Abstract
Nematodes of the genus Raphidascaroides Yamaguti, 1941 parasitising doradid catfishes (Siluriformes: Doradidae) in Brazil were studied based on morphological and molecular evaluation of newly collected material. A new species, Raphidascaroides moraveci n. sp., is described from the intestine of Platydoras armatulus (Valenciennes) from River Miranda, River Paraguay basin, Pantanal, Mato Grosso do Sul. The new species differs from all of the congeners in having short spicules (163–217 μm in length) representing less than 1% of the total body length and in the posterior region of cloacal opening covered by small rudimentary spines. In addition, it differs from the other congeneric species in the number and arrangement of the caudal papillae and the structure of lips and tail. Raphidascaroides moraveci n. sp. is the third species described from freshwater fishes and the second one in the Neotropical Region. New morphological data on R. brasiliensis Moravec & Thatcher, 1997 from Megalodoras uranoscopus (Eigenmann & Eigenmann) and Platydoras costatus (Linnaeus) (both new host records) from River Xingu, River Amazon basin, Pará, are provided including scanning electron micrographs of taxonomically important structures. The differentiation of the new species is supported by molecular data (partial sequences of the small and large subunits of the rRNA gene).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Raphidascaroides Yamaguti, 1941 (Nematoda: Anisakidae) includes intestinal nematodes parasitic mainly in marine fishes, with only two species described from freshwater fishes, namely R. bishaii Khalil, 1961 from Gymnarchus niloticus Cuvier (Gymnarchidae) in the Sudan and R. brasiliensis Moravec & Thatcher, 1997 from Pterodoras granulosus (Valenciennes) (Doradidae) in Brazil (Khalil, 1961; Moravec & Thatcher, 1997). Seventeen nominal species have been described, but only nine of them are considered valid (Bruce, 1990; Moravec & Thatcher, 1997).
During a survey of the parasites of freshwater fishes in Brazil, specimens of Raphidascaroides were collected from three different species of doradid catfish (Siluriformes: Doradidae) in the lower River Xingu, one of the largest southern tributaries of the River Amazon, and River Miranda, a tributary of River Paraguay, Pantanal (River Paraná basin). Morphological examination of these nematodes has revealed that specimens collected in fish from Pantanal represent a new species which is described in the present paper. In addition, new morphological data on R. brasiliensis found in fish from River Xingu are provided together with the first assessment of the phylogenetic relationships of species of Raphidascaroides with other anisakid nematodes.
Materials and methods
Collection, processing and morphological examination
Fishes were collected by hook and trawler nets in two different localities. One Megalodoras uranoscopus (Eigenmann & Eigenmann) (total body length of 47 cm) and one Platydoras costatus (Linnaeus) (total body length of 21.5 cm) were collected in River Xingu, Pará, Brazil; one Platydoras armatulus (Valenciennes) (total body length of 23.5 cm) was collected in River Miranda, Mato Grosso do Sul, Brazil. Host nomenclature and classification follows Froese & Pauly (2014). Nematodes were washed in saline, fixed in hot (almost boiling) 4% formaldehyde solution and preserved in 70% ethanol. For morphological observations, nematodes were cleared in glycerine. The middle body parts of some specimens were excised and fixed in molecular grade 96–99% ethanol for genetic studies; the anterior and posterior parts were fixed in 4% formalin for identification.
Drawings were made using a drawing tube attached to a microscope Olympus BX51. Measurements are given in micrometres, unless otherwise stated. Some specimens (two males and two females of each parasite infrapopulation) for scanning electron microscopy (SEM) were dehydrated through a graded ethanol series, dried in hexamethyl disilazane, coated with gold and examined in a JEOL JSM-740 1F, at an accelerating voltage of 4 kV. Four paratypes (two males and two females) of Raphidascaroides brasiliensis Moravec & Thatcher, 1997 deposited in the Helminthological Collection of the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice (acronym IPCAS) (Cat. No. N-686) were re-examined. Newly collected specimens were deposited in the Coleção Helmintológica do Instituto Oswaldo Cruz (acronym CHIOC) and in IPCAS. The systematic classification of the parasites follows Anderson et al. (2009).
