Abstract
An efficient and stable palladium species catalyst immobilized on functionalized hyper–cross-linked polymers (HCPs-Pd) has been successfully developed and applied in the Suzuki–Miyaura coupling reaction of diverse types of aromatic halides with aryl boronic acid in this work. The results demonstrated that HCPs-Pd exhibited high catalytic activity, which benefited from the microporous structure of the catalyst guaranteed high dispersibility of active palladium, and high specific surface area, large pore volume, good chemical stability. Under optimal reaction conditions, 93.0% of biphenyl yield in the Suzuki–Miyaura reaction with bromobenzene (C6H5Br) and phenylboronic acid (C6H7BO2) as raw material was obtained. The good stability of the HCPs-II-Pd catalyst was verified by five cycles experiments. Perhaps this work provides new insights into the high-yield preparation of aromatic biphenyl compounds employing HCPs-Pd as an efficient and stable catalyst in the Suzuki–Miyaura reaction.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As an essential part of the porous organic polymers (POPs), the hyper–cross-linked polymers (HCPs) have been shown to display superior properties, including high surface area (SBET) and pore volume, special porosity and easy to prepare and chemical modify. Therefore, they have remarkable potentials for the adsorption materials and catalytic materials [1,2,3,4]. Compared with other materials, the diversity of synthesis is the great advantage of HCPs, which can have different chemical structures through connecting different functional groups [5, 6].
Compared with the material HCPs, the catalytic activity and stability of the immobilized precious metal were significantly improved, because of its organic cage frameworks and the coordination between the heteroatoms in HCPs and metal ions, which could fix the ultra-fine metal nanoparticles in the cages and make the metal particles firmly anchored to maintain an excellent activity in the long term, as well as serving as a catalyst support for the production of heterogeneous catalytic converters. [7,8,9,10].
In recent years, precious metal immobilized on functionalized HCPs has attracted interest due to a range of significant advantages, including the ability to utilize a variety of synthesis methods, the presence of small pore size, excellent stability, low economic cost and optimum processing conditions [11, 12]. It was widely used in organic synthesis [13, 14], light/heterogeneous catalysis [15, 16], adsorption and separation [17, 18], CO2 conversion [19, 20] and other fields. Particularly, as a heterogeneous catalyst in the field of organic synthesis, immobilization HCPs catalyst with precious metals has more advantages than traditional homogeneous catalysts, such as stable catalyst, mild reaction conditions, low emissions of three wastes and low environmental pollution [21, 22]. Gu [23] synthesized the hyper–cross-linked organic polymers (HDS-3.6) by Friedel–Crafts alkylation, it exhibited the good catalytic performance in the synthesis of ethyl levulinate (70.3% yield), and its catalytic performance did not significantly decrease. Lyubimov [24] developed a novel palladium nanoparticles catalyst by reduction of [Pd(π-allyl)Cl]2 with hydrogen in a hyper–cross-linked polystyrene matrix, and it showed high catalytic activity in the hydrogenation of benzene. (Benzene conversion was 100%.) Nobre [25] obtained active catalysts by combining Pd(OAc)2 and iminophosphine ligands, which indicated high catalytic activity in Suzuki–Miyaura coupling reactions, and high-yield coupling products can be obtained under mild conditions (25–50 °C) using aryl bromide, iodide or benzyl chloride as substrates. Jia [26] prepared (HCP-PPh3-Ru) catalyst through a one-step external cross-linking reaction, and it exhibited excellent catalytic activities in the synthesis of 2,4-diaryl substituted pyridine and diazodicarbonyl cycloaddition reaction. The results revealed that the ligand PPh3 and Ru immobilized on HCP materials was quite robust in the organic transformations reaction.
The Suzuki–Miyaura cross-coupling of arylboronic acid with organohalide is one of the most important carbon–carbon bond-forming reaction in organic synthesis [27]. Based on the above-mentioned investigated and our earlier experimental research on Suzuki–Miyaura coupling reactions (POPs as the mainly catalysts) [28,29,30,31], an efficient and stable palladium catalyst immobilized on functionalized hyper–cross-linked polymers (HCPs-Pd) had been successfully exploited, which solved the problem of complex process preparation required for the previously prepared POPs catalyst monomers. The research results from the catalytic Suzuki–Miyaura coupling reactions over palladium catalyst immobilized on functionalized hyper–cross-linked polymers are thoroughly reported in this paper.
