Abstract
Four novel conjugates of adamantane connected at a bridgehead position via four-bond linker to structurally different ligands interacting with colchicine binding site of tubulin were synthesized. All compounds were found to inhibit cancer cell proliferation and, notably, to promote the formation of different atypical tubulin assemblies — clusters, curly, or “entangled” microtubules. The distinction in the observed effects for conceptually equivalent structural templates can be partially explained on the basis of computer molecular modeling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
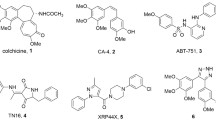
Cellular dimeric protein α,β-tubulin plays a crucial role in the formation of cytoskeleton and the process of cell division [1, 2]. The protein is known for its ability to constitute assemblies of different type and shape. Besides typical structures, i.e., microtubules (MT) formed by tubulin polymerization in cells, tubulin makes atypical assemblies — rings, tubulin sheets, paracrystals, and others [3]. The latter are composed only under special media conditions (pH, different cations, temperature, etc.) in vitro or in the presence of some anticancer agents [3,4,5,6]. For example, interaction of taxol with its binding site in β-subunit of tubulin stabilizes preexisted MT and promotes the protein assembly into stable MT bundles [4]. Another group of tubulin ligands binds with vinca-domain at the border of two α,β-dimers. This binding causes MT destabilization and a formation of either tubulin paracrystals (e.g., vinblastine) or other tubulin aggregates (cryptophycins, phomopsin etc.) [5]. There exists also a big group of compounds, which interact with binding site of colchicine (Fig. 1) and normally promote MT depolymerization only [6]. However, these compounds can cause the formation of tubulin assemblies under special conditions (e.g., [7]). Last decade several new derivatives of ligands of colchicine binding site displaying unusual effect on MT network were described (see [8] and ref. herein and [9,10,11,12,13] for recent examples).
Most of these new compounds represent colchicine derivatives with lipophilic (alicyclic or aromatic) substituents attached via linker chain to C7 atom of the parent molecule and are able to promote MT disassembly with further tubulin assembly into clusters [8,9,10,11,12]. The arising of this ability is attributed to additional interactions of lipophilic group with α-tubulin subunit. For colchicine—adamantane conjugates like tubuloclustin (Fig. 1(1b)) —the morphology of unusual tubulin assemblies was studied and direct correlation of the clusterization strength caused by compounds and their mitostatic activity in cancer cells was demonstrated (see [8] and ref. herein). Tubulin assemblies induced by derivatives of other than colchicine ligands of colchicine binding site are much less studied. Recently, we demonstrated that podophyllotoxin and 2-methoxyestradiol analogues of tubuloclustin cause the formation of weak clusters or slightly curved MT correspondingly [12, 13]. This observation made interesting additional investigations in this field. In the present work, we synthesized novel conjugates of colchicine, podophyllotoxin, and 2-methoxyestradiol with adamantane connected at a bridged position via rather short “four-bond” linker of approximately equal length. The linker was attached to the main molecule so that the substituent should be pointed at the direction of α-tubulin subunit of tubulin dimer (according to pharmacophore model of colchicine domain inhibitors [14]). The effect of the compounds on the MT net of cancer cells was studied and molecular modeling was carried out to explain the results of biotests.
Experimental part
Chemistry
All reaction temperatures correspond to internal temperatures unless otherwise noted. The high-grade commercial starting materials were used without further purification, the solvents were technical grade and were purified by standard procedures prior to use. Liquid column chromatography was performed on silica gel Acros (40–60 μm). 1D and 2D (gHSQC, gHMBC) NMR spectra were recorded on spectrometer Agilent 400-MR (400.0 MHz for 1H; 100.6 MHz for 13C) at room temperature; chemical shifts were measured with reference to the solvent (CDCl3, δH = 7.24 ppm, δC = 77.0 ppm). Chemical shifts are given in ppm (δ); multiplicities are indicated by s (singlet), d (doublet), t (triplet), and m (multiplet) and spin-spin coupling constants (J) are reported in Hz. Assignment of NMR signals of colchicine fragment in the target conjugates was made using the atomic numbering of colchicine, podophyllotoxin, and 2-methoxyestradiol given in Fig. 1. Electron impact mass spectra were obtained with typical voltage of 70 eV. Elemental analysis was performed on CNH analyzer Carlo-Erba ER-20.
