Abstract
A simple and efficient method for liquid-phase catalytic nitration of 1-nitronaphthalene with NO2 to 1,5-dinitronaphthalene under mild conditions has been developed. The results indicated that the sulfated zirconia (SO42−/ZrO2) as solid superacid catalyst exhibits superior catalytic performance with dioxygen and acetic anhydride. 93.8% conversion of 1-nitronaphthalene and 52.8% 1,5-dinitronaphthalene selectivity were achieved. Furthermore, the physicochemical properties of SO42−/ZrO2 were determined by XRD, Py-FT-IR, BET, FT-IR, Raman spectroscopy and ICP-OES technologies. The possible nitration reaction mechanism over SO42−/ZrO2 catalyst was proposed. The present work provides an easy-to-implement, mild and eco-friendly approach for the efficient preparation of valuable 1,5-dinitronaphthalene, which has extensive industrial application prospects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The nitration of aromatic hydrocarbons for the manufacture of aromatic nitro-compounds is indispensable industrial process due to its widely application in pharmaceuticals, perfumes, dyes, explosives and plastics fields [1, 2]. Particularly, the nitration of 1-nitronaphthalene (1-NN) or naphthalene to 1,5-dinitronaphthalene (1,5-DNN) is an important reaction process. 1, 5-DNN as a raw material is used in the preparation of 1,5-naphthalene diisocyanate, which is a key building blocks upstream intermediate for constructing advanced polyurethanes with high durability, elasticity, dynamic capability and wear-resistance property [3,4,5]. Besides, 1,8-dinitronaphthalene (1,8-DNN) can be also hydrogenated to 1,8-diaminonaphthalene which is mainly used in the production of dyes and plastics [6]. At present, the nitration reaction process still used the conventional “mixed acid” method (nitric acid and sulfuric acid as nitrating agent and catalyst, respectively) [7, 8]. Nevertheless, this method suffers from a series of shortcomings such as lack of selectivity (30–35% 1,5-DNN), overuse of mixed acid and discharging of acidic waste stream. There is an urgent need to exploit a nitration method with eco-friendly, high atomic utilization and mild reaction conditions to improve the selectivity to 1,5-DNN.
In the past few decades, varieties of clean nitrification methods have been attempted to enhance the 1,5-DNN selectivity. For instance, Schal groups employed mass fraction of 78% nitric acid as the nitrating agent for naphthalene without using sulfuric acid, and only 39.5% of 1,5-DNN was obtained [9]. In order to improve the chemoselectivity of 1,5-DNN, great efforts have been devoted to the design and preparation of solid acidic catalysts instead of concentrated sulfuric acid, such as montmorillonite clay modified by phosphoric acid [10], Cu(NO3)2 / K10 montmorillonite [11] and the mixed catalysts of transition metal supported on silica gel [12]. Unfortunately, the 1,8-DNN product was still dominated in these reaction systems. In addition, zeolites were also used in this nitration reaction due to its shape-selective effect. Liu et al. used HZSM-5 modified by a mass fraction of 5% PW (phosphotungstic acid) as catalyst and achieved preferable consequences with 48.5% 1,5-DNN selectivity under the condition of excessive concentrated nitric acid [4]. Brandt et al. found that HY zeolite exhibited an acceptable catalytic performance in a reaction system with 4:1 ratio of nitric acid to 1-NN, and the yield of 1,5-DNN was 50% [13]. However, the satisfactory 1,5-DNN selectivity was yet to be obtained and concentrated nitric acid was still overused in the above studies.
Considering some disadvantages of concentrated nitric acid, such as inherently corrosive and low atomic utilization, nitrogen oxides (NOx) as the “green” nitrating agents are promising candidate for nitric acid. The direct use of NO2 as nitrating agent has potential application in industry in that it dispenses both with the multi-step process for manufacturing concentrated nitric acid from NO2 and the disposal of acidic wastewater [8]. Therefore, considerable attentions have been focused on achieving a cleaner nitration process with high chemoselectivity by using NO2. For example, Squadrito’s team conducted the nitration of naphthalene with NO2 in CCl4 solution, but the naphthalene conversion was lower than 10% with a small amount of 1,3-dinitronaphthalene and 2, 3-dinitronaphthalene which were produced [14]. Suzuki et al. reported a high proportion of 1-NN (98.6%) was obtained with low yield of dinitronaphthalene in the nitration of naphthalene with NO2 [15]. Mori et al. investigated the naphthalene-NO2-O3 nitration system under low temperature in dichloroethane as solvent. Unfortunately, the 1,8-DNN still accounted for a large proportion and 1,5-DNN selectivity was only approximately 22% [16]. Peng et al. reported that HBEA could improve the regioselectivity to 1,5-DNN in a mild and easy-to-operate nitration system with NO2-O2 [17]. Our previous works showed that Ni(CH3COO)2·4H2O or HY zeolite was used as catalyst in catalytic nitration of naphthalene or 1-NN with NO2, the main products were 1,5-DNN, 1,3-dinitronaphthalene (1,3-DNN) and 1,4-dinitronaphthalene (1,4-DNN), and only 35% 1,5-DNN selectivity was achieved (1,3-DNN and 1,4-DNN were used as a carbide additive, ammonium nitrate explosive sensitizer, sulfuric acid dye intermediate and organic intermediate) [18, 19].