Experimental
Reagents and instrument
8-hydroxyquinoline (AR, 99.0%), dimethoxy methane (AR, 98.0%), bromobenzene (AR, 99.5%), phenylboronic acid (AR, 98.0%) and iron chloride (AR, 99.9%) were obtained from Shanghai Aladdin biochemical technology Co., Ltd., China. Benzene (AR, 99.7%) and 1,2-dichloroethane (AR, 99.5%) were purchased from Chengdu Jinshan chemical Co., Ltd., China. Palladium chloride (AR, 98.0%) was obtained from Shanghai Dibai biotechnology Co., Ltd., China. The other reagents were afforded commercially.
Catalyst preparation
-
(i)
Synthesis of HCPs-I [32] AlCl3 (anhydrous, 4.3601 g, 32.70 mmol) and 8-hydroxyquinoline (0.5975 g, 4.12 mmol) were added to a solution of benzene (1.2165 g, 15.60 mmol) in 40 mL chloroform. The above formative mixture was stirred sufficiently at 20 °C, then stirred at 45 °C for 5.0 h in order to obtain the initial network, and continued to raise the temperature to 80 °C for 48 h to complete the reaction. The obtained precipitate after the reaction was washed 3 times with CH3OH, followed by hydrochloric acid in a Soxhlet extractor for 24 h. pH of the product was modulated to neutralized with NaOH solution, then washed repeatedly with CH3OH in a Soxhlet extractor for 55 h at 105 °C and lastly vacuum dried at 60 °C for 24 h (defined as HCPs-I, yield 95.0%).
-
(ii)
Synthesis of HCPs-II [33] FeCl3 (anhydrous, 3.3841 g, 20.86 mmol) was added to a solution of formaldehyde dimethyl acetal (FDA, 3.25 g, 42.72 mmol), benzene (1.2061 g, 15.46 mmol), 8-hydroxyquinoline (0.6083 g, 4.19 mmol) in 1,2-dichloroethane (DCE, 100 mL). The next experimental steps are similar to (i) Synthesis of HCPs-I. (Defined as HCPs-II, yield 96.1%).
-
(iii)
Preparation of HCPs-Pd [34] The immobilized catalyst was obtained by the coordination of Palladium(II) ions with the HCPs ligand. Typically, HCPs (0.25 g) were added to the tetrahydrofuran solution (10 mL) containing Pd(OAc)2 (0.037 g, 0.17 mmol) and then refluxed at 60 °C for 24 h. The next experimental steps are similar to (i) synthesis of HCPs-I, and the catalyst as prepared was designated as HCPs-I-Pd (yield 98.2%). For comparison, the HCPs-II-Pd (yield 98.5%) was prepared in the same way (as shown in Scheme 1).
Catalyst characterizations
XPS spectroscopy was performed using an ESCALAB 250Xi spectrometer. The textural properties of the samples were afforded using the N2 physisorption on an ASAP 2020 at 77 K. The elemental content of the samples was analyzed on a Thermo Scientific iCAP 7400 ICP-OES. FT-IR spectra of the samples were collected by using KBr matrix on a Nicolet-380 instrument (500–4000 cm−1). The HRTEM and SEM images were afforded on a JEM-2100 and Sigma HD, Carl Zeiss, Germany (FE-SEM), respectively.
Typical Suzuki–Miyaura coupling reaction
The reactions were performed in a Schlenk reactor [35]. Typically, HCPs-Pd (20 mg, 0.03 mol% of Pd) catalyst, bromobenzene (0.7801 g, 5.0 mmol), phenylboronic acid (0.7930 g, 6.5 mmol) and anhydrous K3PO4 (1.6960 g, 8.0 mmol) were joined to solvent (15 mL, EtOH/H2O (4:1 v/v)) and reacted for 3.0 h at 80 °C under N2. Bromobenzene was consumed as indicated by TLC and poured into 100 mL ice water with stirring. The mixture was alternately washed with dichloromethane and water by turn (3 × 20 mL). The combined organic phase was filtered and concentrated under reduced pressure after MgSO4 has adsorbed trace amounts of water. The obtained product was purified by flash chromatography (silica gel, light petroleum: diethyl ether = 20:1) until biphenyl was obtained as a white solid (716.0 mg, 93.0%) (As shown in Scheme 2). The data along with spectra of 1H NMR and 13C NMR for each purified product are given in Supporting Information.
