Abstract
Isoxazole and its derivatives derived from natural resources are very few. However, they have several applications in pharmaceutical industries, including antimicrobial, antitumor, anticancer, antifungal, and antibacterial activities. As a result, this research aimed to design a novel one-pot green approach to synthesize new oxazole derivatives. The derivatives were further explored for their antibacterial and antioxidant activities together with their density functional theory (DFT) analysis. Characterization of newly synthesized moieties was done by IR, 1H NMR, 13C NMR, CHN analysis, & single-crystal X-ray Crystallography. Further, these compounds were examined for their antibacterial potential by using Gentamycin as a standard drug against S. aureus and E. coli bacterial strains. The derivatives 4a, 4c, 4d, 4f, 4j, and 4k possessed excellent antibacterial potential against former, while 4c and 5 showed the highest activity against the later one. The derivatives were also analyzed for their antioxidant activities by using free radical scavenging (ABTS. & DPPH assays), and total antioxidant capacity. Here also, 4a, 4d, 4e, 4k, 4l, 4m, and 5 exhibited the most promising results. Finally, the DFT analysis was achieved by using the B3LYP methodology with a 6–311 + G(d,p) basis set to study the electronic structure of molecules and analysis of chemical reactivity descriptors such as hardness (η), Mulliken electronegativity (χ), chemical potential (μ) and electrophilicity (ω). These properties were calculated from the levels of the predicted frontier molecular orbitals and their energy gap.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Developing efficient techniques for synthesizing a wide array of significant substructures for discovery and designing a drug is highly anticipated. Such techniques should be high yielding, environmentally benign, and easy to implement, with a rapid growth in structural diversity, by utilizing widely accessible reactants [1,2,3]. The efficiency of these methods is judged by the involvement of non-toxic and cost-effective catalysts, ease of handling & incorporation of a variety of substrates [4]. The rigidity of synthetic methodology used and the lengthy reaction steps are substantial limitations in a novel synthetic protocol. In the last few decades, the synthetic community has been highly affected by the diversity-oriented synthesis and ideas of adaptable multicomponent processes [5,6,7]. Hence, utilizing multicomponent reactions (MCRs) [8,9,10,11] in designing attractive heterocyclic scaffolds are particularly beneficial for the synthesis of plethora of “drug-like” moieties.
Among the primary precursors, isoxazoles are favored frameworks that show a wide scope of critical organic exercises (shown in Fig. 1) [12,13,14,15,16,17,18,19]. Isoxazole moiety is present in naturally occurring compounds like α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) [20], Ibotenic acid [21], etc. They also act as immunosuppressors (EF1502) [22], inhibitors of Heat Shock Protein90 [23], initiators of neurogenesis (N-cyclopropyl-5-(thiophene-2-yl)isoxazole-3-carboxamide) [24], GABAA antagonist (5-(3-(4-(3-chlorophenyl)piperazine-1-yl)propyl)-3-methyl-7-phenylisoxazolo[4,5-d]pyridazin-4(5H)-one) [25], Muscimol [26] and antinociceptive [27], etc. In addition, the isoxazole derivatives are also known to show a wide range of bioactivities like antibiotic (dicloxacillin) [28], anti-inflammatory (Isoxicam [29], immunomodulatory (Leflunomide) [30], COX-2 inhibitor (Valdecoxib) [31], herbicidal assays [32], etc. Some of the isoxazole derivatives are reported as therapeutic agents, e.g., leflunomide (Antirheumatic drug), valdecoxib (Anti-inflammatory drug) and cloxacillin (Antidiabetic). Also, they have various applications as functional materials [33] like liquid crystalline compounds [34].
Due to their immense utility, isoxazole derivatives have grown prominently in recent years. Their impressive bioactivities are the main reason for their high medicinal chemistry and drug discovery demand. As a result, isoxazoles have been a popular target for organic synthetic chemists all around the world, and substantial research efforts have been directed toward its structural modification and biological assessment. Because of this boom, various techniques for producing isoxazoles and their functionalized analogues have been developed. [35,36,37,38,39,40,41]. However, it is worthwhile to note that most of the reported methods have one or several drawbacks, such as the implementation of expensive catalysts, moisture sensitive, unstable, and hazardous reagents, harsh reaction conditions, prolonged durations, lesser yields & involvement of volatile organic solvents [42, 43].
Recently, we have focused much of our research work on the study and preparation of bioactive N- and O- containing heterocycles and have already reported simple and efficient procedures [44,45,46,47,48,49,50,51,52] to prepare molecules of biological interests [53, 54]. Herein, we are reporting the preparation of isoxazole derivatives via one-pot multicomponent reactions of substituted aldehydes (1) with NH2OH.HCl (2), and ethyl acetoacetate (3) using DABCO as an organocatalyst in an aqueous medium (Scheme 2). After the synthesis, the derivatives were tested for their clinical potential viz. antibacterial and antioxidant properties. Further, DFT analysis was also studied to obtain the optimized molecular structures of the synthesized isoxazole moieties to study their properties.
Results and discussion
Chemistry
At the onset of our investigation, we attempted to access the isoxazole-5(4H)-ones by establishing the model reaction conditions for this multicomponent synthesis. For that purpose, an equimolar amount of benzaldehyde (1a) was reacted with hydroxylamine hydrochloride (2) and ethylacetoacetate (3) with different catalysts (sodium acetate, pyridine, bromine water, green tea, DMAP, iodine, DABCO, and piperidine) and solvents (water, ethanol, DCM, acetonitrile) (Scheme 1) (Table 1). During optimization, we identified DABCO as the most promising catalyst amongst all other tested ones in terms of yield and reaction duration. Also, during literature review we observed that DABCO has never been used as a catalyst for the synthesis of isoxazole-5(4H)-one derivative.
