Abstract
The current investigation uses the density functional theory (DFT) with the M06-2X functional and 6-31G (d, p) basis set to examine the interaction between trichlorfon (TCF) and the cavity of β-cyclodextrin (β-CD). The primary objective of our study is to gain an insight into the molecular characteristics of this interaction by analyzing quantum parameters such as the HOMO–LUMO gap, the HOMO, and the LUMO. Two potential encapsulation modes, designated as A and B models, were identified for TCF. The thermodynamic assessments including complexation energies and alterations in enthalpy, entropy, and Gibbs free energy indicate a stable and advantageous encapsulation procedure. The independent gradient model (IGMH) provides insights into the non-covalent interactions in developing the TCF@β-CD complex. The observed stability can be mainly attributed to a significant intermolecular hydrogen bond emphasized by the NBO and EDA and weak van der Waals forces. The results of our study indicate that β-CD as macrocycle has the potential to be a suitable trap for trichlorfon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The supramolecular chemistry investigates non-covalent interactions within several categories of compounds essential for biological, chemical, and physical applications. Supramolecular chemistry finds significant use in environmental remediation, which serves as a valuable tool for extracting and eliminating agents such as heavy metals, organic contaminants, and radioactive compounds from terrestrial and aquatic environments. Supramolecular host molecules are able to capture coordinated and selective guest entrap [1,2,3]. Among those used as host chemicals, the supramolecular systems are cyclodextrins, cucurbiturils, calixarenes, and pillar arenes [4].
Cyclodextrins (CDs) are cyclic oligosaccharides that have unique structure with hydrophobic cavity and hydrophilic surface (hydrophilic exterior), which allow to form inclusion complexes with various guest molecules. The most frequently used CDs are α, β, and γ [5, 6].
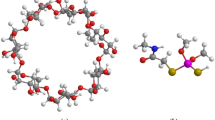
A particular category of cyclodextrins, β-CD (Fig. 1a), consists of eight glycopyranones having a height of about 8 Å and the diameter of the cavity is 6.0–6.4 Å. β-Cyclodextrin (β-CD) has demonstrated notable efficiency in various domains, including food science, pharmaceuticals, and environmental remediation applications [7, 8]. It is a result of its distinctive composition and capacity to establish inclusion complexes with various contaminants, such as heavy metals [9], pesticides, and organic dyes. An example of the application of β-CD in environmental remediation is the removal of trichlorfon (TCF), which is a soil- and water-contaminating pesticide. The addition of β-cyclodextrin (β-CD) to soil or water contaminated with trichlorfon (TCF) results in an inclusion complex between β-CD and TCF molecules. These results in increased solubility and mobility of the TCF molecules, which facilitates their removal from the environment [10, 11]. Several experimental works have been devoted to inclusion complex formation of cyclodextrin with TCF guest adopting various processes [12].
For many years, trichlorfon (O,O-dimethyl-(2,2,2-trichloro-1-hydroxyethyl) phosphonate) has been used extensively in agriculture despite its extremely high toxicity (Fig. 1b) [13,14,15]. Its extensive production is mainly attributed to its positive impact on methods of agriculture. Unfortunately, this substance can cause environmental pollution that harms human health. When TCF is used in excess, residues could be created and linger in contaminated soil, air, and groundwater. TCF’s mutagenic and carcinogenic characteristics could also harm the public’s health, where aplastic anemia in children, non-lymphoma, Hodgkin’s lung cancer, and other severe human diseases have also been linked to TCF exposure [16].
It is critical to comprehend the forces maintaining stability in host–guest interactions to advance the development of novel materials, catalysts, and therapeutic agents. The interactions between hosts and guests play a significant role in various chemical processes. By understanding the fundamental forces in these interactions, we will be able to develop more efficient strategies for their control.
The investigation of host–guest interactions’ complexation behavior has been the focus of various theoretical investigations devoted to understanding the of fundamental processes of stabilizing guest molecules within the cavities of macrocyclic hosts. For this objective, we have employed a range of quantum chemical techniques, such as semi-empirical methods [13] and density functional theory (DFT) methods [17, 18].
