Abstract
An effective and environmentally friendly Fe3O4-PVP nanocomposite functionalized with sulfonic group, Fe3O4-PVP-SO3H, was synthesized and characterized as the heterogeneous nanocatalyst for one-pot synthesis of xanthene derivatives via condensation reaction of various aldehydes and dimedone in ethanol media. The catalyst was efficient, and can be separated easily from the reaction mixture and recovered rapidly by an external magnetic field. It can be used five times without significant loss of its activity. Physicochemical properties were characterized using different techniques including FT-IR, XRD, FE-SEM, EDS, TGA, and VSM to illustrate the structure of the catalyst. The presence of sulfonic group on the surface of the catalyst was confirmed by these analyses. Large number of the acidic groups on the surface of the PVP layer led to the more activity of the catalyst and consequently, the yield of xanthene increases. The products were obtained in high yields with short reaction times.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanocomposites are a specific class of nano-sized materials that have been applied in different area such as catalysts, drug delivery, cosmetic orthodontics, modern construction, electronics, tissue engineering and agriculture. [1,2,3,4,5,6,7]. Magnetic nanocomposites were prepared by modification of magnetic nanoparticles with different organic and inorganic materials with unique functionality [8]. Iron oxide nanopowder (Fe3O4) is one of the most popular nanoparticles because of its application in ferrofluids [9], high-density information storage [10], magnetic resonance imaging [11] as well as the role of catalyst or support of catalysts [12]. Moreover, in recent years, Fe3O4 nanoparticles (MNPs) have been attracted in different fields due to their unique properties such as simple synthesis, high surface area, easy separation and recovery from reaction medium using a simple magnet [13,14,15,16,17,18,19]. However, MNPs have several problems such as the tendency for aggregation and easily air oxidation because of having high chemical activity on their surface which then cause their magnetic properties and dispersibility to decrease [13, 20,21,22]. Thus, it is necessary to overcome these problems by chemically stabilization of MNPs via functionalization with different materials such as polymer, silica, carbon, metal oxide and oxide absorber [12, 23,24,25,26,27,28]. These problems were overcome using one of the promising candidates, polymer coating, which stabilizes MNPs due to its specific characteristics [20, 29,30,31]. Polymers like PVP can tackle the challenge due to its solubility (soluble in water and polar solvents), stability, low production cost, easy functionalization, and low toxicity. Also, PVP is an excellent stabilizer and has been used for coating of MNPs [32,33,34]. This polymer made from the monomer N-vinylpyrrolidone that has polar amide group in the ring. These functional groups along their backbones are soluble in many solvents and give them chelating characteristics [35,36,37]. It was reported that PVP molecules prevent of random agglomeration of iron oxide particles and facilitate the cluster formation [33]. This stabilizer prevents aggregation of MNPs through the repulsive forces due to its hydrophobic carbon chains that disperse within solvents and interact with each other. Moreover, the addition of PVP to MNPs reduces average particle size by preventing agglomeration [38]. The increasing amount of PVP, during chemical reduction synthesis of iron oxide nanoparticles, controls the size and oxidation of the final nanoparticles and changes the final morphology. In addition, crystal growth of iron oxide was restricted in the presence of PVP, and its oxidation limited. Significantly, PVP can apply as a growth modifier, nanoparticle dispersant, and reducing agent related to the particular synthetic conditions [34, 38]. Also, the type of materials and the synthesis method are important for modifying the performance of Fe3O4 nanoparticles [39]. The uniqueness of the powder with large surface-area is detrimental for the catalytic applications.
Xanthene derivatives constitute an important class of organic oxygen-containing compounds that have been used for pharmaceutical applications possessing antiviral and antibacterial properties [40,41,42]. In addition, these heterocyclic compounds have been widely used as sensitizers in photodynamic therapy [43], pH sensitive fluorescent materials for visualization of biomolecules [44, 45], and luminescent dyes [46].
In continuation of our ongoing research on using heterogeneous nanocatalyst [47,48,49,50,51] herein, the efficient synthesis of xanthenes catalyzed by Fe3O4-PVP-SO3H via one-pot reactions of aldehydes and dimedone was reported. This work was cleanly performed in ethanol as the solvent under reflux conditions. Moderate catalytic activity, catalyst recyclability, easy reaction conditions, simple magnetically catalyst separation and reduction in the amount of acidic waste make the catalyst as an effective green catalyst for the synthesis of xanthene derivatives.