DABCO, i.e., 1,4-Diazabicyclo[2.2.2]octane, is a bicyclic heterocyclic compound containing nitrogen atoms as heteroatom. Two tertiary nitrogens are present in it, providing it a high nucleophilic and basic character. As a result, it possesses broad catalytic activities. Particularly for the synthesis of heterocyclic compounds [55]. Xanthenes, 2-arylidene-2H-thiazolo[3,2-a]quinazolines, tetrahydropyrimidines, etc., are some heterocyclic derivatives which were synthesized efficiently using DABCO as a catalyst [56]. The combination of DABCO as organocatalyst and water as a solvent resulted in remarkable results (95% yield of 4a) within 60 min (entry 9, Table 1). Later, we also studied the effect of concentration of catalyst on the synthetic procedure (Table 1). When we perform the same reaction without DABCO, it takes 8 h to afford only a 20% yield (entry 10, Table 1). However, with the increase in catalyst amount (up to 15 mol%), the resultant product 4a was produced in very high yields (90%, entry 16, Table 1). However, a minor decrease in yield was noticed with an increase in reaction time by 30 min. On further increasing the catalytic amount, the yield starts decreasing with an increase in the reaction time (82%, entry 17, Table 1). The product 4a obtained was thoroughly characterized with the help of infrared (IR), 1H and 13C nuclear magnetic resonance (NMR) spectroscopy.
After optimizing the reaction conditions, we decided to synthesize a library of 3,4-Disubstituted isoxazole-5(4H)-one derivative (4a–4n) with optimized reaction conditions (Scheme 2).
To synthesize a library of 3,4-disubstituted isoxazole-5(4H)-one derivative (4a-4n), a variety of aldehyde (benzaldehyde, anisaldehyde, 4-hydroxybenzaldehyde, 4-bromo-2-fluorobenzaldehyde, 3-hydroxybenzaldehyde, 4-methylbenzaldehyde, 4-dimethylaminobenzaldehyde, 2-hydroxybenzaldehyde, 2,6-dichlorobenzaldehyde, 4-bromobenzaldehyde, naphthaldehyde, 3,4-dihydroxybenzaldehyde, thiophene-2-carboxaldehyde, furfural, 4-chlorobenzaldehyde, 3-nitrobenzaldehyde, 4-nitrobenzaldehyde, 2-chlorobenzaldehyde, formaldehyde, butyraldehyde, and pyridine-2-carboxaldehyde) were used. The results are given in Table 2.
From the results, it has been found that nature and position of substituents on the phenyl ring affected the product yield and reaction durations. In case of aromatic aldehydes bearing electron-donating groups such as –OH, –OCH3, –N(Me)2, CH3 the product was obtained in high yields. However the 2-hydroxy benzaldehyde yielded the corresponding product in relatively lower yield than 3-hydroxy benzaldehyde and 4-hydroxy benzaldehyde. That can be attributed to its crowded steric hindrance. While in case of aromatic aldehyde with electron withdrawing functional groups such as Br, F, Cl, NO2 the desired product were obtained in lesser yields compared to aromatic aldehydes with electron donating groups. Furthermore, the reaction does not perform well with 3-nitrobenzaldehyde, 4-nitrobenzaldehyde, 2-chlorobenzaldehyde, formaldehyde, butyraldehyde and pyridine-2-carboxyaldehyde as no product was observed. It is worth mentioning here that when the reaction was carried out with the 2,6-dichlorobenzaldehyde, (E)-2,6-Dichlorobenzaldehyde oxime (5) was formed instead of the (Z)-4-(2,6-dichlorobenzylidene)-3-methylisoxazol-5(4H)-one (4i) (Scheme 3).
The proposed synthesis mechanism of isoxazole derivatives via a one-pot three-component reaction catalyzed by DABCO is explained in Scheme 4. First, the reaction initiates with the hydroxylamine hydrochloride's nitrogen (2) nucleophilic attack over the carbonyl oxygen of the ethyl acetoacetate (3), resulting in the formation of the oxime intermediate. In the next step, DABCO abstracts the proton from the oxime intermediate, due to which carbanion intermediate is generated. Then this carbanion intermediate undergoes the Knoevenagel condensation with an aldehyde (1), and the Knoevenagel product is formed as an intermediate. After that, the intermediate undergoes rearrangement, hydrolysis, and intramolecular cyclization steps to afford the final product 4.
Single-crystal XRD
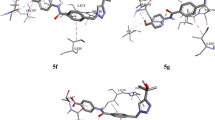
Single-crystal XRD data of compound 4j is obtained at 296 K using BRUKER D8 VENTURE SC-XRD. The study revealed that the crystal system of the compound 4j is orthorhombic with Fdd2 space group. Unit cell dimension of the crystal system is a = 25.108(4) Å, b = 33.746(5) Å, and c = 5.1802(7) Å. Unit cell volume of the crystal system is 4389.1(10) Å3. C–C bond distance ranges from 1.333 to 1.500 Å. Detailed bond angles and lengths will be provided along with the supplementary file. CIF has been deposited to the Crystallographic Data Centre. CCDC deposition number of compound 4j is 2117145. Crystallographic Data of compound 4j can be obtained from https://www.ccdc.cam.ac.uk/structures/. The crystal structure of the compound 4j is given in Fig. 2 and crystal data is given in the Table 3.
DFT studies
Density functional theory is an effective tool for chemical calculations of molecules and better representation of polar bonds. Most of the quantum chemical calculations were done by the DFT approach. For example, the DFT studies of isoxazole derivatives (4a–4n) and 5 have been done by using the B3LYP method with a 6–311 + G (d,p) basis set (Table 4) with the help of Gaussian 16 and Gauss view software.