On the other hand, the solid superacid sulfated zirconia (SO42−/ZrO2, SZr) is an appealing catalyst owing to its superacidic properties and uniquely textural features, which displays high catalytic activity and regioselectivity in aromatic nitration, alkylation and isomerization [20, 21]. And to our best knowledge, few researches regarding SZr catalysts in nitration process of 1-NN or naphthalene with NO2 have been reported. Herein, we developed a simple and efficient method for selective catalytic nitration of 1-NN with NO2 to 1,5-DNN over solid superacid SZr catalyst promoted by molecular oxygen and acetic anhydride (Ac2O) under mild conditions (Scheme 1). The detailed results will be presented in this paper.
Experimental
Reagents and instruments
1-NN (AR, 99%), ZrOCl2·8H2O (AR, 98%), Fe (NO3)3·9H2O (AR, 98.5%), Ca[CH3COO]2 (AR, 98%) and TiCl4 (AR, 99%) were obtained from Macklin Biochemical Technology Co., Ltd. The liquid nitrogen dioxide (NO2 purity > 99.9%) was obtained from the Gas Company of Chengdu Keyuan Gas Co., Ltd. Zeolites (Hβ, HY and HZSM-5) and other reagents were obtained commercially. The obtained reaction products were quantified by GC (Agilent, GC-7890B, DB-1701 capillary column) with FID using nitrobenzene as internal standard.
Preparation of solid superacid catalysts
All solid superacid catalysts (SZr, SO42−/Fe2O3 (SFe) and SO42−/TiO2 (STi)) were prepared by coprecipitation-immersion process. Typically, ZrOCl2·8H2O or metallic nitrate was dissolved in deionized water with constant stirring until dissolved. The ammonia aqueous (NH3·H2O, 28%) was added drop-wise to the salt solutions until pH value 9–10. After overnight aging, the precipitate was repeatedly washed by water with ethanol until no detection of Cl− and dried at 110 °C for 12 h. The dried sample was immersed in 1.0 mol/L H2SO4 (1 g/15 ml) for 6 h. Finally, the resulted samples were dried and calcined at 550 °C for 3 h. The obtained solid sample was defined as SZr catalyst. Besides, tetragonal ZrO2 was prepared by adding additional precipitate of Ca[CH3COO]2 to the precursor hydroxide suspension, then washed with ethylenediamine aqueous solution and calcined at 600 °C for 1 h.
Characterization of catalysts
The textural properties of catalysts were measured by physisorption measurement on an ASAP 2020 (Micrometrics, USA) using nitrogen as adsorbent at 77 K. The X-ray diffraction (XRD) patterns were obtained by D/Max-2550 V+ (Japan Rigaku) diffractometer equipped with a monochromatic copper Kα radiation (λ = 1.5418 Å). Thermogravimetric-derivative thermogravimetric (TG/DTG) curves of samples were carried out on Mettler 1600HT. FT-IR spectra of samples were obtained on the Nicolet 380 spectrometer in the 400–4000 cm−1 range. The Raman spectra with 325 nm excitation laser, and the scan time was 60–180 s for a single spectrum were operated on a Renishaw Raman spectrometer. The infrared spectra of pyridine adsorption of samples obtained on Thermo Nicolet 380 FT-IR spectrometer were used to identify the acidity sites of samples. The ICP-OES technology was operated on an Agilent 730 instrument for element content.