N-[2-(1-adamantyl)acetyl]-N-deacetylcolchicine (4)
To a solution of N-deacetylcolchicine (50 mg, 0.140 mmol) in CH2Cl2, 2-(1-adamantyl)acetic acid (33 mg, 0.169 mmol) and N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) (50 mg, 0.202 mmol) were added. The mixture was stirred for 48 h. The filtrate was evaporated and chromatographed with a gradient (ethyl acetate/petroleum ether (40–70 °C) 1:2, then CH2Cl2/methanol in a gradient ratio from 99:1 to 98:2). Yield 30% (0.026 g), yellowish oily liquid. NMR1Н (CDCl3,δ, ppm,J/Hz): 1.54–1.65 (12H, m, Ad), 1.76–1.84 (1H, m), 1.95 (5H, m, Ad+CH2), 2.21–2.29 (1H, m), 2.40–2.45 (1H, m), 2.50–2.55 (1H, m), 3.65 (3H, s, OCH3), 3.91 (3H, s, OCH3), 3.94 (3H, s, OCH3), 3.99 (3H, s, OCH3), 4.65–4.71 (1H, m, H7), 6.20 (1H, m, NH), 6.54 (1H, s, Ar), 6.82 (1H, d, J = 10.8 Hz, Ar), 7.30 (1H, d, J = 10.8 Hz, Ar), 7.50 (1H, d, J = 19.5 Hz, Ar). NMR13С (CDCl3,δ, ppm): 28.52; 29.94; 32.78; 36.61; 37.35; 42.51; 51.44; 51.77; 56.01; 56.27; 61.34; 61.60; 107.22; 112.27; 125.60; 131.13; 134.13; 135.06; 136.33; 141.53; 151.19; 151.31; 153.34; 163.91; 170.28 (CONH); 179.24 (C=O). MS (EI),m/z: 533 (M+), 556 (M+ + Na). Anal. calcd. for C32H39NO6, %: С, 72.02; Н, 7.37. Found: С, 72.08; Н, 7.40.
4-O-(1-adamantylacetyl)-L-podophyllotoxin (5)
To the solution of 0.046 g (0.239 mmol) 2-(1-adamantyl)acetic acid in CH2Cl2 (10 ml), 0.080 g (0.193 mmol) of podophyllotoxin was added at room temperature. To the resulting mixture, 0.068 g (0.330 mmol) of DCC and a catalytic amount of DMAP (0.01 g) were added. After stirring at room temperature for 12 h, 5–10 μl of acetic acid was added and then, after 15 min, the solvent was removed in vacuo. The residue was dissolved in ethyl acetate (10–20 ml) and kept at 0–4 °C for 1 h. The N, N′-dicyclohexylurea precipitate was filtered and washed with cold ethyl acetate (2 × 10 ml), and the filtrate was washed by saturated NaCl solution (10 ml), water (10 ml), then dried with Na2SO4 and evaporated. The residue was chromatographed [eluent: ethyl acetate—petroleum ether (40–70 °C) 1:8–1:5]. Obtained 0.065 g of 5 (yield 57%), waxy solid, m.p. 123 °C. NMR1Н (CDCl3,δ, ppm,J/Hz): 1.62–1.68 (9H, m, Ad), 1.72–1.75 (3H, m, Ad), 2.00 (3H, m, Ad), 2.18 (1H, d, J = 13.0, AdCH2), 2.21 (1H, d, J = 13.0, AdCH2), 2.77–2.87 (1H, m, H3), 2.94 (1H, dd, J = 14.5, 4.3, H2), 3.76 (6H, s, OCH3), 3.82 (3H, c, OCH3), 4.24 (1H, dd, J = 10.3, 9.4, H3a), 4.33 (1H, dd, J = 9.4, 6.9, H3a), 4.61 (1H, d, J = 4.3, H1), 5.87 (1H, d, J = 9.1, H4), 5.98 (1H, d, J = 1.0, OCH2O), 6.00 (1H, d, J = 1.0, OCH2O), 6.40 (2H, s, H2′,6′), 6.54 (1H, s, H8), 6.50 (1H, s, H5). NMR13C (CDCl3,δ, ppm.): 28.45, 33.00, 36.56, 38.87, 42.53, 43.69, 45.55, 48.88 (AdCH2CO2), 56.04 (2OCH3), 60.71 (OCH3), 71.58, 73.25 (C4), 101.53 (OCH2O), 107.17, 108.00, 109.62, 128.40, 132.28, 134.80, 137.06, 147.51, 148.03, 152.60, 172.18, 173.64 (AdCH2CO2). Mass (MALDI-TOF),m/z: 590 [M]+, 613 [M+Na]+, 629 [M+K]+. Found, %: C 69.10; Н 6.52. Calculated, %: C34H38O9, %: C 69.14; Н 6.48.