Experimental
General
The chemical materials, reagents, and solvents in this research were purchased from Merck and Sigma-Aldrich companies and used without any purification. 1H NMR and 13C NMR spectra were recorded on a Bruker 300 MHz spectrometer in CDCl3 as the solvent. Melting points were determined with Electrothermal 9300 (Electrothermal, Essex, UK). FT-IR BRUKER, Model tensor 27spectrometer was applied to record IR spectra of all samples with a scanning range of 400–4000 cm−1 with using KBr pellets. X-ray diffraction (XRD) patterns were obtained by a Philips X-ray analytical diffractometer using Cu/Kα radiation at room temperature in the range of 2θ from 10 to 80° to prove the catalyst contents. VSM spectrum was obtained by a vibrating-sample magnetometer (VSM, LBKFB model-Meghnatis Daghigh Kavir Company) at room temperature and measured the magnetic properties of the catalyst. Thermogravimetric analysis (TGA) was carried out by BAHR STA 503 thermal analysis system heated from 25 to 500 °C using a heating rate of 10 °C /min under N2 flow. Field emission scanning electron microscopy (FE-SEM) images and energy-dispersive X-ray (EDX) analysis of samples were obtained with a TESCAN MIRA3 digital scanning microscope and Bruker XFlash6130 to determine the morphology and location the elements of the catalyst, respectively. The size, shape and morphology features of nanocomposite were identified using TEM Philips EM 208S and HR-TEM FEI TECNAI F20. Plasma atom emission spectrometer (Perkin-Elmer, DV-530) was used to present the chemical composition of the Fe3O4-PVP-SO3H composite.
Synthesis of Fe3O4-PVP nanocomposites
The Fe3O4 NPs were produced with modification according to the previously reported method [52]. At the room temperature, 0.54 g of FeCl3.6H2O (2 mmol) was dissolved in 25 mL of ethylene glycol, in the presence of N2 gas to remove oxygen. Then, 0.04 g of CTAB (0.108 mmol) and 0.23 g of PVP (0.0057 mmol) were added into the above solution, respectively. The solution color was changed from yellow to green. Next, 10 mL of the aqueous solution of NaOH (0.2 M) was added to the solution, resulting in a darker solution. Then, the solution was heated to 100 °C under stirring and 0.322 g of sodium citrate (1.25 mmol) as a reducing agent added to the solution. The temperature of the solution was increased to 140 °C under the mechanical stirring vigorously for 24 h. After the reaction was complete, the reaction mixture was allowed to cool for collecting the iron oxide powders using an external magnetic field. The black iron oxide precipitate was washed thoroughly with ethanol two times and dried overnight under vacuum desiccator at room temperature (Fig. S1). To understand and observe the effect of PVP and CTAB on the structure and morphology of Fe3O4 nanoparticles, the iron oxide nanoparticles without the PVP and CTAB capping agents have been also synthesized using the same procedure.
Synthesis of Fe3O4-PVP-SO3H nanocomposite
To a suspension solution of 0.6 g Fe3O4-PVP nanocomposite in 15 mL CH2Cl2, a solution of 0.15 mL chlorosulfonic acid (2.2 mmol) in 10 mL CH2Cl2 was added and the mixture stirred for 2 h at room temperature. The obtained precipitate was filtered by an external magnet and washed two times with CH2Cl2 (2 × 10 mL). The black powder was produced after drying it in a vacuum desiccator at room temperature.
Measuring the acidity of the catalyst
To determine the number of SO3H groups on the catalyst, the Fe3O4-PVP-SO3H (0.1 g) was added to an aqueous solution of NaOH (10 mL, 0.2 M) in an Erlenmeyer flask. Then the system was stirred for 1 h at room temperature. The catalyst was then removed using an outer magnet and to the resulted a clear solution, two drops of phenolphthalein indicator added. The solution was titrated to a neutrality point using 17.7 mL of an aqueous HCl solution (0.1 M). The blank titration (without adding the catalyst) was also carried out and the volume of used HCl solution (0.1 M) found to be 20 mL. Therefore, the volume of used HCl solution (0.1 M) for computing the number of SO3H groups on the catalyst was observed to be 2.3 mL. The H+ loading of the nanocatalyst can be computed based on Eq. 1:
in which M1 (mol. L−1) and V1 (L) are the used concentration and volume of the NaOH solution (0.2 M), respectively, and M2 (mol. L−1) and V2 (L) are the used concentration and volume of the HCl solution (0.1 M), respectively, in the titration process. Therefore;
General procedure for preparation of xanthene derivatives
A mixture of aldehyde (1 mmol), dimedone (2 mmol) and Fe3O4-PVP-SO3H nanocomposite (30 mg) as the catalyst was stirred in 2 mL ethanol at 80 °C under reflux condition. The progress of the reaction was continuously monitored by TLC: ethyl acetate/n-hexane (1:5). After completion of the reaction, the catalyst was separated by an external magnet. Then, the mixture was cooled to room temperature and 5 mL of ice water added drop by drop and decanted to isolate the crude product. The crude product was put in the oven at 80 °C till dried. The resulting solid as the crude product was recrystallized from EtOH to afford the pure product. The obtained products were characterized by m.p., 1H NMR, and 13C NMR techniques.