In this work, we used the density functional theory (DFT) computational method using the M06-2X functional by means of the 6-31G (d, p) basis set [19, 20], in order to examine the inclusion phenomena involving β-cyclodextrin (β-CD) and trichlorfon (TCF). The accuracy of the DFT method has been discussed in previous works for related inclusion complexes and is revealed to be a good method [15]. Experimental evidence has already confirmed the creation of a complex with a 1:1 stoichiometry [12].
This research offers valuable insights into the chemical interactions responsible for incorporating TCF (trichlorfon) into β-CD (beta-cyclodextrin). The provided data has the potential to inform the development of novel materials based on β-CD suitable for environmental TCF removal. The primary objective of this study is to improve our comprehension of the structural geometry and crucial interactions that are fundamental to the stability of the complex being investigated by IGMH. Furthermore, it provides detailed insights into the various factors influencing the inclusion process.
Calculation details
The structures of trichlorfon and β-CD were extracted using ChemOffice 3D Ultra [21] and the PubChem chemical database [22]. The complex TCF@β-CD was initially modeled using the Hyperchem 7.5 program [23]. The trichlorfon as a guest molecule was deliberately manipulated to traverse the β-CD host cavity along the Z-axis, ranging from − 8 to 8, in two orientations labeled A and B (Fig. 2). The inclusion process followed the methodologies outlined in the previous literature [24]. The PM7 [13] methodology optimized each resultant structure using MOPAC2016 [25]. It allowed for the discovery of the complexes with the highest thermodynamic stability. Geometry optimization and energy calculations were carried out using Gaussian 09 software [26].
In order to achieve greater computational accuracy, the personalized guest, host, and preeminent complexes underwent additional refinement using density functional theory (DFT). The M06-2X functional [27, 28] was chosen for its ability to describe accurately non-covalent interactions, particularly the important hydrogen-bonding processes. The calculations were carried out using the 6-31G (d, p) basis set [29, 30], in ambient and aqueous conditions. To comprehensively analyze the dynamics of solvents, the conductor polarizable continuum model (CPCM) [31, 32] was integrated for water.
The quantitative representation of the energetic parameter involved in the complexation of trichlorfon with β-cyclodextrin was established based on the lowest energy conformation, as given in Eq. (1) [33].
The equation includes three components: the host–guest interaction energy \(\left({E}_{{\text{TCF}},\upbeta -{\text{CD}}}\right) ,\) the energy of the host molecule\(\left({E}_{\upbeta -{\text{CD}}}\right)\), and the energy of the guest molecule (\({E}_{{\text{TCF}}}\)).
Further investigation was conducted to gain a more comprehensive understanding of the details of charge redistribution of β-CD and TCF assembly, which is accomplished through natural bond orbital (NBO) [34, 35] investigation. In addition, the independent gradient model based on the Hirschfeld partition (IGMH) [36] is a very effective tool for visualizing diverse interactions within chemical systems. It gives rise to a better accuracy and computing efficiency compared to the independent gradient model (IGM) [37, 38]. The foundation of IGMH is established upon of the use of the Eq. (2):
where λ2 is the second eigenvalue of the Hessian matrix of the electron density and ρ(r) is the electron density. The isosurface of the IGMH is shown with coloration corresponding to the IGMH(r) value: blue denotes attractive interactions, green represents non-bonding interactions [39], and red signifies repulsive interactions, as shown in Fig. 3.The Multiwfn package [40] was used to investigate the IGMH analysis, which was then visualized with the VMD program [41].
Energy decomposition analysis is a powerful tool for understanding the nature of the chemical bonding [42, 43] and other chemical phenomena. It was performed at the dispersion corrected method M06-2X/6-31G(d, p) level to decompose the total binding energy (ΔEbind) into four terms: Pauli repulsion (ΔEPauli), electrostatic (ΔEelstat), orbital (ΔEorb), and dispersion (ΔEdisp) terms [44]. The last three terms contribute to the attractive energy, while Pauli’s repulsion is the destabilizing term. This study used EDA to investigate the interactions between the pesticide trichlorfon and the host β-cyclodextrin.