4-O-[2-(adamant-1-yl)ethyl]-L-podophyllotoxin (6α) and 4-O-[2-(adamant-1-yl)ethyl]-L-epipodophyllotoxin (6β)
Twenty-nine microliters of boron trifluoride etherate was added to the solution of 0.070 g (0.169 mmol) of podophyllotoxin in 4 ml of dry CH2Cl2 at 0 °C. After 5 min, 0.046 g (0.256 mmol) of 2-adamant-1-yl ethanol was added. The reaction mixture was stirred for 6 h at room temperature, then 20 ml of water was added and extracted with methylene chloride (3 × 20 ml). The organic layers were combined and dried over Na2SO4, and the solvent was evaporated in vacuo. The residue was chromatographed (eluent ethyl acetate: petroleum ether 40–70 °C gradient 1:6–1:4). Obtained 0.062 g of compound 46 (64% yield) in a 3:2 ratio (according to 1H NMR spectroscopy), a colorless oily liquid. 6α,β: NMR1Н (CDCl3,δ,J/Hz, ppm; chemical shifts for 6β are in square brackets): 1.40 (1H, m, AdCH2), 1.52 (7H, m, Ad), 1.60–1.63 (3H, m, Ad), 1.69–1.72 (4H, m, Ad), 1.94 (3H, m, Ad), 2.79–2.95 (2H, m, AdCH2CH2O+H3), 3.37–3.42 (0.40H, m, H2), 3.47–3.57 (1.60H, m, AdCH2CH2O+H2), 3.74 (3.6H, s, OCH3) [3.73 (2.4H, s, OCH3)], 3.81 (1.8H, s, OCH3) [3.79 (1.2H, s, OCH3)], 4.32–4.35 (0.6H, m H3a) [4.39 (0.4H, m, J = 3.2 Hz, H3а)], 4.56–4.59 (1.2H, m, H1+3а) [4.60–4.63 (0.80H, m, H1+3а)], 5.97 (1H, d, J = 1.3, OCH2O) [5.96 (1H, d, J = 1.3, OCH2O)], 6.38 (1.2H, s, H2′,6′) [6.25 (0.80H, s, H2′,6′)], 6.49 (0.60H, s, H8) [6.54 (0.40H, s, H8)], 7.06 (0.60H, s, H5) [6.82 (0.40H, s, H5)]. NMR13С (CDCl3,δ, ppm; chemical shifts for 6β are in square brackets): 28.50 [28.53], 31.64 [31.60], 36.95 [36.96], 37.89 [38.30], 42.73 [42.71], 43.91 [43.88], 44.09 [43.82] (AdCH2), 45.47 [41.09], 56.04 [56.16] (2OCH3), 60.68 (OCH3), 64.05 [66.26] (AdCH2CH2O), 71.57 [67.61], 79.82 [74.46] (C4), 101.29 [101.37] (OCH2O), 107.02 [110.70], 107.99 [108.15], 109.47 [109.45], 131.13 [129.59], 131.48 [132.14], 135.41 [135.49], 136.90 [137.02], 147.48 [146.59], 147.58 [148.17], 152.49 [152.42], 174.11 [175.08] (C=O). (MALDI-TOF),m/z: 576 (M+), 599 (M+ + Na), 615 (M+ + K). Found, %: С 70.78; Н 7.04. Calculated, %: C34H40O8, %: С 70.81; Н 6.99.