Results and discussion
Preparation and characterization of Fe3O4-PVP-SO3H nanocomposite
The polyol process was used for the preparation of iron oxide nanopowders due to create iron oxide nanoparticles of high magnetization and hydrophilic surfaces [32, 53]. In this study, the effects of PVP and CTAB were evaluated on the synthesis of MNPs. It was observed that CTAB and PVP as the coating and stabilizer agents changed MNPs morphology and protected them from oxidation. It is interesting to note that PVP chains cannot interact with MNPs due to their neutral charge and hydrophilic properties, while CTAB can interact easier than PVP with MNPs because it is a cationic surfactant. Moreover, CTAB was converted to cetyltrimethylammonium hydroxide (CTAOH) by alkaline environment during the synthesis [54]. This interaction resulted in smaller crystallite sizes, as well as prevented from oxidation and agglomeration of MNPs [39, 55]. Thus, MNPs were synthesized in the presence of both PVP and CTAB and well protected from agglomeration and oxidation (Figs. 2b and 4a). The preparation of Fe3O4-PVP-SO3H nanocomposite as the solid acid nanocatalyst was depicted in Scheme 1.
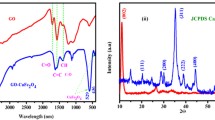
FT-IR The presence of key functional groups was ascertained through FT-IR spectroscopy. The FT-IR spectra of PVP, Fe3O4-PVP and Fe3O4-PVP-SO3H have been presented in Fig. 1. The FT-IR spectrum of PVP (Fig. 1a) indicates the bands around 3458 and 1658 cm−1 that are denoted to hydroxyl and carbonyl groups in PVP while the bands located at 1423 and 1288 cm−1 related to bending vibration of CH2 groups and stretching vibration of the C-N bond of PVP, respectively, [56, 57]. The spectrum of Fe3O4-PVP (Fig. 1b) shows a new strong band at 584 cm − 1 attributed to Fe–O stretching vibration and the peak 1658 shifted to 1649 cm−1 indicating the interaction among Fe3O4 and PVP. The FT-IR spectrum of Fe3O4-PVP-SO3H (Fig. 1c) shows the appearance extra absorbance peaks at 1074 and 1230 cm−1, corresponding to the stretching vibrations of the S–O of sulfonic acid groups [58]. Also, a moderate peak at 1639 cm−1 which denoted to the imine groups in catalyst is appeared. The obtained results from FT-IR spectra confirm that SO3H groups have functionalized on the surface of the Fe3O4-PVP nanocomposite.
SEM The surface morphology was characterized and the fundamental physical properties of the surface were analyzed by scanning electron microscopy (SEM) (Fig. 2). Cheng et al. [32] showed that iron oxide particle size and its dispersion are controlled by tuning the concentration of sodium citrate and adjusting the electrostatic repulsion between particles in the polyol synthesis of iron oxide powders. They concluded by TEM images that the particle size decreased and the particles became loosely packed when, the amount of sodium citrate was increased. Also, Graeve et al. indicated that the small amount of PVP had the similar effect as the effect of sodium citrate in Cheng et al.’s work [39]. In our study, the obtained SEM image of Fe3O4-PVP nanocomposites (Fig. 2b) not only clearly describes the presence of PVP and CTAB decreased the particle size but, also shows that these nanocomposites have a spherical morphology with loosely packed surfaces, confirming the uniformly coating of PVP as protective layer on the Fe3O4 particles. The Stabilizer has been widely applied to control the size and morphology of nanocrystals due to its great effect on the growth of nanocrystals in the synthesis process. According to Fig. 2c, Fe3O4-PVP-SO3H nanocomposites did not keep the morphological properties of Fe3O4-PVP and lost their spherical morphology due to the little aggregation of the nanocomposites upon addition of chlorosulfonic acid.