Typical experimental process
Nitration of 1-NN with HNO3
The nitration of 1-NN with 65% HNO3 was carried out in a two-necked 50-ml flask with a temperature controller and a Graham condenser at the following reaction process: 1-NN (1.73 g), acetic anhydride and catalyst were placed in the flask and stirred under ice bath, and the 65% HNO3 was slowly added into the mixture. The mix reagent was stirred for a given time and temperature under reflux. When the reaction was complete, the reaction was quenched with distilled water and the flask was cooled by ice bath. The obtained precipitate was filtered, dried and dissolved with mixed solvent of acetonitrile (MeCN) and dichloroethane (v/v = 3:2) for further quantitative analysis.
Nitration of 1-NN with NO2
The nitration process of 1-NN with NO2 was performed in a 100 ml stainless steel reactor. Typically, 1-NN (1.73 g), Ac2O (10 g), liquid NO2 and catalyst were weighed to the reactor, respectively. Then, the reaction kettle was placed in a water bath with a preset temperature (35 oC) with continuously stirring. Oxygen pressure (0.5 MPa) was input into the reactor through the inlet pipe until the reaction was completed. Finally, the reaction products were fully dissolved by the above method. The catalyst was separated and recycled over centrifugation, and the solution was analyzed by GC and GC–MS, respectively.
Results and discussion
Catalytic performance of catalysts in different systems
Table 1 investigates the catalytic nitration of 1-NN to 1,5-DNN over different solid catalysts and nitration systems. The nitration products mainly included 1,5-DNN, 1,8-DNN, 1,4-DNN, 1,3-DNN, 1,6-dinitronaphthalene (1,6-DNN), 1,7-dinitronaphthalene (1, 7-DNN) and a small amount of 2,3,5-trinitronaphthalene. Initially, we investigated the nitration of 1-NN with excessive HNO3 (1-NN/HNO3 = 1/10) with or without catalyst and obtained a poor 1,5-DNN selectivity at low 1-NN conversion (entries 1–2). With the assistance of Ac2O, 1-NN conversion was obviously improved and the addition amount of HNO3 was also greatly reduced (entry 3). Alternatively, the introduction of NO2 resulted in a distinctly change in dinitronaphthalene distribution as compared to the HNO3, but it did not show positive effect on 1-NN conversion due to the low activity of NO2 (entry 5). With the introduction of molecular oxygen, however, the 1-NN conversion increased rapidly from 16.1% to 82.9% (entry 6). The result indicated that the introduced dioxygen significantly enhanced the nitration ability of NO2. To verify the effect of solvent on the nitration reaction, MeCN and acetic acid (Ac) were also participated (entries 7–8). The results showed that only the introduction of Ac2O could promote the conversion of 1-NN. Moreover, in NO2-MeCN and NO2-N2-MeCN system, the target products were not detected, implying the 1-NN was not converted. These results indicated that both O2 and Ac2O were indispensable when using NO2 as a nitrating agent. The possible reason was that the molecular oxygen was responsible for assisting NO2 to form nitroxyl cation (NO2+), and the role of Ac2O was to stabilize NO2+ to form acetyl nitrate (AcONO2) which was a highly reactive nitrating agent [22]. Furthermore, the addition of Ac2O could effectively capture water molecules produced by the reaction, which might poison the acidic sites and yield unnecessary HNO3 [23,24,25].
As metal oxides (ZrO2, Fe2O3 and TiO2) and acidic zeolites (HY, Hβ and HZSM-5) were tested in this nitration system, 1-NN conversion was further increased in varying degrees, while the selectivity to 1,5-DNN was not desirable (entries 10–15). To our delight, the 1-NN conversion and selectivity were significantly improved when solid superacids were introduced to the nitration system (entries 16–18). Particularly, the SZr catalyst exhibited preferable catalytic performance in NO2-O2-Ac2O system, and 93.8% 1-NN conversion and 52.8% 1,5-DNN selectivity were achieved. Alternatively, the results of 1-NN nitration over SZr catalyst were not satisfactory in 65% HNO3, 65% HNO3-Ac2O and NO2-Ac2O system (entries 4, 6 and 11). Collectively, both O2-Ac2O and SZr catalysts were indispensable for enhancing the 1-NN conversion and the 1,5-DNN selectivity.