The optimized structures, the lowest unoccupied molecular orbital (LUMO), and the highest occupied molecular orbital (HOMO) of the synthesized compounds are shown in Figs. 3, 4, and 5, respectively. LUMO is the electron acceptor orbital, which shows how much capacity a molecule has to accept an electron, whereas HOMO. Is the electron donor orbital which shows how much tendency a molecule has to give an electron. The LUMO and HOMO frontier orbitals were distributed evenly over the entire molecule (shown in Figs. 4 and 5) in all the molecules considered for this study. The energy gap between LUMO and HOMO of all molecules is shown in Fig. 6. From the results obtained, the energy gap of a compound of 4g is the lowest among all the synthesized compounds, i.e., 3.22 eV; therefore, the charge transfer interaction (CTI) or electron transition (ET) activities were expected to undergo easily, while maximum energy gap is obtained for compound 5 (5.1), so CTI/ET is expected to be difficult in it.
Quantum mechanical descriptors (QMD) such as electrophilicity index, chemical potential, Mulliken electronegativity, hardness, electron affinity, and ionization potential were calculated using the Koopman's theorem [57,58,59,60,61,62,63]. QMD provide critical insights into the chemical reactivity and electronic properties of molecules. For example, from the electrophilicity index value, it is clear that compound 4d has the maximum value (7.83), so it is likely to be expected to interact effectively with the nucleophilic species, while the lowest value (4.06) is obtained for the compound 5. Therefore, it has minor reactivity towards the nucleophiles. Further, from the Mulliken electronegative value of the CT reaction, it has been observed that compound 4d with the value of 5.38 has the highest tendency to act as electron acceptors. Apart from this, quantum mechanical descriptors also predict the stability of molecules. From the summarized results shown in Table 4, it has been observed that the chemical potential of compound 4d has the highest negative value, i.e., − 5.38, which negative values of the chemical potential indicate that under normal conditions, molecules are highly stable and do not have potential to decompose again into relative elements. Most of the reported compounds have negative values and are expected to be relatively stable.
Antibacterial activity
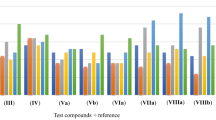
The antibacterial potential of the synthesized isoxazole derivatives was estimated by measuring the minimum inhibitory concentration (MICs) against both gram-positive (Staphylococcus aureus) and gram-negative bacteria (Escherichia coli). Table 5 shows the MIC value for the isoxazolederivatives, which were found to be 40–70 μg/mL for both bacterial strains. The inhibition was further confirmed using the agar disc diffusion method and the measured values are shown in Table 5 and Fig. 7. To compare the antibacterial activity of the synthesized compounds, we have taken the standard antibacterial drug ‘Gentamycin’ as a reference (represented by G in Fig. 7) in our study.
Antibacterial activity against the gram-positive bacteria
Outcomes of the zone of inhibition (mm) from the antibacterial studies have demonstrated that compounds 4a, 4c, 4d, 4f, 4j, and 4k have shown the maximum zone of inhibition (ZOI). The inhibition zone shown by these compounds (4a, 4c, 4d, 4f, 4j and 4k) is even greater than the reference drug, while compounds (4e, 4h, and 4m) have ZOI similar to or near the reference drug. Further, in our studies, 4b has shown moderate activity, while 4g and 4l have shown minor activity. However, compounds 5 and 4n do not show any activity. The maximum zone of inhibition shown by 'Gentamycin' against Staphylococcus aureus is 19 mm, so this value is used to compare the results. Compounds 4a, 4c, 4d, 4f, 4j, and 4k display the highest activity (20–24 mm) compared to the reference drug.
Antibacterial activity against the gram-negative bacteria
From the zone of inhibition values of the synthesized isoxazole derivatives, it has been observed that these are more active against gram-positive bacteria than gram-negative ones. Compound 5 has shown the maximum zone of inhibition (19 mm) which is slightly more significant than the reference gentamycin drug. Here Gentamycin has shown a maximum inhibition zone of 18 mm, so this value is used to compare the results. Furthermore, compounds 4c, 4e, 4h, and 4k also show high activity as they have similar or nearby inhibition zone values. Our studies have shown moderate activity in compounds 4g, 4j, 4l, and 4m. A minor activity is shown by compound 4a, while compounds 4b, 4d, 4f, and 4n do not show any activity against the gram-negative bacterial strain. Compound 4n is the only synthesized isoxazole derivative that does not respond against both bacterial strains.
Antioxidant activity
The TAC, DPPH radical, and ABTS radical scavenging methods were used to determine the possible antioxidant activity of the isoxazole derivatives (4a–4n) and 5 that were prepared. The outcomes of the antioxidant activity by these three methods are shown in Table 6 (also shown their comparison in Fig. 8). Here Gallic acid and ascorbic acid are used as standard compounds. Results are compared with these standards to analyze the antioxidant potential of synthesized compounds. Antioxidant potential of total antioxidant capacity (TAC) was represented as mg ascorbic acid equivalent (AAE)/g dry compound (DC) while ABTS and DPPH assays were represented as % inhibition. Antioxidant potential was evaluated in triplicates of all the samples. Antioxidant activity experiments have been carried out in triplicates to determine the synthesized derivatives' antioxidant potential accurately.
2,2-diphenyl-1-picrylhydrazyl (DPPH) assay
Any organic molecule antioxidant potential depends on its capacity to provide hydrogen or electrons to the DPPH radical. When a molecule donates an electron or hydrogen to a DPPH free radical, it converts the DPPH free radical into a diamagnetic scaffold. A change in colour of DPPH solution indicates an interaction between isoxazole derivatives and DPPH free radicals. When the dark violet colour of the DPPH solution turns light yellow or colourless, indicating the scavenging potential of isoxazole derivatives. At 517 nm their scavenging potential was measured by measuring the absorbance and compared with the standard [64]. Results of scavenging potential of DPPH assay revealed that all the compounds except for compound 4g have shown the inhibition. Maximum inhibitions were shown by the compound 4d (85.88 ± 0.03), 4k (85.40 ± 0.16), and 4m (84.98 ± 0.40) but had lower inhibition compared to the standards.