Results and discussion
Effects of reaction conditions on the reaction with bromobenzene and phenylboronic acid as raw material
The catalytic performances of HCPs-Pd catalyst with different solvent and base for the Suzuki–Miyaura reaction were examined (Table 1). Firstly, the influence of base species on the reaction process was investigated (entries 1–6), and it showed that K3PO4 had better effects than K2CO3 and NaOH. Subsequently, different solvents were used for the reaction, and it showed that 4/1 (v/v) EtOH and H2O solutions were the best with a yield of 93.0% (entries 7–14). Finally, other reaction conditions were explored (entries 15–24), including catalyst dosage, reaction temperature and time, no nitrogen protection and room temperature reaction, and commercial palladium carbon catalysts have also been used in this reaction (entry 25). The optimal reaction conditions were obtained, 5.0 mmol bromobenzene, 6.5 mmol phenylboronic acid, 8.0 mmol K3PO4, 20 mg HCPs-II-Pd catalyst, 3.0 h, 80 °C, the product yield reached 93.0%.
The recycle of HCPs-II-Pd catalyst and thermal filtration experiment in the reaction
The recycle of HCPs-II-Pd catalyst was studied under above optimum reaction conditions. The used catalyst was separated by centrifugation and washed several times in acetone and methanol. It was dried at 105 °C and reused for the next reaction. Figure 1 shows the reusable results of the catalyst. It was evident that HCPs-II-Pd catalyst exhibited good stability in the reaction, and 89.5% of biphenyl yield with high TON (> 2400) was still obtained after five runs.
In addition, in order to verify the absence of free palladium in the catalytic reaction system, two comparative experiments (normal reaction and reaction after thermal filtration of the catalyst) were conducted to further verify the stability of the catalyst [36]. For normal reactions, quenching was occurred after 1.0 h (Table 1, entry 18), and the final yield of biphenyl was 65.2%. Comparative experiment showed that after 1.0 h of reaction, the catalyst was removed by thermal filtration, and the reaction continued for 3.0 h. After quenching the reaction, the final yield of biphenyl was 66.7%. Furthermore, ICP testing was conducted on the filtered reaction solution, and the results showed that it did not contain Pd. Comparing two experiments, it was found that the change in product yield was relatively small, and the HCPs-II-Pd catalyst did not shed palladium species, indicating good stability of the catalyst [37].
Performance comparison of catalysts reported in the studies
Table 2 shows the catalytic results of different catalysts in the studies. It can be seen that different catalytic systems require different reaction conditions, and the yield of the product is different. Although precious metal clusters or boron nitride nanosheets, graphite oxide has high catalytic performance, their preparation process is complex and poor stability, or relatively low TON value, which limits their widely application. We reported an efficient and stable catalyst system for synthesis of aromatic biphenyl compounds employing HCPs-Pd in the Suzuki–Miyaura reaction in this work.
Exploration of reactions with different substrates
Under the above conditions, a series of exploratory reactions were conducted to explore the catalytic reaction range of the catalyst (Scheme 3). As shown in Table 3, the catalytic reaction of HCPs-II-Pd has been proven to be suitable for the reactions of some compounds 1 and 2 containing different functional groups, resulting in the synthesis of product with high catalytic activity, and the structures and copies of 1H NMR spectra of target compounds 3a-3j are listed in Supplementary Information.
Catalyst characterization
N2 adsorption–desorption isotherms of four samples are shown in Fig. 2A. The typical type-IV isotherm with H4 hysteresis loop was observed for all samples [44]. In addition, the non-local density functional theory method was used to get the pore diameter distribution curves (Fig. 2B). It can be evident that a regular distribution of the pore diameters in the micro- and meso-sizes is found for all samples [45].