Energetic and structural analyses
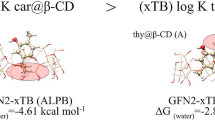
The PM7 calculations provide optimum conformations with the lowest energy configuration, indicating their high stability. The predicted complexation energies for the A and B models [45] is shown in Fig. 4.
Both models A and B exhibit energy profile with negative complexation energy values. Frequency calculations were performed after optimization to confirm that the resulting structures are both minimums of the energies. Structures A and B have the most stability when positioned at Z = − 2 and 5 Å, respectively. The associated complexation energies for these positions are − 19.82 and − 21.90 kcal/mol, respectively.
The re-optimization performed at the M06-2X level of theory with 6-31G (d, p) basis set obtained for the complexation gave energies of − 13.44 and − 19.73 kcal/mol in a vacuum for the A and B configurations, respectively. The complexation energies were determined to be − 19.82 kcal/mol and − 21.90 kcal/mol for the two identical structures in the CPCM aqueous phase. Thus, it can be concluded that the stability of the TCF@β-CD complex is higher for the B model compared to that of the A one.
The thermodynamic favorability of the TCF@β-CD complex formation is higher in an aqueous environment compared to that in a vacuum. The structural investigation of the most stable complex configuration (model B at a distance of 5 Å) was conducted using the M06-2X computational approach, employing the 6-31G (d, p) basis set. According to the findings depicted in Fig. 5, the study demonstrates that TCF exhibits partial enclosure within the β-CD cavity. The values of enthalpy (ΔH°), entropy (ΔS°), and Gibbs free energy (ΔG°) were calculated at a temperature of 298.15 K and a pressure of 1 atm. The results are presented in Table 1.
The presence of negative values for ΔH° indicates that the complexation process is characterized by exothermic behavior, indicating heat release. The orientation B exhibits greater negative ΔH° values, corresponding to a strong interaction between trichlorfon and β-CD. This enhanced interaction may be attributed to van der Waals or hydrophobic interactions. The entropy (ΔS°) of the TCF molecule exhibits a reduction when it performs inclusion complexation with β-CD due to the encapsulation of the molecule inside the cavity of β-CD. The Gibbs free energy shift (ΔG°) has been determined to be negative, reflecting the complexation process’s spontaneous nature [46, 47].
Electronic quantum parameters
Frontier molecular orbitals (FMO) [48, 49] correspond to the highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs). The energy gap between HOMO and LUMO can be used to determine the kinetic stability, chemical reactivity, and the hardness of molecules [50]. A large HOMO–LUMO energy gap is an indicator of high molecular stability.
In the context of basic molecular orbital theory methodologies, the energy of the HOMO, denoted as EHOMO, is connected to the ionization energy (IE) using Koopmann’s theorem. Similarly, the energy of the LUMO, referred to ELUMO, has been employed to approximate the electron affinity (EA) [51].
The hardness (η) is determined as one-half of the energy value gap of LUMO and HOMO energies, which were computed using the provided formulas.
The electrophilicity of complexes was employing the following equation:
The calculation results are gathered in Table 1. The value of the HOMO–LUMO energy gaps in both phases are the highest for orientation B, which agree with the complexation energies.
The inclusion process of TCF@β-CD has changed electronic properties linked to TCF and β-CD. The EHOMO of the complex TCF@β-CD is higher than that of TCF, and the ELUMO is lower than that of TCF, suggesting an elevation in excitation energy. We noticed that the dipole moment of TCF@β-CD is slightly smaller than TCF’s. This might be because of the good structural symmetry of β-CD, which limits the delocalization of the TCF electrons.
We found that the values of the chemical potentials for both orientations are negative, which shows that the inclusion process is spontaneous in nature.
The overall hardness η for the orientation A is the highest, which explains the stability of this orientation compared to orientation B. The electrophilicity index ω is a good descriptor of the reactivity of a molecule. Electrophilicity is defined as the ability of a molecule to bind strongly to a nucleophilic entity by electron transfer. In other words, it is the ability to acquire electrons to stabilize itself. The higher its value, the more electrophilic the entity is. According to the obtained results, orientation B is the most electrophilic.