DNA sequencing and phylogenetic analyses
Tissue samples from the specimens used for molecular analyses were taken as follows: one sample of Raphidascaroides moraveci n. sp. collected from P. armatulus (code number BRMS4), two samples of R. brasiliensis collected from M. uranoscopus and P. costatus, respectively (code numbers PAX11 and PAX14, respectively). The anterior and posterior ends of these specimens, i.e. hologenophores (the voucher specimens from which the molecular sample is directly derived; see Astrin et al., 2013 for more details) were identified based on their morphology. The taxa for which sequences have been retrieved from GenBank are listed in Table 1. Genomic DNA was isolated using DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany), following manufacturer’s instructions. The SSU rRNA gene (18S) was amplified using the protocol and primers PhilonemaF + PhilonemaPCRr described in Černotíková et al. (2011). The LSU rRNA gene (28S) was amplified using the primers D2A (5′-ACA AGT ACC GTG AGG GAA AGT-3′) and D3B (5′-TGC GAA GGA ACC AGC TAC TA-3′) of Nunn (1992). The cycling parameters for amplification of the LSU rDNA were as follows: denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, annealing at 50°C for 1 min and elongation at 72°C for 1 min, followed by a post-amplification extention step at 72°C for 6 min. PCR products were purified through an enzymatic treatment with exonuclease I and shrimp alkaline phosphatise (Werle et al., 1994) and Sanger sequenced at GATC Biotech (Konstanz, Germany) using the PCR primers and two internal primers (WF760 and WR800, see Černotíková et al., 2011) in the case of SSU rDNA. Contiguous sequences were assembled in Genious (Geneious ver. 7 created by Biomatters, available from http://www.geneious.com/) and deposited in the GenBank database under accession numbers KP726274, KP726276, KP726278 (SSU rDNA) and KP726275, KP726277, KP726279 (LSU rDNA).
The SSU and LSU rDNA datasets were aligned separately using the E-INS-i algorithm of the program MAFFT (Katoh et al., 2002) implemented in Geneious and ambiguously aligned positions were excluded. Gene alignments were then concatenated and subjected to maximum likelihood (ML) and Bayesian inference (BI) analyses. ML and BI trees were calculated under the GTR+I+G model of evolution using PHYML (Guindon & Gascuel, 2003) and MrBayes (Huelsenbeck & Ronquist, 2001) Geneious plugins, respectively. The model of evolution was chosen using jModelTest 2 (Guindon & Gascuel, 2003; Darriba et al., 2012). BI analysis was run for 2 × 106 generations, sampling every 500th tree and discarding a ‘burn-in’ fraction of 5 × 105 trees. ML nodal support was estimated by 100 non-parametric bootstrap replications.
-
Family Anisakidae Railliet & Henry, 1912
-
Genus Raphidascaroides Yamaguti, 1941
Raphidascaroides moraveci n. sp.
Type-host: Southern striped raphael Platydoras armatulus (Valenciennes) (Siluriformes: Doradidae).
Site in host: Intestine.
Type-locality: River Miranda (19°34′S, 57°0′W) (River Paraná basin), Mato Grosso do Sul, Brazil.
Intensity of infection: 19 nematodes found in the only fish specimen examined.
Type-material: Holotype, allotype, 6 paratypes and 1 hologenophore in CHIOC (36728a–c); 6 paratypes in IPCAS (N-1079).
Etymology: The specific name is in honour of Dr. František Moravec from the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, for his valuable contribution to the taxonomy of nematode parasites of fish.