2,2‘-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay
ABTS free radicals were scavenged by any compound which is having antioxidant potential. With the scavenging of ABTS free radicals, there is a decrease in the absorption of the sample. All the samples were investigated at 734 nm, and the results obtained were compared with the standards and analyzed [65]. As the synthesized molecule starts interacting with ABTS free radicals, the blue color of the ABTS free radicals starts changing to colorless or pale yellow. Evaluation of the results revealed that 4a, 4j, 4k, and 4m showed the highest antioxidant potential in this protocol. However, the most encouraging thing was that the derivatives 4j and 4m showed 100% inhibition. Compound 4a (94.87 ± 0.16) and compound 4k (84.89 ± 0.80) also showed high antioxidant potential but had inhibition lower than the standards. In addition, compounds 4b, 4c, 4d, 4e, 4f, 4g, 4h, 4l, and 5 also showed moderate to good antioxidant potential. However, 4n was the only compound in this protocol that did not show inhibition.
Total antioxidant capacity (TAC)
In the total antioxidant capacity assay, the presence of antioxidants will reduce the phosphomolybdate ions and form a molybdate-phosphate complex of green color, which was analyzed using a spectrophotometer at 695 nm [66]. Derivatives 4e and 4n showed the highest total antioxidant capacity in this protocol, which were only 42.99 ± 0.13 and 43.56 ± 0.03. In this, all the synthesized derivatives showed activity but were found to be very low. This protocol showed less antioxidant activity than the other two protocols. The main reason behind the difference in the results of all the three protocols could be that the conditions and mechanisms they followed were different.
From the analysis of these three antioxidant activity protocols, it can be concluded that 4a, 4d, 4j, 4k, 4l, and 4m compounds have shown great potential. Results revealed that these derivatives could be considered antioxidant agents. However, toxicological and in vivo essential studies should be performed before considering these compounds.
Conclusions
Overall, a proficient and green synthetic methodology for the synthesis of 4-arylidene-3-methyl isoxazole-5(4H)-ones via one-pot three-component condensation of ethyl acetoacetate, aryl aldehyde, and hydroxylamine hydrochloride by using DABCO as catalyst under reflux condition has been developed. Additionally, the antioxidant and antibacterial activity of the synthesized isoxazole were tested and evaluated, which confirms that these derivatives have immense potential to act as antioxidants and antibacterial agents. Moreover, in future the synthesized derivatives can be explored further for their anti-inflammatory properties. The DFT analysis also confirmed the same, revealing the synthesized derivatives' molecular structure and related properties. This reaction has various merits, such as easy work-up, mild conditions, safety, lesser reaction time, high yields, and use of water with its eco-friendly point of view.
Experimental section
General
The solvents and starting materials used in this protocol are of commercially available synthesis grade and have been used without any further purification. The purity of the synthesized compounds is ensured with the help of Thin-layer chromatography. The melting point of the compounds is observed with the help of digital melting point apparatus. IR spectra have been recorded with the help of Shimadzu FTIR spectrophotometer. 1H NMR and 13C NMR spectra have been recorded on 500 MHz Bruker Avance NEO apparatus taking Me4Si as internal standard. Elemental analyses have been recorded using Perkin Elmer 2400 automatic carbon, hydrogen, nitrogen analyzer.
General procedure
Synthesis of isoxazole derivatives was (4a–4n) attained by using various aldehydes (1) (1 mmol), hydroxylamine hydrochloride (2) (1 mmol), and ethylacetoacetate (3) (1 mmol) by using 10 mol% DABCO as the catalyst in the presence of 5 ml water under reflux conditions. The progress of reaction was monitored by TLC using ethyl acetate and hexane (30:70) solvent system. The solid crude product obtained was simply purified via recrystallization using ethanol.