Furthermore, the chemical composition and structural characteristics of the samples are listed in Table 4. The Pd content of the HCPs-I-Pd catalyst is essentially the same as that of the HCPs-II-Pd catalyst from the results of ICP test. The increase in pore volume and the decrease in BET specific surface area of HCPs-Pd may be due to the modification of Pd, which blocks the small pore size of HCPs [46]. These results indicated that hyper–cross-linked polymers containing palladium Pd components have been successfully prepared [46, 47]. In addition, HCPs-II-Pd has a higher specific surface area than HCPs-I-Pd, which may be one of the reasons for its better catalytic activity.
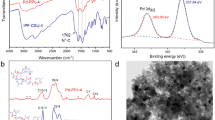
Figure 3 mainly explains XPS characterization of the Pd element in two HCPs-Pd samples. Figure 3A shows the characteristic peaks of C 1s and Pa 3d at binding energies 284.8 and 338.1 eV, respectively. It can be observed that both HCPs-I-Pd and HCPs-II-Pd exhibit the characteristic peaks of Pd. The complex Pd 3d spectra are shown in Fig. 3B. The presence of Pd(II) is correlated with the peaks at 343.7 and 338.2 eV, and the other peak at 342.2 and 336.6 eV is attributed to Pd(0), respectively [13, 15, 48], maybe it is a synergistic catalytic action between Pd(0) and Pd(II) in the catalytic reaction [49]. It can be inferred that Pd has successfully coordinated with HCPs to form HCPs-Pd [48, 50].
SEM-Mapping was used to study the morphologies of four materials. As shown in Fig. 4, it indicated that HCPs-Pd have no specific morphology, as if they are irregularly stacked spherical particles. To provide direct evidence of the successful introduction of Pd into HCPs, new evidence was obtained by elemental mapping of SEM images of two samples (Fig. 5) [51]. It indicates that the distribution of C, N, O and Pd can be clearly observed, confirming the existence and uniform distribution of Pd in two samples. It is worth noting that one of the reasons for its superior performance in the reaction process is the homogeneity of the four elements [52].
Figure 6 depicts the TEM images of four samples. The presence of Pd on hyper–cross-linked polymers is determined, and the lattice spacings of around 0.2 nm can be detected and attributed to the Pd species [53], as shown in the HRTEM images (Fig. 6B, D). The results demonstrate that the distribution of Pd species in HCPs-Pd samples is uniform. The uniform dispersion of active Pd species in the catalyst may be one reason for the good catalytic activity in the reaction [54,55,56].
FT-IR shows a large number of distinctive peaks of four samples (Fig. 7). Compared two curves of HCPs-I and HCPs-I-Pd, it is found that the intensity of the infrared peaks at 1624, 1492, 877 and 736 cm−1 positions increased [43, 57]. The typical band at 3449 cm−1 is assigned for the O–H group in salen backbone. Peaks from 2910 to 3030 cm−1 resulted from the C–H stretching vibration of the benzene rings, while the peak at 2857 cm−1 came from the –CH2– stretching vibration. In addition, a series of peaks around 1589 to 1624 cm−1 are ascribed to the existence of –C=N– stretching [43, 58]. The peaks at 1443 and 1680 cm−1 are the C=C stretching vibration of aromatic ring, and the peak at 1276 cm−1 is assigned for the C=O group in salen backbone [48]. According to literature reports, palladium mainly coordinates with nitrogen and oxygen [20, 48]. The peaks around 1150–877 cm−1 corresponded to C–H in-plane bending of the benzene skeleton. The bands around 1250–950 and 900–650 cm−1 can be attributed to benzene skeleton stretching C–H out-of-plane bending and in-plane bending vibrations of the benzene ring, respectively [59]. Interestingly, compared the curves of HCPs-II and HCPs-II-Pd, it is found that the infrared peak of HCPs-II-Pd shifted red at positions 782 and 736 cm−1 and is a separate infrared peak (741 cm−1). In addition, the peak intensity also weakens at 606 cm−1 [59, 60]. These changes may be caused by the coordination of Pd on HCPs [61, 62].