Description (Figs. 1, 2)
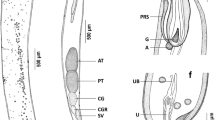
General. Large, whitish nematodes. Cuticle thick, with fine transverse striations throughout body. Lateral alae absent. Anterior end with 3 well-developed lips, dorsal lip slightly narrower than subventral (Figs. 1B, E, 2A–D). Lips relatively narrow, longer than wide, with lateral flanges broadest at base; pulp with 2 anterior lobes. Dorsal lip with 2 subdorsal double papillae (Figs. 1B, 2D); subventral lips with amphid located near pulp base, adjacent to weakly developed single circular formation and single conspicuous subventral double papilla (Figs. 1E, 2C). Interlabia well-developed, narrow, length > 1/3 of lip length (Figs. 1C, 2B). Oesophagus muscular, long, broader at its posterior part (Fig. 1A). Nerve-ring encircling oesophagus between 1/8 and 2/8 of its length (Fig. 1A). Excretory pore slightly posterior to level of nerve-ring (Fig. 1A). Subspherical ventriculus located between oesophagus and intestine; ventricular appendix long (Fig. 1A).
Raphidascaroides moraveci n. sp. ex Platydoras armatulus. Holotype (male) and allotype (female). A, Anterior end of male, lateral view; B, C, Cephalic end of female, dorsal and ventral views; D, Vulval region; E, Dorsal lip of male; F, Posterior end of male, lateral view; G, Tail of male, ventral view; H, Tail of female, lateral view; I, Egg; J, Tail of male, lateral view
Raphidascaroides moraveci n. sp. ex Platydoras armatulus. Scanning electron micrographs. A, Anterior end, apical view; B, Cephalic end, ventral view, C, Cephalic end, subventral view; D, Cephalic end, dorsal view; E, Tail of male, subventral view (asterisk indicates small rudimentary spines); F, Clocal region of male, subventral view (asterisk indicates small rudimentary spines); G, Posterior end of male, sublateral view; H, Tail of male, ventral view (asterisk indicates small rudimentary spines); I, Tail of female, sublateral view. Abbreviations: a, amphid; c, cephalic papilla; d, dorsal lip; e, excretory pore; i, interlabia; p, phasmid; s, subventral lip; u, unpaired papilla
Male [Based on 5 specimens; measurements of holotype in parentheses.] Body length 26.2–29.6 (27.5) mm, maximum width 617–720 (697). Lips 141–162 (141) long; interlabia 54–68 (56) long. Oesophagus 3.1–3.7 (3.6) mm long, with maximum width 159–188 (159). Entire length of oesophagus represents 11–13 (13)% of total body length. Nerve-ring and excretory pore located at 478–597 (478) and 525–659 (525), respectively, from anterior end. Ventriculus 79–106 (90) long, 116–138 (122) wide; ventricular appendix 623–739 (694) long. Spicules equal, short, well sclerotised, stout (Fig. 1F), 163–217 (163) long, representing 0.6–0.8 (0.6)% of total body length. Gubernaculum absent. Precloacal papillae: 22 pairs of subventral papillae, smaller pairs near cloacal opening; large median unpaired papilla slightly anterior to cloacal opening (Figs. 1F, G, J, 2E, F, H). Adcloacal papillae: 2 pairs, single small subventral pair followed by larger adjacent pair (Figs. 1G, J, 2E, H). Postcloacal papillae: 4 pairs organised in 2 subventral vertical lines, first and second pairs larger than third and fourth (Figs. 1G, 2H). Phasmidial pores conspicuous, located laterally in tail at level of last postcloacal pair of papillae (Figs. 1G, J, 2E, G, H). Region immediately posterior to cloacal opening covered by small rudimentary spines (Figs. 1G, J, 2E, F, H). Tail conical, relatively short, 257–296 (264) long, ending in rounded tip (small mucron), with lateral longitudinal expansions of body wall (Figs. 1F, G, J, 2E, G, H), internally supported by fine, transverse musculature (Figs. 1F, G, J).
