Abstract
The mechanism (regio- and stereoselectivity) of 1,3-dipolar cycloaddition (1,3-DC) of 2-ethylthio-4-phenyl-1-azetin 1 with benzonitrile oxide 2a, 2-aminobenzonitrile oxide 2b and 2-azidobenzonitrile oxide 2c has been investigated by density functional theory-based reactivity indices and activation energy calculations at B3LYP/6-31G(d,p) level of theory in the gas and solvent phase. Thermodynamic and kinetic parameters of the possible ortho/meta regioisomeric and endo/exo stereoisomeric pathways have been determined. In order to rationalize complete endo selective fashion provided by these 1,3-DC cycloadditions, a natural steric analysis between NLMOs i,j for TS1ox and TS1on and also a second-order interaction energy, E 2, analysis between the donor–acceptor orbitals in these TSs were carried out. In all cases, the ortho pathways are more favorable compared to the meta alternatives and it is found that the endo pathway is preferred. Our results show that these cycloadditions follow an asynchronous one-step mechanism with a nonpolar character. Theoretical data are in good agreement with the experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cycloaddition reactions play a fundamental role in organic synthesis and mechanistic studies [1]. The 1,3-DC reaction, which is the joining of a 1,3-dipole with a dipolarophile, is an efficient way for the synthesis of five-membered heterocyclic structures [2–4]. In particular, the nitrile oxides are the important dipoles which react with double carbon–nitrogen bonds and afforded the 1,2,4-oxadiazole [5, 6]. These aromatic rings have various biological and medical applications [7, 8] and have been introduced into drug discovery programs for several different purposes. In some cases, they have been used as potent EthR inhibitors that boost ethionamide activity in the treatment of multi-drug-resistant tuberculosis [9]. In other cases, oxadiazoles have been displayed to act as a flat, aromatic linker to put substituents in the suitable position [10] and also regulating molecular properties by positioning them in the periphery of the molecule [11]. They have also been used as replacements for carbonyl including compounds like esters, amides, carbamates and hydroxamic esters [12, 13].
The 1,3-DC reactions have customarily been studied by the frontier molecular orbital (FMO) theory and transition state theory (TST). Although TST remains the most greatly used and the most rigorous approach for the investigation of the mechanism of these reactions, the transition state locating is not always more comfortable. Furthermore, transition state calculations often require a lot of time when big substituents are existent in reactive systems. The reactivity indexes, as described by the density functional theory (DFT) [14], such as Fukui indices, local softnesses and local electrophilicity, have been used to rationalize the reactivity and regiochemistry of cycloaddition reactions [15–17]. For instance, several treatments of 1,3-DC reactions of nitrile oxide with various dipolarophiles can be found in the literature [18–20]. In continuation of our previous works on the theoretical investigation of 1,3-DC reactions [21–28], we present the results of an extended investigation on the reactivity, regio- and stereoselectivity of 1,3-DC reaction between 2-ethylthio-4-phenyl-1-azetin 1 with benzonitrile oxide 2a, 2-aminobenzonitrile oxide 2b and 2-azidobenzonitrile oxide 2c based on activation energy calculations and DFT-based reactivity indices. Experimentally, it has been found that the cycloaddition reactions of 1 with 2a, 2b and 2c give only (5R,7R)-5-(ethylthio)-2-aryl-7-phenyl-4-oxa-1,3 diazabicyclo[3.2.0]hept-2-ene P1on as shown in Scheme 1. These results showed that the ortho and endo pathways are dominant [29].
Computational methods
All calculations were done with GAUSSIAN03 program package [30]. Geometric optimization of the reactants was done by DFT methods at the B3LYP/6-31G (d,p) level of theory [31]. The transition states (TSs) for the 1,3-DC reactions have been localized at the B3LYP/6-31G(d,p) level of theory. Frequency calculations described the stationary points to confirm that the TSs had one and only one imaginary frequency. The intrinsic reaction coordinates (IRC) [32] calculation was done in forward and backward path to determine that each saddle point joints to the two related minima by the second-order González–Schlegel integration method [33, 34]. Solvent effects were considered at the B3LYP/6-31G (d,p) level of theory by geometry optimization of the gas-phase structures using a self-consistent reaction field (SCRF) [35] on the basis of the polarizable continuum model (PCM) of Tomasi’s group [36]. Because the studied cycloaddition reactions were carried out in diethyl ether, we selected its dielectric constant at 298.0 K, ε = 4.3. The electronic chemical potential µ was assessed in terms of the one electron energy of the HOMO and LUMO, using Eq. (1) [37]:
The global electrophilicity ω for dipoles and dipolarophile was determined using Eq. (2) [38]:
The global nucleophilicity index Ν [39], based on the HOMO energies obtained within the Kohn–Sham scheme [40], is defined as Ν = ε HOMO (Nu) − ε HOMO (TCE) in which (Nu) denotes nucleophile. This relative nucleophilicity index refers to tetracyanoethylene (TCE).