TEM and HR-TEM
Figure 3 shows the TEM and HR-TEM images of the Fe3O4-PVP-SO3H nanocomposite synthesized through electrostatic interactions between cetyltrimethylammonium bromide (CTAB) stabilized Fe3O4 nanoparticles and PVP chains by the polyol process and then modified by the addition of chlorosulfonic acid. Figure 3a, b indicates layers of the continuous network of the PVP on the Fe3O4 nanoparticles surface so that they look much brighter than Fe3O4 nanoparticles. Evidently, the spherical magnetic nanoparticles are observed by dark spots and some of them are shown more dark seem to be agglomerated but, most they are not. However, there are many Fe3O4 nanoparticles with spherical structures who are uniformly dispersed due to the strong interaction between the nanoparticles and the PVP chains. These uniformly dispersed nanoparticles confirm that PVP successfully can prevent of coagulation. To observe the structure with precise detail, the typical HRTEM images of Fe3O4-PVP-SO3H nanocomposite are shown in Fig. 3c, d. The structures of Fe3O4-PVP-SO3H nanocomposite exhibit the crystallinity of Fe3O4 nanoparticles with several spherical shaped nanocrystals in sizes less than 10 nm [39] and amorphous nature of the PVP layer. So that, Fe3O4 NPs were homogenously distributed in PVP matrix which resulted to regular arrangement of Fe3O4 nanoparticles. Furthermore, it must be noted that the SAED pattern with bright spots represented in Fig. 3d can clarify the crystalline nature of Fe3O4 as indicated by bright spots with uniform diffusive circles.
EDX
EDX is used as a useful technique to determine the elements presented in the nanocomposites. Figure 4 indicates the EDX pattern of Fe3O4-PVP-SO3H nanocomposites. This pattern clearly shows the elemental compositions are Fe, N and C and the peaks attributed to S, demonstrating the presence of sulfonic acid group along with PVP in the nanocomposite and the successful synthesis of Fe3O4-PVP-SO3H nanocatalyst.
XRD
XRD analysis was used in order to investigate the crystallographic structure of the catalyst. XRD patterns of Fe3O4-PVP and Fe3O4-PVP-SO3H are shown in Fig. 4. The XRD of pure PVP indicates the amorphous nature of the polymer owing to appearance of a broad diffraction peak at 2θ = 11 and 21° (Fig. 5a) [59, 60]. The pattern of Fe3O4-PVP (Fig. 4a) indicates the presence of peaks in 2θ = 30, 36, 43, 53, 57, 63 and 75°, that is related to (200), (311), (400), (422), (511), (440) and (511) planes of Fe3O4 nanoparticles (JCPDS 19-0629) [56, 61, 62]. The broad peak at 2θ = 21° is related to PVP. It can be seen that the XRD patterns of Fe3O4-PVP and Fe3O4-PVP-SO3H show the same diffraction patterns indicating that the structure of nanocomposite remained intact during the coating and loading of PVP and SO3H.
VSM
The magnetic hysteresis measurement of Fe3O4-PVP-SO3H is obtained by VSM with the field sweeping from -10,000 to + 10,000 Oe (Fig. 6). As shown in this figure, the saturation magnetization values for Fe3O4, Fe3O4-PVP and Fe3O4-PVP-SO3H are 61.3, 29.0 and 19.7 emu g−1, respectively. The intensity of magnetization of bare Fe3O4 was reduced from 61.3 to 19.7 emu g−1 because of the increased thickness of grafting by PVP and SO3H. The nanocomposites exhibit the superparamagnetic characteristics since the hysteresis loop for the particles was completely reversible. Thus, the Fe3O4-PVP-SO3H can easily separate and recycle from the products using an external magnetic field.
TGA
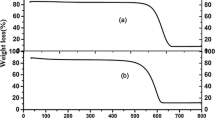
The TGA analysis in the temperature range of 25–500 °C for Fe3O4-PVP-SO3H was shown in Fig. 7. There are three stages of weight losses. The first mass weight loss (12%) up to145 ºC is assigned exclusively to physically adsorbed water or ethanol molecules on the catalyst surface and the solvent trapped on the surface and /or polymer matrix pores of nanocomposites. The second mass weight loss of 27% was observed at 145–275 ºC that can be related to the degradation and decomposition of the sulfonic moieties in Fe3O4-PVP-SO3H. The third one with mass weight loss (19.0%) occurred at bigger than 275–500 ºC and it is corresponded to PVP decomposition. These results can prove the attachment of sulfonic acid group and PVP moiety onto the surface of Fe3O4 and show the catalyst has around 58% of organic material. Therefore, it can be concluded that the excellent grafting of PVP and sulfonic groups on the Fe3O4 exist.