2-(Adamant-1-yl)- (17β)-17-hydroxy-2-methoxyestra-1,3,5(10)-triene-3-yl acetate (7)
A solution of 2-(1-adamantyl)acetic acid (32.2 mg, 0.166 mmol) and DCC (34.15 mg, 0.166 mmol) in CH2Cl2 was added dropwise to a solution of 2-methoxyestradiol (50 mg, 0.166 mmol) and DMAP (0.02 mg) in CH2Cl2 for 15 min. After addition, the mixture was stirred for 30 min and then the solvent was evaporated, and ethyl acetate was added and cooled to 4 °C. The precipitated N,N′-dicyclohexylurea was filtered off, and the filtrate was evaporated and chromatographed first using column chromatography on silica gel (petroleum ether (40–70 °C): ethyl acetate 1:15–1:10) and then on TLS glass plates pre-coated with silica gel with fluorescent indicator (Macherey–Nagel, Germany). Yield 15% (0.012 g), colorless oily liquid. NMR1Н (CDCl3,δ, ppm,J/Hz): 0.79 (3H, s), 0.84–0.89 (1H, m), 1.16–1.24 (1H, m), 1.31–1.57 (9H, m), 1.67–1.73 (6H, m), 1.76–1.77 (7H, m), 1.85–1.89 (1H, m), 1.95–2.00 (1H, m), 2.02 (3H, m), 2.11–2.15 (1H, m), 2.16–2.29 (1H, m), 2.32 (2H, s), 2.77–2.81 (2H, m), 3.74 (1H, m), 3.80 (1H, c), 6.72 (1H, s), 6.90 (1H, s). NMR13С (CDCl3,δ, ppm): 11.86, 23.58, 26.32, 27.31, 28.54, 30.14, 30.45, 33.32, 36.56, 37.92, 38.75, 41.82, 42.24, 43.46, 44.58, 49.78, 55.68, 85.70, 110.24, 121.68, 129.28, 136.84, 138.96, 148.15, 168.55 (AdCH2CO2). (MALDI-TOF),m/z: 478 (M+), 501 (M+ + Na), 517 (M+ + K).
Biology
Cytotoxicity was measured using quantitative colorimetric MTT (3-(4,5-dimethylthiazolyl-2)-2,5-diphenyl-2H-tetrazoliumbromid, Roth GmbH, Karlsruhe, Germany) assay [15]. A549 human lung epithelial carcinoma cells (CCL-185™) were cultured with Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and 1% antibiotic penicillin/streptomycin at 37 °C under a 5% CO2 humidified atmosphere. The cells were seeded in 96-well plates at a density of 3000 cells per well. Stock solutions of test compounds were prepared in DMSO at concentration 20 mM. Cells were treated for 24 h with selected compounds at 1 nM–100 μM (8 wells for each concentration). DMSO (0.5%) served as a negative control. Optical density was measured at 550 nm with 690-nm reference filter using EL808 Ultra Microplate Reader (BioTek Instruments, Winooski, USA). For each compound, experiments were repeated three times and EC50 values were determined by sigmoid curve fitting using Excel-based software.
Cell growth inhibition assay
A549 cells were incubated with 1 μM or 10 μM of each tested (or control) compound during 24 and 48 (0.5% DMSO was used as a negative control). After culturing, the cells were resuspened in PBS and counted directly by phase-contract microscopy using hemocytometer.