Results and discussion
Theoretical studies of the regio- and stereochemistry of these 1,3-DC reactions will be based on two sections. Firstly, we focus our attention on the energetic aspects of the 1,3-DC reactions. In the second section, we investigate the bond lengths, bond orders, steric analysis and charge transfers in the TS structures.
Activation energy calculations
In this present study, the transition states are located through vibrational frequency analysis. Each such transition state is distinguished by a single imaginary frequency. There are two regioisomeric pathways—ortho and meta—and two stereoisomeric pathways—endo and exo—as shown in Scheme 1. For the different structures of the nearing reactants, transition states and the products, a useful naming system has been utilized according to our previous theoretical investigations [21, 22, 26, 27]. Abbreviated structural symbols for the products (Pyon, Pyox, Pymn, Pymx), the transition states (TSyon, TSyox, TSymn, TSymx) and the oriented reactants (1, 2a, 2b, 2c) have been followed throughout this discussion. In these reactions, cycloadditions of dipolarophile 1 with nitrile oxides (2a, 2b, 2c) in ortho channel and endo/exo stereoselectivity approach lead to products Pyon and Pyox. Similarly, cycloaddition reactions of dipolarophile 1 with nitrile oxides (2a, 2b, 2c) with meta channel and endo/exo stereoselectivity approach lead to products Pymn and Pymx. The transition states TSyon, TSyox, TSymn and TSymx are related to products Pyon, Pyox, Pymn and Pymx, respectively, and also, y = 1, 2 and 3 are used for (2a, 2b and 2c) nitrile oxide derivatives, respectively (Scheme 1).
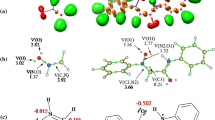
The corresponding activation barriers, structures and energy profile are given in Table 1, Figs. 1 and 2, respectively. All the reactions progressed exothermically with large ΔH values (Table 1). In accord with Hammond’s postulate, the TSs should then be more adjacent to the reactants. As shown in Table 1, for the all these reactions, the ortho cyclization modes are more favorable than the meta ones by about 17 kcal mol−1, leading to the formation of the ortho cycloadducts Pyon and Pyox, while the endo approach modes giving P1on, P2on and P3on are more favorable, by ca. 2 kcal mol−1, than the exo ones. Moreover, in order to confirm our calculations, we calculated above activation energies for reaction between 1 and 2a with larger basis set (6 − 311 + G(2d,p)), we obtained same results. Therefore, 1,3-DC reactions show complete ortho regioselectivity and high endo stereoselectivity. These results are in agreement with the experimental data [29].
In order to rationalize complete endo selectivity along ortho regioisomeric pathway, a natural steric analysis was carried out on the B3LYP/6-31G (d,p)-generated wave function of both optimized TS1on and TS1ox (as a model) using Gen NBO5.0 W software [41]. Important pairwise steric exchange energies, ∆Ei,j, between NLMOs i,j for TS1on and TS1ox in 1,3-DC reaction of 1 toward 2a including optimized structures of TS1on and TS1ox together with corresponding atoms numbering are given in Table S1 from supplementary information. As shown, the sum of important pairwise steric exchange energies is 288.99 and 299.11 kcal mol−1 for TS1on and TS1ox, respectively, indicating that TS1ox suffers from a larger destabilizing steric interaction than TS1on about 10.12 kcal mol−1. The greatest value of pairwise steric exchange energy in both TS1ox and TS1on is associated with the interaction between σ C1–C5/σ C8-N14 and σ C1–C5/σ C8-N13, respectively (see Table S1 from supplementary information). In order to complete the study of endo selectivity provided by the 1,3-DC reaction of 1 toward 2a, stabilizing electron density delocalization effects were also examined.