Investigation of catalytic activity of Fe3O4-PVP-SO3H
The catalytic performance of Fe3O4-PVP-SO3H was evaluated in the synthesis of xanthene derivatives (Scheme 2). To optimize the reaction conditions for synthesis of xanthenes, the effects of the catalyst amount, solvent and temperature were examined. The reaction of benzaldehyde (1 mmol), dimedone (2 mmol), was chosen as a model reaction.
First, the reaction was carried out in solvents including ethanol, acetonitrile, methanol, dimethyl formamide and water under reflux conditions (Table 1, entries 3, 8–11). As indicated in Table 1, ethanol was the best solvent and the xanthene was obtained in 87% yield after 20 min using 0.03 g of the catalyst. The other solvents were not as effective as EtOH and only gave moderate to low yields of the product. Second, the reaction was heated at different temperatures. It was found that the reflux temperature afforded the best result compared with room temperature and 50 °C (Table 1, entries 5 and 6). This reaction at room temperature even after 60 min gave only trace amount of the product. Finally, to determine the optimum amount of the catalyst, the reaction was examined using 0.02, 0.03 and 0.04 g of the catalyst. Based on the obtained results, the best performance is found when 0.03 g of the catalyst is used. When the amount of the catalyst was increased, the yield of the reaction was slightly raised. It is notable that in the absence of the catalyst the yield of the product was trace (Table 1, entry 1). With the optimal reaction conditions in hand, EtOH as a solvent, reflux temperature, and 0.03 g of the catalyst, the different benzaldehyde derivatives were reacted with dimedone for preparation of various 1,8-dioxo-octahydroxanthenes (Table 2).
To further examine the catalytic behavior of the catalyst for the synthesis of xanthene derivatives, the obtained results were compared with some of those reported in the literature (Table 3). The catalysts of entries 1–4 are homogeneous catalysts and isolation of them after completion of the reaction are difficult and some of them need higher temperatures for the reactions occur. In addition, they are not recoverable and reusable. Fe(HSO4)3 is robust and heterogeneous catalyst, but its isolation needs filtration which takes time to be isolated. This catalyst was easily separated from the products by exposure of the reaction vessel to an external magnet followed decantation of the reaction solution. The catalyst is energy saving and can be isolated faster without needing filtration. It was found that this catalyst is comparable with the others in terms of the reaction time and yield. Also, due to the magnetic feature of this catalyst, it can be readily separated from the reaction mixture and used at least five times without any noticeable loss of the yield in product.
Leaching test
The hot filtration was carried out to examine the catalyst durability using the model reaction under optimized conditions (Fig S2). The reaction was stopped after 5 min and the catalyst separated from the reaction medium, then the reaction continued in the absence of the catalyst for 20 min. A reaction yield of 25% was found. In another testing, the reaction proceeded for 10 min, then the catalyst was removed from the medium, and the reaction let continue for 20 min in the absence of the catalyst. A yield of 50% was obtained. We believe that the leaching was negligible during the reaction, and after the removal of the catalyst, the reaction was not able to proceed further to a higher yield.
To determine the weight percent of Fe element in the fresh and reused Fe3O4-PVP-SO3H composite, ICP-OES analysis was employed. The weight percent of Fe for the fresh catalyst was calculated to be Fe 7.41%, and in the reused catalyst was 7.24%. The slight decreasing in the element content of Fe indicating unavoidable loss for recycled catalyst after five consecutive runs during the process of collection and washing of the catalyst.
Recyclability of the catalyst
To consider the stability and recyclability of Fe3O4-PVP-SO3H, after each reaction, the nanocatalyst was separated from the reaction mixture with a magnet, washed with water and ethanol, dried under vacuum and reused for another reaction to evaluate its recycling performance. As indicated in Fig. 8, the Fe3O4-PVP-SO3H nanocatalyst can be successfully used for at least five times for synthesis of the xanthene without significant loss in its catalytic activity. A small decrease in its performance can be ascribed to the loss of the nanocatalyst after every recycling. Furthermore, the FT-IR spectrum of the reused nanocatalyst matches well with the fresh one and it confirms the recoverability and stability of the nanocatalyst during the reactions (Fig. 9).