Female [Based on 9 gravid specimens; measurements of allotype in parentheses.] Body length 27.6–35.4 (32.3) mm, maximum width 766–928 (826). Lips 150–187 (165) long; interlabia 64–75 (72) long. Oesophagus 3.7–4.5 (4.0) mm long, with maximum width 183–211 (192). Entire length of oesophagus represents 11–14 (13)% of total body length. Nerve-ring and excretory pore located at 514–614 (614) and 593–714 (714), respectively, from anterior end. Ventriculus 138–170 (138) long, 145–174 (174) wide; ventricular appendix 705–893 (868) long. Vulva in second quarter of body, at 7.7–10.1 (9.5) mm from anterior end, at 28–31 (29)% of body length; vulval lips not elevated (Fig. 1D). Vagina posteriorly directed with well-developed musculature (Fig. 1D). Uterus amphidelphic, full of eggs; ovaries tubular (Fig. 1D). Eggs widely-oval, with membranous, smooth, thin shell, non-embryonated (Fig. 1I), measuring 45–53 × 35–50. Phasmidial pores inconspicuous, difficult to observe, located in posterior half of tail (Fig. 2I). Tail conical, relatively long, 370–480 (425), ending in rounded tip (small mucron) (Figs. 1H, 2I).
Remarks
The nematodes from P. armatulus described above belong to Raphidascaroides because they possess an excretory pore slightly posterior to the nerve-ring, three well-developed lips without dentigerous ridges, interlabia, ventriculus with a posteriorly directed ventricular appendix, no intestinal caecum, numerous precloacal papillae and a vulva located at the anterior half of the body (Yamaguti, 1941; Moravec, 1998; Moravec & Nagasawa, 2000; Anderson et al., 2009).
Raphidascaroides moraveci n. sp. differs from all congeners by having males with short spicules (163–217 µm) representing less than 1% of total body length, and with posterior region of the cloacal opening covered by small rudimentary spines. The new species differs from R. brasiliensis and R. bishaii, the only species parasitic in freshwater fishes, in the structure of the lips, which are narrower and longer in R. moraveci n. sp., and in the number and arrangement of the postcloacal papillae in males i.e., R. brasiliensis has 5–6 and R. bishaii only 2–3 pairs of small papillae anteriorly arranged in groups, whereas the new species has four pairs of medium to large-sized papillae arranged in two lateral lines (Khalil, 1961; Moravec & Thatcher, 1997). Furthermore, both males and females of R. bishaii possess tail end covered by rudimentary spines whereas these are absent in R. moraveci n. sp. (Khalil, 1961).
The remaining seven species of Raphidascaroides considered valid have been described from marine fishes off other continents than America (South and East Asia, Australia, Africa) (Bruce, 1990; Moravec & Thatcher, 1997; Bilqees et al., 2005). The new species can be distinguished from these as follows. Raphidascaroides bengali Rajyalakshmi, 1995, R. indicus Rajyalakshmi, 1994, R. chilomycteri Yamaguti, 1961, R. nipponenis Yamaguti, 1941 and R. elongatus Bilqees, Shaukat, Navqi & Medi, 2005 have longer spicules and greater spicule/body length ratio than the new species (spicule length > 450 vs 163–217 µm; minimum spicule/body length ratio of 2.1 vs 0.6–0.8%); they also differ in the morphology and structure of the lips and in the number and arrangement of the caudal papillae, i.e., none of them have characteristic narrow labia with narrow base and five pairs of postcloacal papillae arranged in two subventral longitudinal lines as in the new species (Yamaguti, 1941, 1961; Rajyalakshmi, 1994, 1995; Moravec & Nagasawa, 2000; Bilqees et al., 2005). Raphidascaroides africanus Khalil & Oyetayo, 1988, parasitic in Bostrychus africanus (Steindachner) (Eleotridae) from Nigeria, and R. fisheri Hooper, 1983 described from Platycephalus endrachtensis Quoy & Gaimard (Platycephalidae) in Australia have the tail tip in both sexes covered by rudimentary spines (Hooper, 1983; Khalil & Oyetayo, 1988) that are absent in the new species. Furthermore, R. africanus has lateral alae extending to the posterior end of the body (Khalil & Oyetayo, 1988) and R. fisheri has only two pairs of postcloacal papillae and sub-equal spicules (280 and 320 µm long) (Hooper, 1983).
The new species also possesses lateral expansions of the body wall in the caudal region of males. This structure does not seem to represent an artefact because it was observed in all specimens and was seen also in SEM micrographs (Figs. 2E, G, H). Raphidascaroides moraveci n. sp. is the third species of Raphidascaroides described from freshwater fish and the second freshwater species of the genus known in South America.