Delocalization of electron density between occupied Lewis-type (bond or lone pair) NBO orbitals and formally unoccupied (anti-bond or Rydberg) non-Lewis NBO orbitals corresponds to a stabilizing donor–acceptor interaction. The energy of this interaction can be estimated by the second-order perturbation theory [42]. The calculated important second-order interaction energies (E 2) between the donor–acceptor orbitals in TS1ox and TS1on as well as optimized structures of these TSs together with corresponding atoms numbering are listed in Table S2 from supplementary information. As presented in this table, the sum of important second-order interaction energies is 485.02 and 477.74 kcal mol−1 for TS1on and TS1ox, respectively. These values obviously show that TS1on is stabilized than TS1ox by 7.28 kcal mol−1 explaining the preferable endo selectivity displayed by the studied 1,3-DC reaction. It is interesting to note that the greatest value of the second-order interaction energy in TS1ox and TS1on is related to the delocalization of donor LP (3) O4 to the acceptor π* C2–N3 and donor LP (2) S9 to the acceptor π* C1–N13, respectively.
As the 1,3-DC reactions between dipolarophile 1 and dipoles 2a, 2b and 2c were carried out in diethyl ether, which can have some prevalence on the energies and geometries, solvent effects in these 1,3-DC reactions were evaluated by full optimization of the solvent phase geometries by utilizing the PCM model of Tomasi’s group [36]. The relative energies are summarized in Table 1. With the incorporation of solvent effects, reactants are more stabilized than TSs and Ps [43]. As a result, in diethyl ether, the relative Gibbs free energies associated with ortho and meta TSs are moderately increased between 0.5–1.1 and 0.7–2.2 kcal mol−1, respectively (Table 1).
The geometries of the TSs, the lengths of the C–O, C–N, C–C and N–O forming bonds and their Wiberg bond order values involved in these 1,3-DC reactions are shown in Fig. 1. Analysis of the lengths of the two forming bonds in the TSs shows that they are not formed to the same extent. It is of note that the C–N bond length is shorter than the C–O bond length (in TS1on, TS2on and TS3on) and C–C bond is shorter than N–O bond (in TS1mn, TS2mn and TS3mn). The degree of bond formation on a reaction path is given by the bond order conception [44]. The bond order (Wiberg indices) values of the C–O, C–N, C–C and N–O forming bonds at TSs are shown in square brackets in Fig. 1. These values are within the range of 0.09–0.36 for favorable pathway (TS1on, TS2on and TS3on) and 0.26–0.42 for unfavorable pathway (TS1mn, TS2mn and TS3mn). The differences of bond order for forming bonds at the TSs indicate asynchronous one-step mechanism processes. These results also show that the most favorable TSs (TS1on, TS2on and TS3on) are consistently more asynchronous than the unfavorable ones (TS1mn, TS2mn and TS3mn).
Recently, an interesting and helpful classification for Diels–Alder (DA) reactions based on the polar character, global electron density transfer (GEDT) computed at the TS, of DA reaction between cyclopentadiene and twelve substituted ethylenes has been provided by Domingo et al. [45]. According to this classification, there are three different types of DA reactions: (a) nonpolar DA (N-DA) reactions for which GEDT and activation energy are less than 0.15e and higher than 18 kcal mol−1, respectively; (b) polar DA (P-DA) reactions which are characterized by 0.15e < GEDT < 0.40e and an activation energy ranging from 17 to 5 kcal mol−1; (c) and finally, ionic DA (I-DA) reactions show a GEDT higher than 0.40e and negative activation energy. Therefore, the electronic nature of these 1,3-DC reactions is evaluated by analyzing the global electron density transfer at the TSs along the cycloaddition process. In the gas phase, the GEDTs at the TSs, which passes from the dipolarophile to the dipole moiety, are 0.123 e, 0.130 e and 0.122 e for the favorable TSs TS1on, TS2on and TS3on, respectively, indicating nonpolar character for these 1,3-DC reactions. This low electron density transfer can be related to the electron-rich character of the dipolarophile 1.
IRC calculations were carried out for all studied reactions and presented only for the reaction pathway leading to P1on (Figure S1 from supplementary information). This figure shows saddle point clearly and illustrates that the TSs connect to the associated minima of the concerted mechanism.