Possible catalytic reaction pathway over HCPs-II-Pd catalyst
Scheme 4 shows a possible catalytic reaction pathway for Suzuki–Miyaura coupling reaction over heterogeneous HCPs-II-Pd catalyst. Several excellent reviews have been published in the literature [63,64,65], and it is a synergistic catalytic action between Pd(0) and Pd(II) in the catalyst. Herein, we focus on the use of EtOH and water as a medium for Suzuki–Miyaura coupling reactions in heterogeneous systems, and its advantages toward green chemistry. Substances [I] and [II] coexist with each other. Firstly, reaction raw compound 1 is adsorbed on the catalyst and forms [III]. Under alkaline conditions, [III] removes halogen X to form [IV]. [IV] adsorbs reaction raw material compound 2 to form [V], and the target product is ultimately removed from the catalyst to form biphenyl substances, which then return to the initial state [I].
Conclusions
In conclusion, two porous hyper–cross-linked polymers (HCPs-I, HCPs-II) were successfully designed and prepared by a one-pot Friedel–Crafts alkylation reaction employing anhydrous FeCl3 or AlCl3 in the presence or absence of FDA. Further, a “braided” strategy was used for the preparation of Pd metal–organic catalysts on functionalized HCPs. The new polymer (HCPs-Pd) has excellent catalytic activity, good porosity with high specific surface area, large pore volume, good stability and highly dispersed palladium. Consequently, in the Suzuki–Miyaura coupling reaction of aryl bromides, the HCPs-Pd showed good performance in combination with the mild conditions and hydrophilic reaction media. Furthermore, the HCPs-Pd catalyst can be recycled and reused for multiple runs with no apparent obvious loss of catalytic efficiency. This work also shows that the microporous polymers can not only act as supporting materials, but can also protect the catalyst and have a positive effect on the catalytic activity. In addition, we also expect the work to open up a new path of designing and constructing excellent heterogeneous solid catalysts on a molecular scale.
Data availability
All data generated or analyzed in this study are included in this article.
References
Y. Gu, S.U. Son, T. Li, B. Tan, Adv. Funct. Mater. 31, 2008265 (2020)
Y. Liu, X. Fan, X. Jia, B. Zhang, H. Zhang, A. Zhang, Q. Zhang, J. Mater. Sci. 51, 8579 (2016)
L. Tan, B. Tan, Chem. Soc. Rev. 46, 3322 (2017)
A. Croce, G. Re, C. Bisio, G. Gatti, S. Coluccia, L. Marchese, Res. Chem. Intermed. 47, 419 (2021)
Z. Duan, Y. Wang, Q. Pan, Y. Xie, Z. Chen, Chin. J. Polym. Sci. 40, 310 (2022)
J. Li, X. Wang, G. Chen, D. Li, Y. Zhou, X. Yang, J. Wang, Appl. Catal. B Environ. 29, 87 (2012)
S. Gorji, R. Ghorbani-Vaghei, Appl. Organomet. Chem. 35, e6018 (2021)
C. Yue, Q. Xing, P. Sun, Z. Zhao, H. Lv, F. Li, Nat. Commun. 12, 1875 (2021)
T. Ratvijitvech, Polymer 14, 2749 (2022)
S. Sadjadi, M. Malmir, N. Pourmohammad, S. Ahmadi, M.M. Heravi, Res. Chem. Intermed. 45, 4349 (2019)
X. Huang, X. Hong, H. Lin, X. Cao, Q. Dang, S. Tang, D. Chen, Y. Zhang, Chem. Eng. J. 435, 134990 (2022)