In Fig. 6, we present the HOMO and LUMO frontier orbitals of the TCF@β-CD complex for both orientations A and B by the M06-2X/6-31G (d, p) calculation. According to this figure, we find that the HOMO and LUMO orbitals are largely localized on the guest molecule and slightly extended to the host. The existence of HOMO and LUMO on both partners indicates a charge transfer between them.
Natural bond orbital (NBO) analysis
Natural bond orbital (NBO) analysis [34, 35] is an effective method to understand how molecules bond and interact. It can be employed to investigate charge transfer and conjugative interactions, which are crucial in numerous chemical and biological phenomena. The theory of micro-disturbances of second order E(2) is a computational technique employed in NBO analysis to determine interaction energies between electron donors and acceptors [47].
A positive E(2) value signifies a stabilizing interaction, whereas a negative value signifies a destabilizing interaction [52]. This delocalization of electron density stabilizes the molecule by decreasing its energy. The formula of the E(2) value [53] measures the donor–acceptor interaction’s potency, given as follows:
where: \({q}_{{\text{i}}}\) represents the occupancy of the donor orbital. The term \({F}_{\left({\text{i}},{\text{j}}\right)}\) denotes the off-diagonal element of the NBO Fock matrix. Additionally, \({\varepsilon }_{{\text{j}}}\) and \({\varepsilon }_{{\text{i}}}\) represent the eigenvalues of the NBO Fock matrix for the acceptor and donor orbitals, respectively.
The relaxed geometry of the most stable complex was subjected to natural bond orbital (NBO) analysis at the M06-2X/6-31G (d, p) theoretical level [28]. This analysis was conducted in an aqueous environment using the Gaussian 09 software [26]. Table 2 summarizes the most significant acceptor–donor interactions characterized by high second-order perturbation energies E(2). The interaction between the orbitals of the atoms possessing lone pairs and the antibonding orbitals, as shown in Table 2, demonstrates an energy range of 1.04 to 2.41 kcal/mol. This interaction occurs at.
large interatomic distances, typically 2.3 to 2.8 Å. The model in question revealed a crucial electronic charge density between the orbitals of the lone pairs of oxygen (O83) and the antibonding orbitals σ* of the atom (O7-H 14). This interaction resulted in a substantial stabilization energy of 27.73 kcal/mol, corresponding to the shortest interatomic distance of 1.7 Å [54].
The molecular structure analysis (Fig. 7) demonstrates the existence of hydrogen bonds.
The most important hydrogen bond appears between the oxygen atom (O83) of the donor β-CD molecule and the hydrogen atom (H14) of the acceptor TCF molecule giving rise to a strong interaction quantified by an energy of 27.73 kcal/mol. The spatial separation between these two atomic entities is 1.72 Å.
IGMH study of non-covalent interaction in TCF@β-CD
In order to conduct a more comprehensive examination of the impact of weak interactions [55] on the configuration of the TCF@β-CD complex, this study used the IGMH technique to investigate the weak interactions occurring between the TCF and β-CD units. Additionally, the objective is to ascertain the quantity and positioning of hydrogen bonds formed between these entities. Figure 8 displays the 3-D \({\delta }_{g}^{inter}\) versu sign \(\left({\lambda }_{2}\right)\rho\) isosurface and 2-D scatter plots of the TCF@β-CD complex. The analysis of the isosurface demonstrates a significantabundance of prominently green isosurfaces, suggesting that van der Waals interactions play a dominant role.
Nevertheless, it is worth noting that a small number of light blue isosurfaces can be detected amidst the hydrogen atoms of the TCF molecule and the oxygen atoms of the β-CD molecule. This observation suggests the existence of hydrogen bonds [56], which plays a role for the TCF@β-CD complex’ stabilization.
The isosurface of the TCF@β-CD complex of the most stable was calculated using the Multiwfn software [40]. The resulting isosurface, with an isovalue of 0.005 atomic units depicted in Fig. 8, was visualized using VMD [41], exhibiting a hydrogen bond, as denoted by a blue disk, with a magnitude of 0.1 eV.