Selected spectra data of products
3,3,6,6-Tetramethyl-9(phenyl)-1,8-dioxo-octahydroxanthene (Table 2, entry 1)
1H NMR (CDCl3, 300 MHz), δppm: 0.98 (s, 6H), 1.09 (s, 6H), 2.16 (d, J = 16.3, Hz, 2H), 2.23 (d, J = 16.3 Hz, 2H), 2.46 (s, 4H), 4,75 (s, 1H), 7.09 (t, J = 7.0 Hz, 1H), 7.21 (t, J = 7.0 Hz, 2H), 7.28 (d, J = 7.20 Hz, 2H); 13C NMR (CDCl3, 75 MHz), δppm: 27.7, 29.6, 32.3, 32.6, 41.3, 51.2, 116.1, 126.8, 128.4, 128.8, 144.5, 162.7, 196.8 ppm.
3,3,6,6-Tetramethyl-9(4-chloro-phenyl)-1,8-dioxo-octahydroxanthene (Table 2, entry 2)
1H NMR (CDCl3, 300 MHz), δppm: 0.98 (s, 6H), 1.09 (s, 6H), 2.14 (d, J = 16.1 Hz, 2H), 2.23 (d, J = 16.1 Hz, 2H), 2.45 (s, 4H), 4.70 (s, 1H), 7.19–7.29 (m, 4H); 13C NMR (CDCl3, 75 MHz), δppm: 27.5, 29.4, 31.7, 32.4, 41.1, 50.8, 115.6, 128.6, 130.2, 132.4, 143.1, 162.5, 196.7.
3,3,6,6-Tetramethyl-9(4-nitro-phenyl)-1,8-dioxo-octahydroxanthene (Table 2, entry 3)
1H NMR (CDCl3, 300 MHz), δppm: 0,98 (s, 6H), 1,12 (s, 6H), 2,15 (d, J = 16,3 Hz, 2H), 2,24 (d, J = 16,3 Hz, 2H), 2,49 (s, 4H), 4,81 (s, 1H), 7,46 (d, J = 7,9 Hz, 2H), 8,08 (d, J = 7.,9 Hz, 2H);
13C NMR (CDCl3, 75 MHz), δppm: 27.2, 29.2, 32.3, 32.4, 40.8, 50.6, 114.5, 123.1, 129.3, 146.4, 151.5, 163.0, 196.3.
3,3,6,6-Tetramethyl-9(4-methoxy-phenyl)-1,8-dioxo-octahydroxanthene (Table 2, entry 6)
1H NMR (CDCl3, 300 MHz), δppm: 0,98 (s, 6H), 1,11 (s, 6H), 2,15 (d, J = 15,7 Hz, 2H), 2,23 (d, J = 15,7 Hz, 2H), 2,44 (s, 4H), 3,72 (s, 3H), 4,71 (s, 1H), 6,75 (d, J = 8,6 Hz, 2H), 7,20 (d, J = 8,6 Hz, 2H); 13C NMR (CDCl3, 75 MHz), δppm: 27.4, 29.4, 31.2, 32.3, 40.8, 50.8, 55.1, 113.4, 115.8, 129.3, 136.5, 157.7, 162.0, 196.5.
Conclusion
In this work, we have successfully synthesized a novel, effective and magnetic Fe3O4-PVP support chlorosulfonic acid as the catalyst for the preparation of xanthene derivatives through condensation of aldehydes and dimedone in ethanol at 80 °C. Clean reactions, simple performance, reusability of the nanocatalyst, easy work-up method, high yield of products, and using Fe3O4-PVP-SO3H as the powerful heterogeneous proton donor are outstanding advantages of this produced catalyst. It is worth mentioning that the catalyst has a wide surface area due to its nanospherical structure. Moreover, it was found to be potentially valuable in industrial applications and widespread use in organic synthesis for other compounds because of reusability of the magnetic heterogeneous nanocatalyst with maintaining its efficiency.