H. Ya, L. Zhan, S. Gaa, H. Wana, Z. Ha, Y. Xu, K. Huang, J. Catal. 396, 342 (2021)
T. Mandal, M. Mondal, J. Choudhury, Organometallics 40, 2443 (2021)
K. Wang, W. Cui, Z. Bian, Y. Liu, S. Jiang, Y. Zhou, J. Wang, Appl. Catal. B Environ. 281, 119425 (2021)
Y. Zhang, L. Zhang, X. Zhang, D. Yang, C. Du, L. Wan, C. Au, J. Chen, M. Xie, New J. Chem. 44, 15202 (2020)
Y. Gu, S.U. Son, T. Li, B. Tan, Adv. Funct. Mater. 31, 2170082 (2021)
G. Xiong, S. Gao, Q. Zhang, B. Ren, L. You, F. Ding, Y. He, Y. Sun, Polymer 247, 124787 (2022)
J. Zhang, N. Liu, H. Gong, Q. Chen, H. Liu, Microp. Mesop. Mat. 336, 111836 (2022)
H.R. Penchah, P. Najafi, A. Ghaemi, H.G. Gilani, Environ. Progr. Sustain. 40, e1586 (2021)
R.A. Molla, P. Bhanja, K. Ghosh, S.S. Islam, S.M. Islam, A. Bhaumik, ChemCatChem 9, 1939 (2017)
W. Jiang, W. Sun, Y. Zhou, Y. Li, Res. Chem. Intermed. 45, 5535 (2019)
A. Blocher, F. Mayer, P. Schweng, T.M. Tikovits, N. Yousefi, R.T. Woodward, Mater. Adv. 3, 6335 (2022)
J. Gu, J. Zhang, D. Li, H. Yuan, Y. Chen, J. Chem. Technol. Biot. 94, 3073 (2019)
S.E. Lyubimov, A.A. Zvinchuk, A.A. Korlyukov, V.A. Davankov, O.P. Parenago, Petrol. Chem. 61, 76 (2021)
S.M. Nobre, A.L. Monteiro, J. Mol. Catal. A: Chem. 313, 65 (2009)
Z. Jia, K. Wang, B. Tan, Y. Gu, Adv. Synth. Catal. 359, 78 (2017)
A. Fihri, D. Luart, C. Len, A. Solhy, C. Chevrin, Y. Polshettiwar, Dalton Trans. 40, 3116 (2011)
H. Lin, H. Yao, X. Gao, L. Zhang, Q. Luo, Y. Ouyang, B. Xiang, S. Liu, D. Xiang, Chem. Lett. 50, 1879 (2021)
H. Lin, X. Gao, H. Yao, Q. Luo, B. Xiang, C. Liu, Y. Ouyang, N. Zhou, L. Zhang, D. Xian, Catal Sci. Technol. 11, 3676 (2021)
W. Xu, C. Liu, D. Xiang, Q. Luo, Y. Shu, H. Lin, Y. Hu, Z. Zhang, Y. Ouyang, RSC Adv. 9, 34395 (2019)
C. Liu, L. Zheng, D. Xiang, S. Liu, W. Xu, Q. Luo, Y. Shu, Y. Ouyang, H. Lin, RSC Adv. 10, 17123 (2020)
B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan, T. Li, Adv. Mater. 24, 3390 (2012)
Y. Zhi, K. Li, H. Xia, M. Xue, Y. Mu, X. Liu, J. Mater. Chem. A 5, 8697 (2017)
N.A. Nemygina, L.Z. Nikoshvili, M.G. Sulman, V.G. Matveeva, E.M. Sulman, Chem. Eng. Trans. 52, 691 (2015)
F. Mohajer, G.M. Ziarani, A. Badiei, Res. Chem. Intermed. 49, 1273 (2023)
Q. Fu, Y. Meng, Z. Fang, Q. Hu, L. Xu, W. Gao, X. Huang, Q. Xue, Y. Sun, F. Lu, A.C.S. Appl, Mater. Inter. 9, 2469 (2017)
P.S. Pharande, G.S. Rashinkar, D.M. Pore, Res. Chem. Intermed. 47, 4457 (2021)
V. Faria, D. Oliveira, M. Kurz, F. Gonçalves, C. Scheeren, G. Rosa, RSC Adv. 4, 13446 (2014)
G. Alvarenga, C. Ruas, J. Vicenti, F. Duarte, M. Gelesky, G. Rosa, J. Brazil. Chem. Soc. 27, 787 (2016)
R. Begum, Z. Farooqi, J. Xiao, E. Ahmed, A. Sharif, A. Irfan, J. Mol. Liq. 338, 116780 (2021)
Y. Dong, Z.Z. Wang, X. Li, T. Li, Y. Ren, W. Hu, L. Zhang, X. Zhang, C. Wei, J. Mol. Liq. 368, 120679 (2022)
M. Lin, S. Wang, J. Zhang, W. Luo, H. Liu, W. Wang, C. Su, J. Mol. Catal. A: Chem. 394, 33 (2014)
S. Xu, K. Song, T. Li, B. Tan, J. Mater. Chem. A 3, 1272 (2015)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
N. Koukabi, M. Arghan, Res. Chem. Intermed. 48, 4553 (2022)