Energy decomposition analysis (EDA) of non-covalent interaction in TCF@β-CD
The Morokuma-Ziegler energy decomposition analysis (EDA) [57,58,59] largely used recently [60,61,62,63,64,65,66] was applied to provide a deep insight into the weak interactions of the inclusion complex. The decomposition of the total energetic contribution is listed in Table 3. Electrostatic, orbital, Pauli, solvation, and dispersion energies were − 34.87, − 10.57, 20.87, − 52.73, and − 61.64 kcal/mol, respectively. The total bonding energy of − 84.20 kcal/mol is negative, favorable for formation of the inclusion complex. The solvation energy of − 56.92 kcal/mol is negative, indicating that the complex’s stability is higher in a solution than in the gas phase and synonymous of stabilization effects. The dispersion energy of − 28.82 kcal/mol contributes significantly into the total bonding energy, indicating that this energy is essential to take into account the van der Waals interaction, which is a factor of the stability of the inclusion complex. Electrostatic energy of − 33.75 kcal/mol is the second largest contribution to the total bonding energy due to the presence of hydrogen bonds; thus, the electrostatic interaction is a crucial descriptor for the hydrogen bonds.
However, to some extent, the repulsion effect countered the attractive interaction due to electrostatic and dispersion effects. The major contributions to this total bonding energy were dispersion and electrostatic energies, suggesting that van der Waals interaction and hydrogen bonds exerted a noticeable effect on the stability of the inclusion complex.
Conclusion
This computational study was performed using density functional theory (DFT) to investigate the complexation mechanism between trichlorfon (TCF) and β-cyclodextrin (β-CD). The investigation analyzed thermodynamic parameters, structural characteristics, and interaction energies focusing on hydrogen bonding interactions via the IGMH approach and EDA.
Thermodynamic favorability, as evidenced by the exothermic nature and favorable enthalpy and entropy contributions, drives the spontaneous formation of the TCF@β-CD complex. This sets the stage for in-depth analysis of the underlying intermolecular forces. The exothermic nature of the complexation process further suggests potential for sustained and controlled release, making it even more attractive for real-world applications.
The study by IGMH showed that hydrogen bonds and van der Waals interactions mainly controlled the formation and stability of the TCF@β-CD complex. A significant hydrogen connection was established between the TCF molecule and the β-CD cavity, exhibiting a distance of 1.72 Å. The strong binding between TCF and β-CD may be attributed to the hydrogen bond as well emphasized and quantified by the energy decomposition analysis as evidenced by the dispersion contribution. The study results indicate that β-cyclodextrin can serve as a host molecule for the encapsulation of TCF. TCF’s regulated release and distribution might have potential uses in environmental fieldwork. This study constitutes a valuable contribution to the existing body of research and has the potential to serve as a foundational basis for future experimental investigations.
Data availability
No datasets were generated or analyzed during the current study.