Raphidascaroides brasiliensis Moravec & Thatcher, 1997
Hosts: Megalodoras uranoscopus (Eigenmann & Eigenmann) and Platydoras costatus (Linnaeus) (Siluriformes: Doradidae) (both new hosts records).
Site in host: Intestine.
Localities: River Xingu (near Pimental Dam) (3°12′S, 52°12′W) (River Amazon basin), State of Pará, Brazil (first record after original description); both new locality records.
Intensity of infection: 21 nematodes found in single M. uranoscopus and 16 nematodes found in single P. costatus examined.
Voucher material: 8 males, 8 females and 2 hologenophores are deposited in CHIOC (36726a, b; 36727a, b) and IPCAS (N-686/2/3).
Description (Figs. 3, 4)
Male [Based on 10 specimens from M. uranoscopus and 8 specimens from P. costatus; for measurements see Table 2.] Precloacal papillae: 25–27 pairs of subventral small papillae, sometimes arranged asymmetrically, with 2 pairs at same level (Fig. 3F); single large median papilla anterior to cloacal opening (Figs. 3G, H, 4I, J). Adcloacal papillae: single small lateral pair, followed by single small subventral pair, both pairs sometimes slightly posterior or anterior to cloacal line (Figs. 3G, H, 4I). Postcloacal papillae: 6 pairs, 4 of which anteriorly distributed and subventral, 2 remaining pairs posteriorly located, 1 lateral and 1 sublateral (Figs. 3G, H, 4I, J). Lateral phasmidial pores conspicuous, located at level of first or second postcloacal papillae pairs (Figs. 3G, H, 4I).
Raphidascaroides brasiliensis Moravec & Thatcher, 1997 ex Megalodoras uranoscopus. A, Anterior end, lateral view; B, Cephalic end, dorsal view; C, Cephalic end, ventral view; D, Vulval region; E, Subventral lip; F, Posterior end of male, lateral view (arrowhead indicates phasmid); G, H, Tail of male, ventral and lateral views (arrowheads indicate phasmids); I, Egg; J, Tail of female, lateral view (arrowhead indicates phasmid); K, Tail tip of female, subventral view
Raphidascaroides brasiliensis Moravec & Thatcher, 1997 ex Megalodoras uranoscopus. Scanning electron micrographs. A–D, Cephalic end. A, Cephalic end, apical view; B, Cephalic end, ventral view; C, Cephalic end, dorsal view; D, Cephalic end, subventral view; E, Detail of the anal region of female, ventral view; F, Posterior end of female, ventral view (arrowheads indicate pore-like structures); G, Detail of the pore-like structure; H, Tail tip of female (arrowheads indicate spine-like structures, asterisk indicates mucrons); I, J, Tail of male, lateral and ventral views (arrowhead indicates lateral precloacal papilla). Abbreviations: a, amphid; c, cephalic papilla; d, dorsal lip; i, interlabia; p, phasmid; s, subventral lip; u, unpaired papilla
Female [Based on 8 gravid specimens from M. uranoscopus and 7 specimens from P. costatus; for measurements see Table 2.] Two pore-like structures (not phasmids) located slightly lateral to anal opening (Fig. 4E–G). Small, inconspicuous phasmidial pores located laterally in posterior half of tail (Figs. 3J, 4H). Tail conical, short, ending in small semi-spherical protuberance (mucron) (Figs. 3K, 4H); minute spine-like structures visible only under SEM (Fig. 4H).
Remarks
Nematodes found in the two species of doradid fishes from River Xingu correspond in their morphology and morphometry to those described as R. brasiliensis from Pterodoras granulosus by Moravec & Thatcher (1997). The original description was detailed and SEM micrographs were also provided, with the exception of the tail in female. In the present study, we provide additional details on the morphology of this nematode based on newly collected material from two new hosts, both belonging to the Doradidae. Morphological examination of the new material and its comparison with paratypes of R. brasiliensis deposited in IPCAS revealed slight differences (see Table 2).