Immunofluorescence staining of cellular MT
A549 cells were cultured in 12-well plates on small glass coverslips (11 mm diameter) at a density of 20,000 cells per coverslip. Cells were incubated with tested compounds at concentrations of 10 and 100 μM for 24 and 48 h (0.5% DMSO served as a negative control). The cells were fixed and stained as described in [16]. Fixed cells were labeled for tubulin with mouse monoclonal antibody against α-tubulin at a dilution of 1:300 (Sigma, St. Louis, USA), followed by incubation of Alexa Fluor488 labeled goat anti-mouse IgG at a dilution of 1:300 (Invitrogen, Germany). Images of all samples were acquired with a Nikon Diaphot 300 inverted microscope (Nikon GmbH, Düsseldorf, Germany) equipped with a cooled charge-couple device camera system (SenSys; Photometrics, Munich, Germany).
Molecular modeling
Computer molecular modeling of ligand–tubulin interactions was performed with AutoDock 4.2 software [17], using a model of the colchicine binding site in tubulin (PDB ID: 1SA1). The structures of the compounds were previously submitted to a conformational MMFF force field optimization and then automated molecular docking was carried out. The best model was selected based on the scoring functions calculated by Autodock 4.2.
Results and discussion
The synthesis of colchicine derivative 4 was carried out by EEDQ-assisted amidation reaction of 2-(1-adamantyl)acetic acid with N-deacetylcolchicine, obtained from initial molecule in three steps using slightly modified procedure described in [18] and depicted on Scheme 1. The target conjugate 4 was obtained in a 15% yield, the formation of amide bond having been proved by the downfield shift of C7-proton resonance in its 1H NMR spectra (4.65–4.71 ppm) in comparison with the corresponding peak of N-deacetylcolchicine (~ 3.71 ppm).
Podophyllotoxin ester 5 was synthesized by Steglich esterification of initial lignan 2a with 2-(1-adamantyl)acetic acid in the presence of 1,3-dicyclohexylcarbodiimide (DCC) and 4-dimethylaminopyridine (4-DMAP) (Scheme 2). In 1H NMR spectra of compound 5, the resonance of С4-proton in podophyllotoxin fragment is observed at 5.87 ppm; in 13C NMR spectra, atom С4 is displayed at 73.25 ppm. The corresponding to ester 5 ether derivative of podophyllotoxin was obtained as diastereomeric mixture 6α,β by reaction of 2a with (adamantan-1-yl)ethanol in the presence of boron trifluoride diethyl etherate (Scheme 2).
Though subjected to column chromatography on silica gel, the diastereomeric mixture was not resolved to individual isomers due to almost identical values of retardation factor of individual α and β isomers of 6; the isomeric ratio was 3:2. In 1H NMR spectra of compound 6, the resonances of protons -OCH2СН2Ad are observed at 3.47–3.57 ppm for α-isomer and at 3.37–3.42 ppm for β-isomer correspondingly. In 13C NMR spectra of ether 6, the resonance of atom C4 is shifted upfield with respect to the corresponding peaks of C4 in podophyllotoxin and revealed at 79.82 ppm for 6α and 74.46 ppm for 6β.
Steglich esterification of 2-methoxyestradiol 3a with 2-(1-adamantyl)acetic acid in equivalent amounts led to a target compound 7 (Scheme 3) in a low yield 15% due to the double purification procedure. As though ester 7 partially decomposed during column chromatography, it was additionally purified using thin layer chromatography on glass plates.
The ability of synthesized conjugates to promote a formation of atypical tubulin assemblies in human epithelial lung carcinoma cell line A549 was investigated using immunofluorescence microscopy. The compounds were also evaluated for determination of their cytotoxicity and ability to inhibit the cell growth correspondingly in a standard calorimetric MTT assay and using microscopy for direct cell counting over 24 h and 48 h of culturing. The results are presented in Table 1 and on Fig. 2.