W. Lin, Y. Song, L. Wang, N. Li, Y. Fu, D. Chen, W. Zhu, F. Zhang, Catal. Lett. 153, 2368 (2023)
M. Ghabdian, M.A. Nasseri, A. Allahresani, A. Motavallizadehkakhky, Res. Chem. Intermed. 47, 1713 (2021)
D. Meng, J. Bi, Y. Dong, B. Hao, K. Qin, T. Li, D. Zhu, Chem. Commun. 56, 2889 (2020)
D. Rosa, B. Vargas, M. Silveira, C. Rosa, M. Martins, G. Rosa, Waste Biomass Valori. 10, 2285 (2018)
B. Wu, P. Lyu, K. Wang, X. Qiu, T. Liu, F. Zhang, H. Li, S. Xiao, Res. Chem. Intermed. 44, 6327 (2018)
S. Rana, S. Maddila, K. Yalagala, S.B. Jonnalagadda, Appl. Catal. A: Gen. 505, 539 (2015)
S. Sun, D. Pan, H. Huang, Z. Wang, Y. Xu, Y. Zhao, Res. Chem. Intermed. 48, 3129 (2022)
D. Xu, F. Wang, G. Yu, H. Zhao, J. Yang, M. Yuan, X. Zhang, Z. Dong, ChemCatChem 10, 4569 (2018)
H. Sato, T. Mameda, K. Nakai, T. Misaki, Y. Haruyama, S. Sonobe, T. Kubota, Y. Okamoto, T. Sugimura, Res. Chem. Intermed. 42, 31 (2016)
A. Modak, J. Su, W. Qiu, X. Liu, Catalysts 6, 161 (2016)
G. Chen, K. You, F. Zhao, Z. Chen, H. Luo, Res. Chem. Intermed. 48, 2593 (2022)
C. Xu, M. Hu, Q. Wang, Gu. Fan, Y. Wang, Y. Zhang, D. Gao, J. Bi, Dalton Trans. 47, 2561 (2018)
Z. Jia, K. Wang, T. Li, B. Tan, Y. Gu, Catal. Sci. Technol. 6, 4345 (2016)
F. Zhang, J. Jin, X. Zhong, S. Li, J. Niu, R. Li, J. Ma, Green Chem. 13, 1238 (2011)
V. Sadhasivam, M. Mathappan, M. Harikrishnan, C. Chithiraikumar, S. Murugesan, A. Siva, Res. Chem. Intermed. 44, 2853 (2018)
Y. Cao, Y. Wang, Y. Fu, F. Zhou, J. Huang, Sep. Purif. Technol. 322, 124272 (2023)
J. Zhang, K. Dong, W. Luo, H. Guan, Fuel 234, 664 (2018)
A. Balanta, C. Godard, C. Claver, Chem. Soc. Rev. 40, 4973 (2011)
V. Polshettiwar, A. Decottignies, C. Len, A. Fihri, Chemsuschem 3, 502 (2010)
S. Kotha, K. Lahiri, D. Kashinath, Tetrahedron 58, 9633 (2002)
Acknowledgements
The authors are grateful for the financial support of the Natural Science Foundation of Hunan Province (2021JJ30539, 2023JJ50450) and Innovative Training Project for College Students of China (S202310548066).
Funding
Funding was provided by Natural Science Foundation of Hunan Province (2021JJ30539, 2023JJ50450) and Innovative Training Project for College Students of China (S202310548066).
Author information
Authors and Affiliations
Contributions
LF contributed to conceptualization, investigation, data curation and writing—original draft. SL was involved in writing and editing and data curation. HZ contributed to conceptualization, investigation and data curation. GC was involved in conceptualization, methodology, formal analysis, investigation, writing—original draft, and writing—review and editing. DX contributed to conceptualization, supervision and project administration.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, L., Lu, S., Zou, H. et al. Palladium catalyst immobilized on functionalized hyper–cross-linked polymers with 8-hydroxyquinoline as monomer for Suzuki–Miyaura coupling reactions. Res Chem Intermed 50, 2051–2066 (2024). https://doi.org/10.1007/s11164-024-05270-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-024-05270-0