In females from both M. uranoscopus and P. costatus, we observed a pair of pore-like structures, which are not phasmids, located in the horizontal line of anal opening, a semi-spherical structure (small mucron) and minute spine-like formations (observed only using SEM) located on the tail tip. These features were not mentioned by Moravec & Thatcher (1997) and are difficult to observe using light microscopy. However, re-examination of paratypes of R. brasiliensis confirmed the presence of the pore-like structures in these specimens.
In addition, we observed slight differences in the arrangement and number of caudal papillae in males, the morphology and position of phasmids and the number and arrangement of postcloacal papillae (six pairs in the new material vs five pairs in paratypes). However, these differences are negligible and apparently reflect individual, intraspecific variability of specimens from different fish hosts and of different state of maturity (females studied by Moravec & Thatcher, 1997 were seemingly more mature and thus larger than those found in fishes in River Xingu).
Phylogenetic relationships of Raphidascaroides spp.
Partial sequences of the rDNA of R. moraveci n. sp. (1,562 and 739 bp long for SSU and LSU rDNA, respectively) and R. brasiliensis (1,562 and 763 bp long for SSU and LSU rDNA, respectively), were obtained. The concatenated sequences of the SSU and LSU rDNA for R. brasiliensis from M. uranoscopus and P. costatus were almost identical with only two variable sites in the LSU gene (i.e. < 0.1% sequence divergence) and, logically, they were most similar to those of R. moraveci n. sp. with sequence identity of 98.8%. The topology of the phylogenetic trees generated using ML and BI was identical, in both cladograms species of Raphidascaroides clustered together and formed a well-supported clade (Fig. 5).
Maximum likelihood (ML) phylogenetic tree from phylogenetic analysis of the concatenated sequences of SSU and LSU rDNA for ascaridid nematodes (families Anisakidae, Ascarididae and Toxocaridae). Branch support indicated as ML (100 replications)/ Bayesian posterior probability (for 2 × 106 generations; bur-in = 5 × 105). Symbols: *parasites of freshwater fishes; †parasites of marine fishes
Phylogenetic analysis of the new sequences for Raphidascaroides spp. with those for selected ascaridids (species of the families Ascarididae Baird, 1853, Toxocaridae Hartwich, 1954 and Anisakidae) retrieved from GenBank revealed that Raphidascaroides spp. form a well-supported monophyletic assemblage among the nematodes of the family Anisakidae (Fig. 5). Hysterothylacium pelagicum Deardorff & Overstreet, 1982 from Coryphaena hippurus (Linnaeus) (Coryphaenidae) appeared as a sister branch of Raphidascaroides but with weak support (Fig. 5).
Discussion
Yamaguti (1941) erected the genus Raphidascaroides to accommodate R. nipponensis Yamaguti, 1941 as its type-species and also its subspecies R. nipponensis lophii Yamaguti, 1941 (= R. nipponensis according to Moravec & Nagasawa, 2000). Moravec & Nagasawa (2000) redescribed this species and amended the generic diagnosis of Raphidascaroides based on light and SEM observations of the type- and newly collected specimens.
The new species of Raphidascaroides described here is the third species of the genus reported from freshwater fishes. Similarly as R. brasiliensis, the only species previously known from the Neotropical Region, the new species also occurs in doradid fishes. The two doradids examined in the present study, Megalodoras uranoscopus and Platydoras costatus, represent new definitive host records for R. brasiliensis, currently known only from fishes in the River Amazon basin in Brazil. In contrast, the new species was found in fishes from River Miranda, which is a tributary of River Paraguay, thus belonging to the River Paraná basin.
Petter (1995) reported one juvenile female and two fourth-stage larvae of Raphidascaroides sp. from P. costatus in Paraguay. Moravec & Thatcher (1997) assumed that these immature forms may belong to R. brasiliensis, but the present data indicate that they may also belong to the newly described species, R. moraveci n. sp. However, this assumption needs to be verified by obtaining mature nematodes in P. costatus, a species distributed from the northern part of South America to Argentina, including the River Paraná basin (Froese & Pauly, 2014).