Immunofluorescence microscopy images of the MT network in human lung carcinoma cells A549 treated with tested conjugates or DMSO or control compounds. a Intact MT (0.5% DMSO, negative control). b MT depolymerized (2a, positive control; the same for 1a and 3a); c Spot-like tubulin clusters (4, 100 μM). d MT depolymerized, some shortened and slightly curled MT (6α,β, 10 μM). e “Entangled” microtubules (6α,β, 100 μM), the rests of MT around centrosomes are observed as star-like structures. f Strongly “entangled” MT (5, 10 μM). Bar 20 μm
The results of biotests indicated that the behavior of all compounds in the cells of carcinoma A549 was different. At 10 μM, colchicine derivative 4 caused complete depolymerization of microtubules and also stimulated the formation of small clusters typical for some less active tubuloclustin analogues [12] (Fig. 2c). This result was interesting, because the tubulin-clustering ability was mostly associated with the colchicine binding site derivatives with long linker chain (see, e.g., [9,10,11]). Present data reveal that this effect can be manifested by compound with rather short linker. However, since the clusterization was observed at high concentrations, this linker length seems to be minimal for the effect observation. Conjugate 4 was more cytotoxic than parent molecule (see Table 1) in accordance with the previous observation that the ability to cause tubulin clustering correlates with cytotoxicity level [12].
Interesting result was observed for podophyllotoxin ester 5: at 10 μM, it stimulated the formation of tubulin associates curled and involuted so intensively that they reminded a “tangle of microtubules” (Fig. 2f). The type of tubulin assemblies was totally different from the tubulin clusters induced by compound 4 or podophyllotoxin analogue of tubuloclustin (2a) [12]. The shape of the observed associates did not resemble either regular vinblastine-induced paracrystals or taxol-induced MT bundles. Structurally similar to ester 5, podophyllotoxin ethers 6α,β at concentration 10 μM were found to cause MT depolymerization and the formation of some shortened and slightly curled microtubules (Fig. 2d). However, at high concentrations (100 μM), ethers 6α,β promoted the same effect as ester 5 (Fig. 2e). Thus, instead of either clustering typical for the action of tubuloclustin analogues or diffused tubulin pattern (see Fig. 2b) specific for the action of all classical colchicine binding site ligands in cells, e.g., podophyllotoxin (2a), 2-methoxyestradiol (3a), nocodazole, or combretastatin A-4, we observed the network of unusual MT after cell treatment with compounds 5 and 6 (Fig. 2e, f).
Computer molecular docking of compounds 5 and 6α into the model of the colchicine binding site and intradimer region of α/β-tubulin (PDB ID: 1SA1) indicates that carbonyl oxygen of the ester bond in 5 does not form any hydrogen bonds with protein (Fig. 3a), and adamantane moieties of both compounds are located in the very close areas at the interface of α and β-subunits. So, the differences in concentrations necessary for the formation of “entangled assemblies” seem to relate to various conformational flexibility of linker chains of 5 and 6α.
According to the computer molecular modeling data the position of adamantane core in podophyllotoxin, ester 5 does not match the one in colchicine derivative 4 (Fig. 3), which might explain the differences in the structural type of the tubulin associates induced by the compounds.
2-Methoxyestradiol conjugate 7 did not cause any effect on MT network at concentration 10 μM; however, at 100 μM, slight MT “curling” was observed in A549 cells and this action reminds the effect of 2-methoxyestradiol analogue of tubuloclustin (3a) on microtubule cytoskeleton [13].
In general, the studied structural modification of the ligands of colchicine binding site enables their ability to induce the formation of the assemblies under the conditions when no aggregates are formed by parent molecules. The precise mechanism of the conformational changes in tubulin leading to the curling effect is worth additional study and this work is now in progress.
Conclusion
Four conjugates of adamantane connected at a bridgehead position to structurally different ligands, interacting with colchicine binding site in tubulin via short linker of equal length, were synthesized. Immunofluorescence microscopy study revealed the ability of compounds to promote the formation of different atypical tubulin assemblies—clusters, curly, or “entangled” microtubules in cancer cells A549. The distinction in the observed effects for conceptually equivalent structural templates was partially explained by computer molecular modeling.