(E)-4-Benzylidene-3-methylisoxazol-5(4H)-one (4a)
Yellow needles, mp 140–142 ℃; IR (KBr): 3057 (ArH), 2948 (Aliph. H), 1736 (C=O), 1616 (C=N), 1447, 1384, 1219, 1114, 1021, 987, 879, 759 cm−1; 1H NMR (500 MHz, CDCl3) δ: 8.34 (d, J = 7.75Hz, 2H, ArH), 7.59–7.55 (m, 1H, ArH), 7.51–7.48 (m, 2H, ArH), 7.44 (s, 1H, Ar–CH=), 2.29 (s, 3H, CH3) ppm. 13C NMR (125 MHz, CDCl3) δ: 167.91 (C=O), 161.23 (C=N), 150.08, 134.02, 133.83, 132.30, 130.50, 129.03, 128.93, 119.61, 11.62 (CH3) ppm. Elemental Analysis Calc. (MW: 187.20): C, 70.58; H, 4.85; N, 7.48 Elemental Analysis (found): C, 70.73; H, 4.82; N, 6.67
(E)-4-(4-Methoxybenzylidene)-3-methylisoxazol-5(4H)-one (4b)
Color: Yellow needles, mp 177–179 ℃; IR (KBr): 3083 (ArH), 3030, 1785 (C=O), 1552, 1429, 1313, 1260, 1167, 1013, 927, 841, 774 cm−1; 1H NMR (500 MHz, CDCl3) δ: 8.43 (d, J = 9.1Hz, 2H, ArH), 7.34 (s, 1H, ArCH=), 7.00 (d, J = 9Hz, 2H, ArH), 3.92 (s, 3H, OCH3), 2.28 (s, 3H, CH3) ppm; 13C NMR 125 MHz, CDCl3) δ: 168.77 (C=O), 164.62 (C=N), 161.27, 149.32, 136.96, 125.84, 116.37, 114.67, 55.71 (OCH3), 11.64 (CH3) ppm; Elemental Analysis Calc. (MW: 217.22): C, 66.35; H, 5.10; N, 6.45; Elemental Analysis (found): C, 66.30; H, 5.07; N, 6.34
(E)-4-(4-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4c)
Yellow needles, mp 214–216 ℃; IR (KBr): 3247 (OH), 3034 (ArH), 2959 (Aliph. H), 1724 (C=O), 1623 (C=N), 1545, 1373, 1223, 1122, 890, 774 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 11.02 (s, 1H, OH), 8.45 (d, J= 8.9Hz, 2H, ArH), 7.78 (s, 1H, ArCH=), 6.96 (d, J = 8.85Hz, 2H, ArH), 2.26 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 168.70 (C=O), 163.73 (C=N), 162.11, 151.35, 137.40, 124.45, 116.03, 113.76, 11.14 (CH3) ppm; Elemental Analysis Calc. (MW: 203.20): C, 65.02; H, 4.46; N, 6.89; Elemental Analysis (found): C, 65.97; H, 5.04; N, 6.78
(E)-4-(4-Bromo-2-fluorobenzylidene)-3-methylisoxazol-5(4H)-one (4d)
Color: Yellow needles, mp 155–157 ℃; IR (KBr): 3105 (ArH), 3042, 1754 (C=O), 1620 (C=N), 1556, 1406, 1346, 1227, 1103, 1073, 875, 774 cm−1; 1H NMR (500 MHz, CDCl3) δ: 8.91 (s, 1H, ArH), 7.65 (s, 1H, Ar-CH=), 7.45 (d, J=8.75Hz, 1H), 7.38 (d, J=10Hz, 1H), 2.31 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3) δ: 167.58, 162.87, 161.01, 160.79, 138.68, 133.89, 129.88, 128.44, 121.38, 119.37, 11.54 ppm; Elemental Analysis Calc. (MW: 284.08): C, 46.51; H, 2.48; N, 4.93; Elemental Analysis (found): C, 46.46; H, 2.45; N, 4.82
(E)-4-(3-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4e)
Color: Yellow, mp 199–202 ℃; IR (KBr): 3210 (OH), 3083 (ArH), 2963(Aliph. H), 1736, 1605, 1477, 1346, 1245, 1111, 1032, 901, 777 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 9.90 (s, 1H, OH), 7.93 (s, 1H, ArH), 7.83 (s, 1H, Ar-CH=), 7.78 (d, J=8.05Hz, 1H), 7.37 (t, J = 10Hz, 1H, ArH), 7.07 (d, J=8.5Hz, 1H, ArH), 2.27 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 167.66 C=O, 162.10 (C=N), 157.31 (=C(OH)–), 151.84, 133.51, 129.75, 125.20, 121.28, 119.33, 118.42, 11.17 (CH3) ppm; Elemental Analysis Calc. (MW: 203.20): C, 65.02; H, 4.46; N, 6.89; Elemental Analysis (found): C, 64.97; H, 4.43; N, 6.78
(E)-3-Methyl-4-(4-methylbenzylidene)isoxazol-5(4H)-one (4f)
Yellow needles, mp 129–131 ℃; IR (KBr): 3098 (ArH), 2997 (Aliph. H), 1728 (C=O), 1623 (C=N), 1511, 1410, 1350, 1219, 1107, 1025, 935, 879, 774 cm−1; 1H NMR (500 MHz, CDCl3) δ: 8.27 (d, J=8.3Hz, 2H, ArH), 7.38 (s, 1H, ArCH=), 7.30 (d, J=7.75Hz, 2H, ArH), 2.44 (s, 3H, CH3), 2.28 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3) δ: 168.22 (C=O), 161.26 (C=N), 150.00, 145.72, 134.16, 131.05, 129.96, 129.88, 129.74, 118.38, 22.06, 11.62 (CH3) ppm. Elemental Analysis Cacl. (MW: 201.23): C, 71.63; H, 5.51; N, 6.96; Elemental Analysis (found): C, 71.58; H, 5.48; N, 6.85
(E)-4-(4-(Dimethylamino)benzylidene)-3-methylisoxazol-5(4H)-one (4 g)