DFT-based reactivity indices
HOMO and LUMO energies, electronic chemical potential μ, chemical hardness η, global electrophilicity ω and global nucleophilicity N of the dipolarophile 1 and nitrile oxides 2a, 2b and 2c are given in Table 2.
As it can be seen in Table 2, the electronic chemical potential (µ) of dipolarophile 1 (−0.1165) is greater than those of dipoles 2a (−0.1409), 2b (−0.1248) and 2c (−0.1453), which shows that the charge transfer takes place from dipolarophile 1 to dipoles 2a, 2b and 2c. Consequently, the dipoles 2a, 2b and 2c can act as electrophile in these 1,3-DC reactions. These results are in agreement with GEDT calculations at the TSs. The electrophilicity difference for the dipole/dipolarophile pair, ∆ω, is a useful criterion to describe the high- or low-polar nature of the cycloaddition [46]. The small ∆ω between 1 and 2a, 2b and 2c is 0.66, 0.42 and 0.87 eV, respectively, which shows a nonpolar character for these 1,3-DC reactions.
A useful classification of 1,3-DC reactions into pseudodiradical-type (pr-type) and zwitterionic-type (zw-type) reactions has been proposed [47]. Pr-type reactions occur easily via an earlier TS with nonpolar nature, and zw-type reactions occur via polar TSs. It can be concluded that our investigated reactions participate in pr-type reactions. Recently, Domingo proposed the new local reactivity, Parr functions, in polar organic reactions [48]. In the present study, dipolarophile 1 is not electrophilically motivated; consequently, these 1,3-DC reactions are nonpolar. Therefore, Parr functions cannot give correct information about the regioselectivity in these nonpolar 1,3-DC reactions. He declared that due to the electrophilic/nucleophilic manner of these reactions, the Fukui functions on the basis of finite charge differentiations cause some serious errors and are not match to exchange in charge distribution, as suggested by these Fukui functions. Therefore, the Fukui functions also could not be used.
Conclusion
The 1,3-DC reaction of 2-ethylthio-4-phenyl-1-azetin and nitrile oxide derivatives has been investigated at DFT/B3LYP/6-31G(d,p) level of the theory in the gas and diethyl ether phases. The computed energies, enthalpies and free energies of activation of the transition states indicated experimentally formed products from ortho regioisomeric channels and endo stereoisomeric approaches along the ortho pathways are more favorable cycloadducts. Wiberg bond index and GEDT indicated an asynchronous one-step mechanism over the regioisomeric reaction channels. In order to rationalize complete endo selective fashion provided by these 1,3-DC cycloadditions, a natural steric analysis between NLMOs i,j for TS1ox and TS1on and also a second-order interaction energy, E 2, analysis between the donor–acceptor orbitals in these TSs were carried out. The results evidently showed that the complete endo selectivity explained TS1on leads the endo stereoisomeric approach preferred over the exo one.
References
Padwa A, Pearson WH (2003) Synthetic applications of 1, 3-dipolar cycloaddition chemistry toward heterocycles and natural products, vol 59. Wiley, New York
Huisgen R (1963) Angew Chem Int Ed Eng 2:565
Padwa A (2002) 1, 3-Dipolar cycloaddition chemistry, vol 1. Wiley, New York
Gothelf KV, Jørgensen KA (1998) Chem Rev 98:863
Nishiwaki N, Kobiro K, Hirao S, Sawayama J, Saigo K, Ise Y, Okajima Y, Ariga M (2011) Org Biomol Chem 9:6750
Kumar RS, Ramar A, Perumal S, Almansour AI, Arumugam N, Ali MA (2013) Synth Commun 43:2763
Boström J, Hogner A, Llinàs A, Wellner E, Plowright AT (2012) J Med Chem 55:1817