References
Hadfield JA, Ducki S, Hirst N, McGown AT (2003) Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res 5:309–325
Risinger AL, Giles FJ, Mooberry SL (2009) Microtubule dynamics as a target in oncology. Cancer Treat Rev 35:255–261
Karecla P, Hirst E, Bayley P (1989) Polymorphism of tubulin assembly in vitro. J Cell Sci 94:479–488
Yvon AM, Wadsworth P, Jordan MA (1999) Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell 10:947–959
Gupta S, Bhattacharyya B (2003) Antimicrotubular drugs binding to vinca domain of tubulin. Mol Cell Biochem 253:41–47
Perez EA (2009) Microtubule inhibitors: differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther 8:2086–2095
Saltarelli D, Pantaloni D (1982) Polymerization of the tubulin-colchicine complex and guanosine 5′-triphosphate hydrolysis. Biochemistry 21:2996–3006
Zefirova ON, Lemcke H, Lantow M, Nurieva EV, Wobith B, Onishchenko GE, Hoenen A, Griffiths G, Zefirov NS, Kuznetsov SA (2013) Unusual tubulin-clustering ability of definitely C7-modified colchicine analogues. ChemBioChem 14:1444–1449
Zefirov NA, Hoppe M, Kuznetsova IV, Chernyshov NА, Grishin YK, Maloshitskaya OA, Kuznetsov SA, Zefirova ON (2018) Homologues series of novel adamantane – colchicine conjugates: synthesis and cytotoxic effect on human cancer cells. Mendeleev Commun 28:225–344
Thomopoulou P, Sachs J, Teusch N, Mariappan A, Gopalakrishnan J, Schmalz HG (2015) New colchicine-derived triazoles and their influence on cytotoxicity and microtubule morphology. ACS Med Chem Lett 7:188–191
Vilanova C, Díaz-Oltra S, Murga J, Falomir E, Carda M, Redondo-Horcajo JM, Díaz JF, Barasoain I, Marco A (2014) Design and synthesis of pironetin analogue/colchicine hybrids and study of their cytotoxic activity and mechanisms of interaction with tubulin. J Med Chem 57:10391–10403
Zefirova ON, Nurieva EV, Wobith B, Gogol VV, Zefirov NA, Ogonkov AV, Shishov DV, Zefirov NS, Kuznetsov SA (2017) Novel antimitotic agents related to tubuloclustin: synthesis and biological evaluation. Mol Divers 21:547–564
Nurieva EV, Zefirov NA, Mamaeva AV, Wobith B, Kuznetsov SA, Zefirova ON (2018) Synthesis of steroid analogs of tubuloclustin, their cytotoxicity and effect on microtubules of A549 carcinoma cells. Russ Chem Bull 67:688–693
Nguyen TL, McGrath C, Hermone AR, Burnett JC, Zaharevitz DW, Day BW, Wirf P, Hamel E, Gussio R (2005) A common pharmacophore for a diverse set of colchicine site inhibitors using a structure-based approach. J Med Chem 48:6107–6116
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Al-Haddad A, Shonn MA, Redlich B, Blocker A, Burkhardt JK, Yu H, Hammer 3rd JA, Weiss DG, Steffen W, Griffiths G, Kuznetsov SA (2001) Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility. Mol Biol Cell 12:2742–2755
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock 4 and AutoDockTools 4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791
Bagnato JD, Eilers AL, Horton RA, Grissom CB (2004) Synthesis and characterization of a cobalamin-colchicine conjugate as a novel tumor-targeted cytotoxin. J Org Chem 69:8987–8996
Funding
This work was supported by Russian Fund of Fundamental Research (project no. №18-03-00524) and the German organization DAAD (German Academic Exchange Service) under the auspices of a collaborative agreement between Moscow and Rostock Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Zefirov, N.A., Evteeva, Y.A., Wobith, B. et al. Adamantyl-substituted ligands of colchicine binding site in tubulin: different effects on microtubule network in cancer cells. Struct Chem 30, 465–471 (2019). https://doi.org/10.1007/s11224-018-1219-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1219-9