Color : Red, mp 230–232 ℃, IR (KBr): 3090 (ArH), 2918 (Aliph. H), 1713 (C=O), 1616, (C=N), 1552, 1436, 1380, 1204, 1099, 953, 834, 774 cm−1; 1H NMR (500 MHz, CDCl3) δ: 8.38 (d, J=8.8Hz, 2H, ArH), 7.19 (s, 1H, ArCH=), 6.70 (d, J=8.85Hz, 2H, ArH), 3.15 (s, 6H, –N(CH3)2), 2.22 (s, 3H, CH3) ppm; 13C NMR (125 MHz, CDCl3) δ:: 170.11 (C=O), 161.57 (C=N), 154.25, 149.25, 137.62, 121.54, 111.52, 111.12, 40.09 (N(CH3)2), 11.54 (CH3) ppm; Elemental Analysis Calc. (MW: 230.27): C, 67.81; H, 6.13; N, 12.17; Elemental Analysis (found): C, 67.76; H, 6.10; N, 12.06
(E)-4-(2-Hydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4 h)
Color: Yellow, mp 150–152 ℃; IR (KBr): 3154 (OH), 2984 (Ar–H), 1739 (C=O), 1601 (C=N), 1459, 1350, 1156, 1092, 901, 766 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 10.98 (s, 1H, OH), 8.75–.8.73 (m, 1H, Ar–H), 8.09 (s, 1H, ArCH=), 7.50–7.47 (m, 1H, Ar–H), 7.01 (d, J = 8.35Hz, 1H, Ar–H), 6.95–6.92 (m, 1H, ArH), 2.26 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 168.13 (C=O), 161.99 (C=N), 159.51, 144.85, 136.58, 132.18, 119.37, 118.96, 116.31, 116.02, 11.06 (CH3) ppm; Elemental Analysis Calc. (MW: 203.20): C, 65.02; H, 4.46; N, 6.89; Elemental Analysis (found): C, 64.97; H, 4.43; N, 6.78
(E)-4-(4-Bromobenzylidene)isoxazol-5(4H)-one (4j)
Color: Yellow, mp 154–156 ℃; IR (KBr): 3079 (Ar–H), 2952 (Aliph. H), 1664 (C=O), 1552, 1432, 1391, 1238, 1118, 1069, 849, 744 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.50 (d, J = 8.5Hz, 2H, Ar–H), 7.17 (d, J = 8.55Hz, 2H, Ar–H), 4.78 (s, 1H, ArCH=), 1.90 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 171.10 (C=O), 161.31 (C=N), 150.00, 139.11, 135.06, 131.01, 129.96, 119.42, 10.81 (CH3) ppm; Elemental Analysis Calc. (MW: 252.07): C, 49.65; H, 3.03; N, 5.26; Elemental Analysis (found): C, 49.60; H, 3.00; N, 7.03
(E)-3-Methyl-4-(naphthalen-1-ylmethylene)isoxazol-5(4H)-one (4 k)
Color: Yellow, mp 148–149 ℃; IR (KBr): 3143 (Ar–H), 1702 (C=O), 1612 (C=N), 1537, 1447, 1380, 1294, 1152, 931, 826, 774 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 8.66 (d, J = 8.35 Hz, 1H, Ar–H), 8.46 (d, J = 7.3 Hz, 1H, Ar–H), 8.20–8.15 (m, 1H, Ar–H), 8.06 (d, J = 8.25 Hz, 1H, Ar–H), 7.72–7.69 (m, 1H), 7.67–7.63 (m, 2H, Ar–H/ArCH=), 2.43 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 167.41 (C=O), 161.83 (C=N), 148.49, 133.43, 132.86, 131.26, 130.68, 128.79, 127.63, 126.56, 124.90, 124.55, 123.97, 120.22, 11.20 (CH3) ppm; Elemental Analysis Calc. (MW: 237.26): C, 75.94; H, 4.67; N, 5.9; Elemental Analysis (found): C, 75.89; H, 4.63; N, 5.79
(E)-4-(3,4-Dihydroxybenzylidene)-3-methylisoxazol-5(4H)-one (4l)
Color: Yellow, mp 211–212 ℃; IR (KBr): 3143 (OH), 1702 (C=O), 1612 (C=N), 1537, 1447, 1380, 1294, 1152, 931, 826, 774 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 10.52 (s, 1H, OH), 9.55 (s, 1H, OH), 8.15 (s, 1H, Ar–H), 7.71 (d, J = 8.45 Hz 1H, Ar-H), 7.58 (s, 1H, ArCH=), 6.84 (d, J = 8.45 Hz, 1H, Ar–H), 2.15 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 168.76 (C=O), 162.17 (C=N), 153.18, 151.84, 145.30, 130.30, 125.05, 120.32, 115.76, 113.37, 11.17 (CH3) ppm; Elemental Analysis Calc (MW: 219.20): C, 60.28; H, 4.14; N, 6.39; Elemental Analysis (found): C, 60.23; H, 4.11; N, 6.28
(E)-3-Methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one (4m)
Color: Yellow, mp 144–146 ℃; IR (KBr):: 3075 (Ar-H), 2984 (Aliph. H), 1730 (C=O), 1596, 1403, 1298, 1122, 1029, 992, 859, 774 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 8.32–8.31 (m, 1H, Ar–H), 8.24 (s, 1H, ArCH=), 8.22 (m, 1H, Ar–H), 7.39–7.37 (m, 1H, Ar–H), 2.27 (s, 3H, CH3) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 168.37 (C=O), 161.47 (C=N), 142.88, 141.41, 140.98, 136.06, 128.83, 112.92, 10.95 (CH3) ppm; Elemental Analysis Calc. (MW: 193.22): C, 55.95; H, 3.65; N, 7.25; Elemental Analysis (found): C, 55.90; H, 3.62; N, 7.14
(E)-4-(Furan-2-ylmethylene)-3-methylisoxazol-5(4H)-one (4n)
Color: Black, mp 237–238 ℃; IR (KBr): 3124, 2986, 1711, 1601, 1537, 1441, 1349, 1225, 1175, 1008, 881, 753 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 7.21 (d, J=7.55 Hz, 1H, Ar-H), 7.10 (d, J=8.1 Hz, 1H, Ar–H), 7.00 (t, J = 9.6 Hz, 1H, Ar–H), 6.49 (s, 1H, ArCH=), 2.18 (s, 3H, CH3) ppm; Elemental Analysis (Calc.): C, 61.02; H, 3.98; N, 7.91; Elemental Analysis (found): C, 60.97; H, 3.95; N, 7.80