Scott JS, Birch AM, Brocklehurst KJ, Broo A, Brown HS, Butlin RJ, Clarke DS, Davidsson Ö, Ertan A, Goldberg K, Groombridge SD, Hudson JA, Laber D, Leach AG, MacFaul PA, McKerrecher D, Pickup A, Schofield P, Svensson PH, Sörme P, Teague J (2012) J Med Chem 55:5361
Villemagne B, Crauste C, Flipo M, Baulard AR, Déprez B, Willand N (2012) Eur J Med Chem 51:1
Ono M, Haratake M, Saji H, Nakayama M (2008) Bio Med Chem 16:6867
Orlek BS, Blaney FE, Brown F, Clark MSG, Hadley MS, Hatcher J, Riley GJ, Rosenberg HE, Wadsworth HJ, Wyman P (1991) J Med Chem 34:2726
Warmus JS, Flamme C, Zhang LY, Barrett S, Bridges A, Chen H, Gowan R, Kaufman M, Sebolt-Leopold J, Leopold W, Merriman R, Ohren J, Pavlovsky A, Przybranowski S, Tecle H, Valik H, Whitehead C, Zhang E (2008) Bioorg Med Chem Lett 18:6171
McBriar MD, Clader JW, Chu I, Del Vecchio RA, Favreau L, Greenlee WJ, Hyde LA, Nomeir AA, Parker EM, Pissarnitski DA, Song L, Zhang L, Zhao Z (2008) Bioorg Med Chem Lett 18:215
Ess DH, Jones GO, Houk K (2006) Adv Synth Catal 348:2337
Le TN, De Proft F, Chandra AK, Langenaeker W, Nguyen MT, Geerlings P (1999) J Am Chem Soc 121:5992
Nguyen LT, Proft FD, Chandra AK, Uchimaru T, Nguyen MT, Geerlings P (2001) J Org Chem 66:6096
Chandra AK, Nguyen MT (1998) J Phys Chem A 102:6181
Shang YJ, Wang YG (2002) Synthesis 1663
Shankar BB, Yang DY, Girton S, Ganguly AK (1998) Tetrahedron Lett 39:2447
Kang KH, Pae AN, Choi KL, Cho YS, Chung BY, Lee JE, Jung SH, Koh HY, Lee HY (2001) Tetrahedron Lett 42:1057
Moeinpour F, Khojastehnezhad A (2014) J Iran Chem Soc 11:1459
Rahimizadeh M, Eshghi H, Khojastehnezhad A, Moeinpour F, Bakavoli M, Tajabadi J (2014) J Fluor Chem 162:60
Moeinpour F, Bakavoli M, Davoodnia A, Morsali A (2012) J Theo Comput Chem 11:99
Bakavoli M, Moeinpour F, Davoodnia A, Morsali A (2010) J Mol Struct 969:139
Moeinpour F (2010) Chin J Chem Phys 23:165
Eshghi H, Khojastehnezhad A, Moeinpour F, Bakavoli M (2015) Can J Chem 93:749
Moeinpour F, Khojastehnezhad A (2015) Acta Chim Slov 62:403
Emamian S, Lu T, Moeinpour F (2015) RSC Adv 5:62248
Hemming K, Khan MN, O’Gorman PA, Pitard A (2013) Tetrahedron 69:1279
Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B. 03. Gaussian Inc., Pittsburgh
Schlegel HB (1982) J Comput Chem 3:214
Head-Gordon M, Pople JA (1988) J Chem Phys 89:5777
Gonzalez C, Schlegel HB (1990) J Phys Chem 94:5523
Gonzalez C, Schlegel HB (1991) J Chem Phys 95:5853
Tomasi J, Persico M (1994) Chem Rev 94:2027
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512
Parr RG, Szentpaly LV, Liu S (1999) J Am Chem Soc 121:1922
Domingo LR, Chamorro E, Pérez P (2008) J Org Chem 73:4615
Galli G (1996) Curr Opin Solid State Mater Sci 1:864
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) NBO 5.0. Theoretical Chemistry Institute, University of Wisconsin, Madison
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) J Phys Org Chem 24:611
Wiberg KB (1968) Tetrahedron 24:1083
Domingo LR, Sáez JA (2009) Org Biomol Chem 7:3576
Chemouri H, Mekelleche SM (2012) Int J Quantum Chem 112:2294
Domingo LR, Emamian SR (2014) Tetrahedron 70:1267
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486
Acknowledgments
The financial support for this work was provided by Research Council of Ferdowsi University of Mashhad (Grant No. 3/29765) and Islamic Azad University, Bandar Abbas Branch.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khojastehnezhad, A., Eshghi, H., Moeinpour, F. et al. Density functional theory study of the regio‐ and stereoselectivity of 1,3-dipolar cycloaddition reactions between 2-ethylthio-4-phenyl-1-azetin and some substituted nitrile oxides. Struct Chem 27, 1041–1047 (2016). https://doi.org/10.1007/s11224-015-0703-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0703-8