISIC/2012/003).
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palacios-Abella, J.F., Rodríguez-Llanos, J., Mele, S. et al. Morphological characterisation and identification of four species of Cardicola Short, 1953 (Trematoda: Aporocotylidae) infecting the Atlantic bluefin tuna Thunnus thynnus (L.) in the Mediterranean Sea. Syst Parasitol 91, 101–117 (2015). https://doi.org/10.1007/s11230-015-9568-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-015-9568-x