Raphidascaroides brasiliensis was found in two different hosts in the present study, i.e., M. uranoscopus and P. costatus, and nematodes from these hosts are morphologically almost indistinguishable. In addition, the first molecular data for R. brasiliensis provided here confirmed the conspecificity of the samples from both fish hosts. The molecular analysis also supported the erection of R. moraveci n. sp., which differs in the concatenated sequences of SSU and LSU rDNA from those of two samples of R. brasiliensis.
Based on the phylogenetic analysis, the genus Raphidascaroides is grouped within the anisakid nematodes, in agreement with the traditional classification based on the morphology (sensu Moravec, 1998; Anderson et al., 2009). However, the phylogenetic relationships of Raphidascaroides with other anisakids are still unclear because molecular data for only a few representatives of other genera are available. The present data also indicate that Hysterothylacium Ward & Magath, 1917 may be paraphyletic, because H. pelagicum from the marine fish Coryphaena hippurus (Coryphaenidae) in the Pacific Ocean was the sister lineage of the Raphidascaroides clade, whereas the two remaining species of Hysterothylacium from marine fishes were distant, clustering with species of Goezia Zeder, 1800 and Iheringascaris Pereira, 1935. However, the support of individual clades in the phylogenetic tree was weak indicating that more data on other species and different genes are required to clarify these relationships.
References
Anderson, R C., Chabaud, A. G., & Willmott, S. (2009). Keys to the nematode parasites of vertebrates: archival volume. Wallingford: CABI Publishing, 480 pp.
Astrin, J. J., Zhou, X., & Misof, B. (2013). The importance of biobanking in molecular taxonomy, with proposed definitions for vouchers in a molecular context. Zookeys, 365, 67–70.
Bilqees, F. M., Shaukat, N., Navqi, S. M. H. M., & Medi, R. (2005). Raphidascaroides elongatus sp. n. (Nematoda: Anisakidae) from the fish Pellona elongata Bennet of Korangi Creek, Karachi, Pakistan. International Journal of Biology and Biotechnology, 2, 811–813.
Bruce, N. L. (1990). Hysterothylacium Ward and Magath, 1917, and Ichthyascaris Wu, 1949, ascaridoid nematodes from Australian demersal fishes. Memoirs of the Queensland Museum, 28, 389–426.
Černotíková, E., Horák, A., & Moravec, F. (2011). Phylogenetic relationships of some spirurine nematodes (Nematoda: Chromadorea: Rhabditida: Spirurina) parasitic in fishes inferred from SSU rRNA gene sequences. Folia Parasitologica, 58, 135–148.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772.
Froese, R., & Pauly, D. (Eds) (2014). FishBase. World Wide Web electronic publication. http://www.fishbase.org, version 06/2014.
Guindon, S., & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52, 696–704.
Hooper, J. A. N. (1983). Parasites of estuarine and oceanic flathead fishes (family Platycephalidae) from northern New South Wales. Australian Journal of Zoology Supplementary Series, 90, 1–69.
Huelsenbeck, J. P., & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755.
Katoh, K., Misawa, K., Kuma, K., & Myiata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066.
Khalil, L. F. (1961). On a new nematode, Raphidascaroides bishaii sp. nov. from a freshwater fish, Gymnarchus niloticus, in the Sudan. Journal of Helminthology, 35, 263–268.
Khalil, L. F., & Oyetayo, A. S. (1988). Raphidascaroides africanus sp. nov. (Nematoda: Anisakidae) from the fish Bostrychus africanus in Nigeria and a key to the species of the genus Raphidascaroides Yamaguti, 1941. Journal of African Zoology, 102, 85–91.
Moravec, F. (1998). Nematodes of the freshwater fishes of the Neotropical region. Prague: Academia, 464 pp.
Moravec, F., & Nagasawa, K. (2000). Some anisakid nematodes from marine fishes of Japan and the North Pacific Ocean. Journal of Natural History, 34, 1555–1574.