(Z)-2,6-Dichlorobenzaldehyde oxime (5)
Color: White crystalline needle shaped, mp 147–151 ℃; IR (KBr): 3274 (OH), 3068 (Ar–H), 1581 (C=N), 1436, 1297, 1185, 1093, 915, 877, 774 cm−1; 1H NMR (500 MHz, DMSO-d6) δ: 11.80 (s, 1H, OH), 8.23 (s, 1H -CH=N, 1H), 7.54 (d, J = 8.25 Hz, 2H, Ar–H), 7.42 (t, J = 7.55 Hz, 1H, Ar–H) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 143.66, 133.80, 130.84, 129.27, 128.79 ppm; Elemental Analysis Calc. (MW: 190.02): C, 44.25; H, 2.65; N, 7.37; Elemental Analysis (found): C, 44.20; H, 2.62; N, 7.26
References
D.R. Spring, Chem. Soc. Rev. 34, 472 (2005)
J. Kim, H. Kim, S.B. Park, J. Am. Chem. Soc. 136, 14629 (2014)
W.R. Galloway, M. Diáz-Gavilán, A. Isidro-Llobet, D.R. Spring, Angew. Chem. Int. Ed. 48, 1194 (2009)
T. Gaich, P.S. Baran, J. Org. Chem. 75, 4657 (2010)
I. Ugi, A. Domling, B. Werner, J. Heterocycl. Chem. 37, 647 (2000)
J. Zhu, H. Bienaymé (eds.), Multicomponent Reactions (Wiley, New York, 2005)
A. Dömling, Chem. Rev. 106, 17 (2005). https://doi.org/10.1021/cr0505728
R.W. Armstrong, A.P. Combs, P.A. Tempest, S.D. Brown, T.A. Keating, Acc. Chem. Res. 29, 123 (1996)
L.F. Tietze, M.E. Lieb, Curr. Opin. Chem. Biol. 2, 363 (1998)
S. L. Dax, J. J. McNally, M. A. Youngman, Curr. Med. Chem. 6, 255–270 (1999)
L.F. Tietze, A. Modi, Med. Res. Rev. 20, 304 (2000)
A. Thakur, M. Verma, R. Bharti, R. Sharma, Tetrahedron 119, 132813 (2022)
O.H. Kan[UNK], I. Adachi, R. Kido, K. Hirose, J. Med. Chem. 10, 411 (1967)
A. Vasudeva Adhikari, Indian J. Chem. IJC-B 48B, 430 (2009)
M.M.M. Santos, N. Faria, J. Iley, S.J. Coles, M.B. Hursthouse, M.L. Martins, R. Moreira, Bioorg. Med. Chem. Lett. 20, 193 (2010)
A. Kamal, E.V. Bharathi, J.S. Reddy, M.J. Ramaiah, D. Dastagiri, M.K. Reddy, A. Viswanath, T.L. Reddy, T.B. Shaik, S.N.C.V.L. Pushpavalli, M.P. Bhadra, Eur. J. Med. Chem. 46, 691 (2011)
P. Diana, A. Carbone, P. Barraja, G. Kelter, H.-H. Fiebig, G. Cirrincione, Bioorg. Med. Chem. 18, 4524 (2010)
T. Ishioka, A. Kubo, Y. Koiso, K. Nagasawa, A. Itai, Y. Hashimoto, Bioorg. Med. Chem. 10, 1555 (2002)
T. Kwon, A.S. Heiman, E.T. Oriaku, K. Yoon, H.J. Lee, J. Med. Chem. 38, 1048 (1995)
P. Krogsgaard-Larsen, J.J. Hansen, Excitatory Amino Acid Receptors: Design of Agonists and Antagonists (Taylor & Francis, Abingdon, 1992)
H. Watanabe, T. Shibuya, Pharmacological Research on Traditional Herbal Medicines (Taylor & Francis, New York, 2003)
K.K. Madsen, R.P. Clausen, O.M. Larsson, P. Krogsgaard-Larsen, A. Schousboe, H. Steve White, J. Neurochem. 109, 139 (2009)
M.J. Drysdale, B.W. Dymock, H. Finch, P. Webb, E. Mcdonald, K.E. James, K.M. Cheung, T.P. Mathews, inventors; Vernalis (Cambridge) Limited, Cancer Research Technology Ltd, The Institute Of Cancer Research, assignee. Isoxazole compounds as inhibitors of heat shock proteins (2004). https://patents.google.com/patent/WO2004072051A1/en-20US4325121.pdf
D. Petrik, Y. Jiang, S.G. Birnbaum, C.M. Powell, M.-S. Kim, J. Hsieh, A.J. Eisch, FASEB J. 26, 3148 (2012)
B. Frølund, A.T. Jørgensen, L. Tagmose, T.B. Stensbøl, H.T. Vestergaard, C. Engblom, U. Kristiansen, C. Sanchez, P. Krogsgaard-Larsen, T. Liljefors, J. Med. Chem. 45, 2454 (2002)
G.A.R. Johnston, Neurochem. Res. 39, 1942 (2014)
M.P. Giovannoni, C. Vergelli, C. Ghelardini, N. Galeotti, A. Bartolini, V. Dal Piaz, J. Med. Chem. 46, 1055 (2003)
G. Miranda-Novales, B.E. Leaños-Miranda, M. Vilchis-Pérez, F. Solórzano-Santos, Ann. Clin. Microbiol. Antimicrob. 5, 1 (2006)
G. DiPasquale, C. Rassaert, R. Richter, P. Welaj, J. Gingold, R. Singer, Agents Actions 5, 256 (1975)
S. Sanders, V. Harisdangkul, Am. J. Med. Sci. 323, 190 (2002)
J.J. Talley, D.L. Brown, J.S. Carter, M.J. Graneto, C.M. Koboldt, J.L. Masferrer, W.E. Perkins, R.S. Rogers, A.F. Shaffer, Y.Y. Zhang, B.S. Zweifel, K. Seibert, J. Med. Chem. 43, 775 (2000)
K. Kobinata, S. Sekido, M. Uramoto, M. Ubukata, H. Osada, I. Yamaguchi, K. Isono, Agric. Biol. Chem. 55, 1415 (1991)