References
Lehn JM (1993) Supramolecular chemistry. Science 260(5115):1762–1763
Kolesnichenko IV, Anslyn EV (2017) Practical applications of supramolecular chemistry. ChemSoc Rev 46(9):2385–2390
Khan SB, Lee SL (2021) Supramolecular chemistry: host–guest molecular complexes. Molecules 26(13):3995
Astray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gandara J (2009) A review on the use of cyclodextrins in foods. Food Hydrocoll 23(7):1631–1640
Crini G (2014) A history of cyclodextrins. Chem Rev 114(21):10940–10975
Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N (2018) Cyclodextrins, from molecules to applications. Environ ChemLett 16(4):1361–1375
Köse K, Tüysüz M, Aksüt D, Uzun L (2021) Modification of cyclodextrin and use in environmental applications. Environ SciPollut Res 29:1–28
Liu Y, Lin T, Cheng C, Wang Q, Lin S, Liu C, Han X (2021) Research progress on synthesis and application of cyclodextrin polymers. Molecules 26(4):1090
Zhang C, Tao Y, Li S, Ke T, Wang P, Wei S, Chen L (2020) Bioremediation of cadmium-trichlorfon co-contaminated soil by Indian mustard (Brassica juncea) associated with the trichlorfon-degrading microbe Aspergillus sydowii : Related physiological Answers and soil enzyme activities. Ecotoxicol Environ Saf 188:109756
Fakayode SO, Lowry M, Fletcher KA, Huang X, Powe AM, Warner IM (2007) Cyclodextrins host-guest chemistry in analytical and environmental chemistry. Curr Anal Chem 3(3):171–181
Flaherty RJ, Nshime B, DeLaMarre M, DeJong S, Scott P, Lantz AW (2013) Cyclodextrins as complexation and extraction agents for pesticides from contaminated soil. Chemosphere 91(7):912–920
Raileanu M, Todan L, Crisan M, Braileanu A, Rusu A, Bradu C, Zaharescu M (2010) Sol-gel materials with pesticide delivery properties. J Environ Prot 1(03):302
Rayene K, Imane D, Abdelaziz B, Leila N, Fatiha M, Abdelkrim G, Rabah O (2022) Molecular modeling study of structures, Hirschfield surface, NBO, AIM, RDG, IGM and 1HNMR of thymoquinone/hydroxypropyl-β-cyclodextrin inclusion complex from QM calculations. J MolStruct 1249:131565
Ferencz L, Balog A (2010) Pesticides masked with cyclodextrins–a survey of soil samples and computer aided evaluation of the inclusion processes. Fresen Environ Bull 19(2):172–179
Jiang K, Zhang N, Zhang H, Wang J, Qian M (2015) Investigation on the gas-phase decomposition of trichlorfon by GC-MS and theoretical calculation. PLoS ONE 10(4):e0121389
Najafi NM, Alizadeh R, Talebpour Z, Ghassempour AR (2011) 31P NMR and computer simulations of the structure of trichlorfon and its derivatives. JSC 52:713–717
Thanthiriwatte KS, Hohenstein EG, Burns LA, Sherrill CD (2011) Assessment of the performance of DFT and DFT-D methods for describing distance dependence of hydrogen-bonded interactions. J Chem Theory Comput 7(1):88–96
Tirado-Rives J, Jorgensen WL (2008) Performance of B3LYP density functional methods for a large set of organic molecules. J Chem Theory Comput 4(2):297–306
Hohenstein EG, Chill ST, Sherrill CD (2008) Assessment of the performance of the M05–2X and M06–2X exchange-correlation functionals for noncovalent interactions in biomolecules. J Chem Theory Comput 4(12):1996–2000
Lin YS, Li GD, Mao SP, Chai JD (2013) Long-range corrected hybrid density functionals with improved dispersion corrections. J Chem Theory Comput 9(1)2:63–272
Kerwin SM (2010) ChemBioOfficeultra 2010 suite. J Am Chem Soc 132:2466–2467
Bolton EE, Wang Y, Thiessen PA, Bryant SH (2008) PubChem: Integrated Platform of Small Molecules and Biological Activities. Annu Rep Comput Chem. 4:217–241
Hypercube Inc, HyperChem Professional 7.51, Hypercube Inc, Gainesville, Fla, USA, 2003
Liu L, Guo QX (2004) Use of quantum chemical methods to study cyclodextrin chemistry. J Incl Phenom Macrocycl Chem 50:95–103
Stewart JJP (2016) MOPAC (Molecular Orbital PACkage) is a semiempirical quantum chemistry program based on Dewar and Thiel's NDDO approximation. Stewart Computational Chemistry, Colorado Springs. CO, USA
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehar M (2009) Gaussian 09, Revision D. 01. Gaussian Inc, Wallingford CT 121:150–166