Moravec, F., & Thatcher, V. E. (1997). Raphidascaroides brasiliensis n. sp. (Nematoda: Anisakidae), an intestinal parasite of the thorny catfish Pterodoras granulosus from Amazonia. Brazil. Systematic Parasitology, 38, 65–71.
Nadler, S. A., & Hudspeth, D. S. (1998). Ribossomal DNA and the phylogeny of the Ascaridoidea (Nematoda: Secernentea): implications for morphological evolution and classification. Molecular Phylogenetics and Evolution, 10, 221–236.
Nadler, S. A., D’Amelio, S., Dailey, M. D., Paggi, L., Siu, S., & Sakanari, J. A. (2005). Molecular phylogenetics and diagnosis of Anisakis, Pseudoterranova, and Contracaecum from Northern Pacific mammals. Journal of Parasitology, 91, 1413–1429.
Nunn, G. B. (1992). Nematode molecular evolution. PhD Dissertation. University of Nottingham, UK, 187 pp.
Petter, A. J. (1995). Nematodes de poissons du Paraguay. VIII. Habronematoidea, Dracunculoidea et Ascaridoidea. Revue Suisse de Zoologie, 102, 89–102.
Rajyalakshmi, I. (1994). Raphidascaroides indicus n. sp. (Nematoda: Heterocheilidae) from the stomach of shark Chiloscyllium indicum (Gmelin) of Visakhaptnam. Rivista di Parassitologia, 11, 179–185.
Rajyalakshmi, I. (1995). Raphidascaroides bengali n. sp. Nematoda: Heterocheilidae from marine eel fish. Uroconger lepturus Richardson. Rivista di Parassitologia, 123, 467–473.
Smythe, A. B., Sanderson, M. J., & Nadler, S. A. (2006). Nematode small subunit phylogeny correlates with alignment parameters. Systematic Biology, 55, 972–992.
Werle, E., Schneider, C., Renner, M., Völker, M., & Fiehn, W. (1994). Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research, 22, 4354–4355.
Yamaguti, S. (1941). Studies on the helminth fauna of Japan. Part 33. Nematodes of fishes II. Japanese Journal of Zoology, 9, 343–396.
Yamaguti, S. (1961). Studies on the helminth fauna of Japan. Part 57. Nematodes of fishes, III. Journal of Helminthology, R. T. Leiper Supplement, 217–228.
Acknowledgements
This study was supported by the “Ciência sem fronteiras” Brazilian program for visiting researchers (No. 135/2012; stay of TS at the Universidade Federal Rural de Rio de Janeiro) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grants to JLL (Nos 474077/2011-0, 304254/2011-8, 402665/2012-0). FBP was supported by a CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) fellowship for a five month stay at the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, České Budějovice (IPCAS). TS was also supported by the IPCAS (RVO: 60077344) and the Czech Science Foundation (P505/12/G112). The authors’ thanks are also due to the staff of the Laboratory of Electron Microscopy, IPCAS, for their technical assistance, Blanka Škoríková for help in providing scientific literature, František Moravec for his valuable comments during manuscript preparation and Jan Brabec, all from the IPCAS, for help with molecular analyses, and David Gonzalez-Solís from El Colegio de la Frontera Sur, Chetumal, Mexico, for valuable advice. Thanks are also due to Aldenice Pereira, Fabiano Paschoal, Philippe Vieira Alves and Juliana Moreira for help with fish collection and parasitological examination and Emil José Hernández Ruiz, Universidade Federal do Pará, Altamira, for providing the facilities during the field trip to the River Xingu. Finally, the authors would also like to thank Fernando Paiva and the staff of the Universidade Federal do Mato Grosso do Sul, for providing logistical support and facilities during collecting trip to the River Miranda.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, F.B., Tavares, L.E.R., Scholz, T. et al. A morphological and molecular study of two species of Raphidascaroides Yamaguti, 1941 (Nematoda: Anisakidae), parasites of doradid catfish (Siluriformes) in South America, with a description of R. moraveci n. sp.. Syst Parasitol 91, 49–61 (2015). https://doi.org/10.1007/s11230-015-9555-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-015-9555-2