Y.-G. Lee, Y. Koyama, M. Yonekawa, T. Takata, Macromolecules 42, 7709 (2009)
D.H. Brown, P. Styring, Liq. Cryst. 30, 23 (2003). https://doi.org/10.1080/0267829021000047525
I. Nakamura, M. Okamoto, M. Terada, Org. Lett. 12, 2453 (2010)
J.J. Donleavy, E.E. Gilbert, J. Am. Chem. Soc. 59, 1072 (1937)
D.B. Lowe, S. Magnuson, N. Qi, A.-M. Campbell, J. Cook, Z. Hong, M. Wang, M. Rodriguez, F. Achebe, H. Kluender, W.C. Wong, W.H. Bullock, A.I. Salhanick, T. Witman-Jones, M.E. Bowling, C. Keiper, K.B. Clairmont, Bioorg. Med. Chem. Lett. 14, 3155 (2004)
H. Kiyani, F. Ghorbani, Res. Chem. Intermed. 41, 2653 (2013)
F. Saikh, J. Das, S. Ghosh, Tetrahedron Lett. 54, 4679 (2013)
P. Duan, Y. Yang, R. Ben, Y. Yan, L. Dai, M. Hong, Y.-D. Wu, D. Wang, X. Zhang, J. Zhao, Chem. Sci. 5, 1574 (2014)
N. Kaur, D. Kishore, Synth. Commun. 44, 1019 (2014)
Q. Liu, R.-T. Wu, J. Chem. Res. 35, 598 (2011)
H. Kiyani, F. Ghorbani, J. Saudi Chem. Soc. 21, S112 (2017)
R. Bharti, T. Parvin, J. Heterocycl. Chem. 52, 1806 (2014)
R. Bharti, T. Parvin, RSC Adv. 5, 66833 (2015)
R. Bharti, T. Parvin, Synth. Commun. 45, 1442 (2015)
R. Bharti, T. Parvin, Mol. Diversity 20, 867 (2016)
R. Bharti, P. Kumari, T. Parvin, L.H. Choudhury, RSC Adv. 7, 3928 (2017)
R. Bharti, P. Kumari, T. Parvin, L.H. Choudhury, Curr. Org. Chem. 22, 417 (2018)
M. Verma, R. Sharma, R. Bharti, A. Tangri, Mater. Today Proc. 37, 2321 (2021)
A. Thakur, R. Bharti, R. Sharma, Orbital Electron. J. Chem. 13, 335 (2021)
A. Thakur, M. Verma, R. Bharti, R. Sharma, Curr. Org. Chem. 26, 299 (2022)
M. Verma, A. Thakur, R. Sharma, R. Bharti, Curr. Org. Synth. 18, 86 (2021)
M. Verma, A. Thakur, S. Kapil, R. Sharma, A. Sharma, R. Bharti, Mol. Divers. (2022). https://doi.org/10.1007/s11030-022-10461-1
U.V. Mallavadhani and N. Fleury-Bregeot, 1,4-Diazabicyclo [2.2.2]octane. In Encyclopedia of Reagents for Organic Synthesis, (Ed.). (2010). https://doi.org/10.1002/047084289X.rd010m.pub
D.K. Jangid, Curr. Green Chem. 7, 146 (2020)
T. Koopmans, Physica 1, 104–113 (1933). https://cir.nii.ac.jp/crid/1573105974633508352
A.S.A. Dena, W.M.I. Hassan, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 163, 108 (2016)
A.S. Abo Dena, Z.A. Muhammad, W.M.I. Hassan, Chem. Pap. 73, 2803 (2019)
H. Muğlu, B.Z. Kurt, F. Sönmez, E. Güzel, M.S. Çavuş, H. Yakan, J. Phys. Chem. Solids 164, 110618 (2022)
M.S. Çavuş, H. Yakan, C. Özorak, H. Muğlu, T.K. Bakır, Res. Chem. Intermed. 48, 1593 (2022)
H. Muğlu, M. Akın, M.S. Çavuş, H. Yakan, N. Şaki, E. Güzel, Comput. Biol. Chem. 96, 107618 (2022)
H. Muğlu, H. Yakan, A.G.A. Misbah, M.S. Çavuş, T.K. Bakır, Res. Chem. Intermed. 47, 4985 (2021)
J.R. Soare, T.C.P. Dinis, A.P. Cunha, L. Almeida, Free Radical Res. 26, 469 (1997)
P. Bhardwaj, M.S. Thakur, S. Kapoor, A.K. Bhardwaj, A. Sharma, S. Saxena, O.P. Chaurasia, R. Kumar, Pharmacogn. J. 11, 536 (2019)
P. Prieto, M. Pineda, M. Aguilar, Anal. Biochem. 269, 337 (1999)
Acknowledgements
The authors are thankful to Department of Chemistry, University Institute of Sciences, Chandigarh University for providing basic facilities and resources. The authors are grateful to Punjab University and IIT Madras for providing the analytical facilities for characterization of products.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
"A.T. and M. V. wrote the main manuscript text, and P. S prepared figures and Schemes. R. B and R. S supervised and provided all the research facilities. A. S did the antioxidant activities of the newly synthesized molecules. N. P. N. performs the antibacterial activities. V. A. and R. B did the DFT Studies and validated the data. All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakur, A., Verma, M., Setia, P. et al. DFT analysis and in vitro studies of isoxazole derivatives as potent antioxidant and antibacterial agents synthesized via one-pot methodology. Res Chem Intermed 49, 859–883 (2023). https://doi.org/10.1007/s11164-022-04910-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04910-7