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. AccChem Res 41(2):157–167
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. TheorChemAcc 120:215–241
Beeke AD (1993) Density-functional therhe role of exact exchange. J ChemPhys 98(7):5648–6
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785
Cossi M, Rega N, Scalmani G, Barone V (2003) Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J ComputChem 24(6):669–681
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J PhysChem 102(11):1995–2001
Messiad FA, Ammouchi N, Belhocine Y, Alhussain H, Ghoniem MG, Said RB, Rahali S (2022) In search of preferential macrocyclic hosts for sulfur mustard sensing and recognition: a computational investigation through the new composite method r2SCAN-3c of the key factors influencing the host-guest interactions. Nanomaterials 12(15):2517
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev PhysChem 35(3):399–440
Lu T, Chen Q (2022) Independent gradient model based on Hirshfeld partition: a new method for visual study of interactions in chemical systems. J ComputChem 43(8):539–555
Lefebvre C, Rubez G, Khartabil H, Boisson JC, Contreras-García J, Hénon E (2017) Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. PhysChemChemPhys 19(27):17928–17936
Lefebvre C, Khartabil H, Boisson JC, Contreras-García J, Piquemal JP, Hénon E (2018) The independent gradient model: a new approach for probing strong and weak interactions in molecules from wave function calculations. ChemPhysChem 19(6):724–735
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7(3):625–632
Lu T, Chen F (2012) Multiwfn: A multifunctional wavefunction analyzer. J ComputChem 33(5):580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Zhao L, Hermann M, Schwarz WE, Frenking G (2019) The Lewis electron-pair bonding model: modern energy decomposition analysis. Nat Rev Chem 3(1):48–63
Fang D, Piquemal JP, Liu S, Cisneros GA (2014) DFT-steric-based energy decomposition analysis of intermolecular interactions. TheorChemAcc 133:1–14
Zhao L, von Hopffgarten M, Andrada DM, Frenking G (2018) Energy decomposition analysis. Wiley Interdiscip Rev ComputMolSci 8(3):e1345
Benaïssa A, Bouhadiba A, Naili N, Chekkal F, Khelfaoui M, Bouras I, Madjram MS, Zouchoune B, Mogalli S, Malfi N, Nouar L, Madi F (2023) Computational investigation of dimethoate and β-cyclodextrin inclusion complex: molecular structures, intermolecular interactions, and electronic analysis. Struct Chem 34(3):1189–1204
Safia H, Ismahan L, Abdelkrim G, Mouna C, Leila N, Fatiha M (2019) Density functional theories study of the interactions between host β-Cyclodextrin and guest 8-Anilinonaphthalene-1-sulfonate: molecular structure, HOMO, LUMO, NBO, QTAIM and NMR analyses. J MolLiq 280:218–229
Kabouche Z, Belhocine Y, Benlecheb T, Assaba IM, Litim A, Lalalou R, Mechhoud A (2023) A DFT-D4 investigation of the complexation phenomenon between pentachlorophenol and β-cyclodextrin. CTA 10(2):202310209
Fukui K (1982) Role of frontier orbitals in chemical reactions. Science 218(4574):747–754
Fleming I (2011) Molecular orbitals and organic chemical reactions. John Wiley and Sons
Politzer P, Murray JS (2007) The average local ionization energy: concepts and applications. ComputTheorChem 19:119–137
Zhan CG, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J PhysChem A 107(20):4184–4195
Abdelmalek L, Fatiha M, Leila N, Mouna C, Nora M, Djameleddine K (2016) Computational study of inclusion complex formation between carvacrol and β-cyclodextrin in vacuum and in water: charge transfer, electronic transitions and NBO analysis. J MolLiq 224:62–71
Fakhari S, Nouri A, Jamzad M, Arab-Salmanabadi S, Falaki F (2021) Investigation of inclusion complex of metformin into selective cyclic peptides as novel drug delivery system: structure, electronic properties, AIM, and NBO study via DFT. J Chin ChemSoc 68(1):67–75
Nadia L, Djameleddine K, Rayenne D (2014) Theoretical study of the inclusion processes of octopamine with β-cyclodextrin: PM6, ONIOM, and NBO analysis. C R Chim 17(12):1169–1175