References
S.M. Lee, E. Pippel, U. Gösele, C. Dresbach, Y. Qin, C.V. Chandran, T. Bräuniger, G. Hause, M. Knez, Science 324, 488 (2009)
Z. Shao, F. Vollrath, Nature 418, 741 (2002)
R.V. Lewis, Chem. Rev. 106(9), 3762 (2006)
F.G. Omenetto, D.L. Kaplan, Nat. Photonics 2, 641 (2008)
N. Lin, F. Hu, Y. Sun, C. Wu, H. Xu, X.Y. Liu, Adv. Funct. Mater. 24, 5284 (2014)
J. Kim, Y. Piao, T. Hyeon, Chem. Soc. Rev. 38, 372 (2009)
A. Nouri Parouch, N. Koukabi, E. Abdous, Res. Chem. Intermed. 46, 3295 (2020)
M.H. So, Y. Liu, C.M. Ho, K.Y. Lam, C.M. Che, ChemCatChem 3, 386 (2011)
G. Kandasamy, S. Soni, K. Sushmita, N.S. Veerapu, S. Bose, D. Maity, J. mol. Liq. 274, 653 (2019)
M. Ozaki, MRS Bull. 14, 35 (1989)
T. Neuberger, B. Schopf, H. Hofmann, M. Hofmann, B. von Rechenberg, J. Magn. Magn. Mater. 293, 483 (2005)
C. Hui, C. Shen, J. Tian, L. Bao, H. Ding, C. Li, Y. Tian, X. Shi, H. J. Gao, Nanoscale 3, 701 (2011)
P. Akbarzadeh, N. Koukabi, M.M. Hosseini, J. Heterocyclic Chem. 57, 2455 (2020)
B. Karami, K. Eskandari, A. Ghasemi, Turk. J. Chem. 36, 601 (2012)
X. Meng, W. Lei, W. Yang, Y. Liu, Y. Yu, J. Colloid Interface Sci 600, 382 (2021)
G. Han, R. Sui, Y. Yu. L. Wang, M. Li, J. Li, H. Liu, W. Yang, J. Magn. Magn. Mater. 528, 167824 (2021)
X. Liu, H. Liu, Y. Wang, W. Yang, Y. Yu, J. Colloid Interface Sci. 581, 619 (2021)
S. Bao, W. Yang, Y. Wang, Y. Yu, Y. Su, J. Hazard. Mater. 409, 124470 (2021)
X. Meng, Y. Liu, G. Han, W.Yang, Y. Yu, Carbon, 162, 356 (2020)
M. Bagherzadeh, H. Haddadi. M. Iranpour, Prog. Org. Coat. 101, 149 (2016)
D. Ling, M.J. Hackett, T. Hyeon, Nano Today 9, 457 (2014)
G. T. Hermanson, Bioconjugate techniques. Academic press (2013)
Z. Shahedi, Y. Mansoori, J. Part. Sci. Technol. 4, 67 (2018)
L. Shen, B. Li, Y. Qiao, Mater. 11, 324 (2018)
S.T. Firdovsi, M. Yagoub, A.E. Parvin, Chinese J. Chem. 25, 246 (2007)
K. Saravanan, B. Tyagi, H.C. Bajaj, Catal. Sci. Technol. 2, 2512 (2012)
A.P. Kumar, J.H. Kim, T.D. Thanh, Y.I. Lee, J. Mater. Chem. B. 1, 4909 (2013)
N.E. Leadbeater, M. Marco, Chem. Rev. 102, 3217 (2002)
J. Liu, S.Z. Qiao, Q.H. Hu, G.Q. Lu, Small 7, 425 (2011)
C. Shuai, W. Yang, C. He, S. Peng, C. Gao, Y. Yang, F. Qi, P. Feng, Mater. Des. 185, 108275 (2020)
W. Yang, Y. Zhong, P. Feng, C. Gao, S. Peng, Z. Zhao, C. Shuai, Polym. Test 76, 33 (2019)
C. Cheng, Y. Wen, X. Xu, H. Gu, J. Mater. Chem. 19, 8782 (2009)
Y. Zhu, W. Zhao, H. Chen, J. Shi, J. Phys. Chem. C 111, 5281 (2007)
A. Ruíz-Baltazar, R. Esparza, G. Rosas, R. Pérez, J. Nanomater 2015, 240948 (2015)
M.A. Amin, K. Khaled, Corros. Sci. 52, 1762 (2010)
H.H. Hassan, Electrochim. Acta 51, 526 (2005)
S. Refaey, F. Taha, A.A. El-Malak, Appl. Surf. Sci. 242, 114 (2005)
K.M. Koczkur, S. Mourdikoudis, L. Polavarapu, S.E. Skrabalak, Dalton Trans. 44, 7883 (2015)
K. Seo, K. Sinha, E. Novitskaya, O.A. Graeve, Mater. Lett. 215, 203 (2018)
O. Evangelinou, A.G. Hatzidimitriou, E. Velali, A.A. Pantazaki, N. Voulgarakis, P. Aslanidis, Polyhedron 72, 122 (2014)