Hobza P, Zahradník R, Müller-Dethlefs K (2006) The world of non-covalent interactions: 2006. Collect CzechoslovChemCommun 71(4):443–531
Karas LJ, Wu CH, Das R, Wu JIC (2020) Hydrogen bond design principles. Wiley Interdiscip Rev ComputMolSci 10(6):e1477
Khireche M, Zouchoune B, Ferhati A, Nemdili H, Zerizer MA (2021) Understanding the chemical bonding in sandwich complexes of transition metals coordinated to nine-membered rings: energy decomposition analysis and the donor–acceptor charge transfers. TheorChemAcc 140(9):122
Frenking G, Froehlich N (2000) The nature of the bonding in transition-metal compounds. Chem Rev 100(2):717–774
Frenking G, Wichmann K, Fröhlich N, Grobe J, Golla W, Van DL, Läge M (2002) Nature of the metal− ligand bond in M (CO) 5PX3 complexes (M= Cr, Mo, W; X= H, Me, F, Cl): synthesis, molecular structure, and quantum-chemical calculations. Organometallics 21(14):2921–2930
Zaiter A, Zouchoune B (2018) Electronic structure and energy decomposition of binuclear transition metal complexes containing β-diketiminate and imido ligands: spin state and metal’s nature effects. Struct Chem 29:1307–1320
Zerizer MA, Nemdili H, Zouchoune B (2022) Electron transfers’ assessment between stannol ring of triple-decker complexes and M (CO) 5 (M= Cr, Mo, W), MnCp (CO) 2 and CoCp (CO) metallic fragments: Bonding and energy decomposition analysis. Polyhedron 223:115960
Mecheri S, Zouchoune B, Zendaoui SM (2020) Bonding and electronic structures in dinuclear (X)[(Ind) M2L2] complexes (M= Ni, Pd, L= CO, PEt3, X= Cl, Allyl, Ind= indenyl, Cp= cyclopentadienyl): analogy between four-electron donor ligands. TheorChemAcc 139(1):12
Mecheri S, Zouchoune B (2023) Terminal and bridging ligand effects on M (I)- M (I) multiple bonding: a DFT investigation of the coordination in (X)[M2Cl] L2 complexes (M= Cr, Fe, L= CO, PEt3, X= Cl, allyl, Cp, and indenyl). Int J Quantum Chem 123(10):e27089
Mecheri S, Zouchoune B (2023) Donor-acceptor electron transfers and bonding performance of cyclopentadienyl and cyclo-P5 middle decks in (CpFeE5) ML3 and (CpFeE5) FeCb (E5= Cp, P5 and ML3= Cr (CO) 3, Mo (CO) 3, CrBz, MnCp, MoBz) triple-decker complexes: Bonding and energy decomposition analysis. Polyhedron 244:116586
Naili N, Kahlal S, Zouchoune B, Saillard JY, Braunstein P (2023) Carbonylmetallates as versatile 2-, 4-or 6-electron donor metalloligands in transition-metal complexes and clusters: a global approach. Chem - Eur J 29(24):e202203557
Tabrizi L, Zouchoune B, Zaiter A (2019) Experimental and theoretical investigation of cyclometallated platinum (ii) complex containing adamantanemethylcyanamide and 1, 4-naphthoquinone derivative as ligands: synthesis, characterization, interacting with guanine and cytotoxic activity. RSC Adv 9(1):287–300
Acknowledgements
The authors are gratefully thankful to the High-Performance Computing (HPC) resources of UCI-UFMC (Unité de Calcul Intensif of the University Freres Mentouri Constantine1).
Funding
None.
Author information
Authors and Affiliations
Contributions
Faiza Chekkal: original draft writing and data curation. while Amina Benaissa, Noura Naili, and Mohamed Amine Zerizer performed the theoretical calculation, conceptualization, and technique.Bachir Zouchoune conducted the investigation and supervision. Analyzing data, Nawel Redjem.
Corresponding authors
Ethics declarations
Ethical approval
The present does not include any experiments using animal or human participants by any authors.
Competing interest
The authors assert that they do not possess any identifiable conflicting financial interests or personal associations that could have influenced the research presented in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chekkal, F., Naili, N., Benaissa, A. et al. A proposed process for trichlorfon and β-cyclodextrinInclusion complexation by DFT investigation. Struct Chem 35, 1539–1549 (2024). https://doi.org/10.1007/s11224-024-02300-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-024-02300-w