J.M. Khurana, D. Magoo, K. Aggarwal, N. Aggarwal, R. Kumar, C. Srivastava, Eur. J. Med. Chem. 58, 470 (2012)
A. Jarrahpour, E. Ebrahimi, E.D. Clercq, V. Sinou, C. Latour, L.D. Bouktab, J.M. Brunel, Tetrahedron 67, 8699 (2011)
A. Noack, H. Hartmann, Chem. Lett. 31, 644 (2002)
J.F. Callan, A.P. De Silva, D.C. Magri, Tetrahedron 61, 8551 (2005)
J. Liu, Z. Diwu, W.Y. Leung, Bioorg. Med. Chem. Lett. 11, 2903 (2001)
S. Samantaray, P. Kar, G. Hota, B.G. Mishr, Ind. Eng. Chem. Res. 52, 5862 (2013)
M.M. Khodaei, A. Alizadeh, H. Afshar Hezarkhani, Appl. Organometal. Chem. 34, e5262 (2020)
M.M. Khodaei, A. Alizadeh, M. Haghipour, Res. Chem. Intermed. 46, 1033 (2020)
M.M. Khodaei, M. Dehghan, Polyhedron 162, 219 (2019)
M.M. Khodaei, A. Alizadeh, M. Haghipour, J. Organomet. Chem. 870, 58 (2018)
M.M. Khodaei, M. Dehghan, New J. Chem. 42, 11381 (2018)
Y. Zhu, W. Zhao, H. Chen, J. Shi, J. Phys. Chem. C. 111, 5281 (2007)
S. Li, T. Zhang, R. Tang, H. Qiu, C. Wang, Z. Zhou, J. Magn. Magn. Mater. 379, 226 (2015)
V.K. Balskrishnan, X. Han, G.W. VanLoon, J.M. Dust, J. Toullec, E. Buncel, Langmuir 20, 6586 (2004)
L.Q. Pham, J.H. Sohn, C.W. Kim, J.H. Park, H.S. Kang, B.C. Lee, Y.S. Kang, J. Colloid Interface Sci. 365, 103 (2012)
M. Bagherzadeh, O. Mousavi, Z. Shams Ghahfarokhi, New. J. Chem. 44, 15148 (2020)
C. Cui, Y. Du, T. Li, X. Zheng, X. Wang, X. Han, P. Xu, J. Phys. Chem. B. 116, 9523 (2012)
S. Das, T. Dutta, R. Borah, J. Mol. Liq, 289, 111099 (2019)
R. Mangalam, M. Thamilselvan, S. Selvasekarapandian, S. Jayakumar, R. Manjuladevi, Ionics 23, 2837 (2017)
K. Mallick, M.J. Witcomb, M.S. Scurrell, Eur. Polym. J. 42, 670 (2006)
H. Zhang, X. Zhong, J.J. Xu, H.Y. Chen, Langmuir 24, 13748 (2008)
L. Wang, L. Wang, J. Luo, Q. Fan, M. Suzuki, I.S. Suzuki, M.H. Engelhard, Y. Lin, N. Kim, J.Q. Wang, C.J. Zhong, J. Phys. Chem. B 109, 21593 (2005)
A. Hasaninejad, M. Dadar. A. Zare, Chem. Sci. Trans. 1, 233 (2012)
Z.A. Piralghar, M.M. Hashemi, A. Ezabadi, Polycyclic Aromat. Compd. 40, 1510 (2020)
F. Mohamadpour, M. Feilizadeh, Chem. Methodol. 4, 647 (2020)
A. Zare, A.R. Moosavi-Zare, M. Merajoddin, M.A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M.H. Beyzavi, E. Rostami, A. Arghoon, R. Roohandeh, J. Mol. Liq. 167, 69 (2012)
P.J. Das, J. Das, RSC Adv. 5, 11745 (2015)
A. Thakur, A. Sharma, A. Sharma, Synth. Commun. 46, 1766 (2016)
H. Eshghi, M. Bakavoli, H. Moradi, Chin. Chem. Lett. 19, 1423 (2008)
F. Nemati, S. Sabaqian, J. Saudi Chem. Soc. 21, S383 (2017)
Acknowledgements
The authors acknowledge the financial support from Razi University of Kermanshah.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karegar, M., Khodaei, M.M. Synthesis of Fe3O4-PVP nanocomposite functionalized with sulfonic group as an effective catalyst for one-pot synthesis of xanthene derivatives. Res Chem Intermed 47, 4537–4555 (2021). https://doi.org/10.1007/s11164-021-04542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04542-3