Optimization of reaction conditions
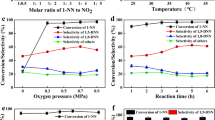
The effects of reaction factors on the nitration of 1-NN to 1,5-DNN are shown in Fig. 1. Clearly, 1-NN conversion gradually increased with the elevated reaction parameters, while the 1,5-DNN selectivity, by and large, increased first and then decreased. The molar ratios of 1-NN to NO2 and reaction temperature were key factors for the nitration reaction. With the increase in reaction temperature, concentration of NO2 and reaction time, the by-products increased obviously, which result in the reduction for selectivity to 1,5-DNN. Moreover, the effect of oxygen pressure is also investigated in Fig. 1d. 1-NN conversion was enhanced with the increased oxygen pressure, implying that introduction of dioxygen could effectively activate nitrification of NO2. In addition, the effects of catalyst dosage are summarized in Table 2. From the above experimental and analysis results, the appropriate conditions were achieved, namely 1:3 of molar ratio of 1-NN to NO2, 4 h of reaction time, 35 °C of reaction temperature, 0.5 MPa of oxygen pressure and 0.3 g of catalyst dosage. Under the optimal reaction conditions, 93.8% of 1-NN conversion and 52.8% 1,5-DNN selectivity were achieved over SZr catalyst.
The effects of reaction factors on the nitration. Reaction condition: a temperature: 35 °C, time: 4 h, oxygen pressure: 0.5 MPa, catalyst: 0.3 g, Ac2O: 10 g; b temperature: 35 °C, oxygen pressure: 0.5 MPa, molar ratio of 1-NN to NO2: 1:3, catalyst: 0.3 g, Ac2O: 10 g; c oxygen pressure: 0.5 MPa, time: 4 h; molar ratio of 1-NN to NO2: 1:3, catalyst: 0.3 g, Ac2O: 10 g; d temperature: 35 °C, time: 4 h; molar ratio of 1-NN to NO2: 1:3, catalyst: 0.3 g, Ac2O: 10 g
Recycling of solid superacid SZr catalyst
The stability of SZr catalyst in the liquid-phase nitration reaction was examined under optimal reaction conditions, as shown in Fig. 2. After each catalytic reaction, the used catalyst can be simply recycled by filtration. As can be seen from the results, the 1-NN conversion and 1,5-DNN selectivity only decreased slightly after five times. Moreover, the catalytic performance of spent SZr catalyst recovers to its original level by simple calcination under the same reaction conditions (5a, Fig. 2). The results showed that SZr catalyst exhibited good stability and regeneration.
Characterization of samples
The XRD patterns of samples are presented in Fig. 3. All samples exhibit the same diffraction peaks at 2θ value 30.2, 35.2, 50.4, 60.2 and 62.8°, belonging to the pure tetragonal phase ZrO2 (JCPDS No. 88–1007) [26, 27]. This indicates that the crystallinity of sample is not changed after the introduction of sulfate in the tetragonal phase ZrO2. In addition, compared to fresh SZr, no obvious changes in terms of the crystal phase intensity have been observed on the used and regenerated samples, indicating the crystal phase of the catalyst has a strong stability.
FT-IR spectra of samples are given in Fig. 4. A broad peak at 3435 cm−1 is ascribed to the stretching vibration of surface hydroxyl group (νO-H), and the intense peak at 1635 cm−1 is attributed to the δH-O–H bending frequency of adsorbed water molecule on catalyst surface [28]. Additional bands (around 450–800 cm−1) are the characteristic peaks of crystalline ZrO2 [29]. Particularly, the characteristic peaks at 740 and 500 cm−1 are assigned to the Z–rO–Zr asymmetric and Zr–O stretching modes, respectively, which systematically further explain the formation of ZrO2 phases [30]. The spectra of SZr samples exhibit the broad peaks having shoulders at 1010, 1045, 1140, 1235 and 1384 cm−1 are attributed to chelating bidentate sulfate group (SO42−) which is coordinated with ZrO2 support surface [31, 32]. However, there are no characteristic peaks of sulfate group in the FT-IR spectrum of pure ZrO2 sample. The results demonstrate that SO42− has been successfully bonded with ZrO2. In addition, the used and regenerated catalysts exhibit the same FT-IR peaks as fresh SZr catalyst; that is, the framework of SZr catalyst still remains perfectly during the nitration reaction.
The thermal stability of catalysts in the temperature range of 30–800 oC is tested, and the obtained results are shown in Fig. 5. Clearly, all samples show a mass loss stage in temperature range 30–100 oC, which is ascribed to the desorption of water adsorbed on the sulfate group or metal oxides surface. However, the mass percentage for SZr samples (ca. 7–10%) is more than pure ZrO2 (ca. 3%), demonstrating that the amount of water adsorbed on the surface of SZr catalyst is more than that on the surface of pure ZrO2. Moreover, the used SZr sample exhibits another large mass loss stage in the range of 450–600 °C, which shows the decomposition of organic species with high boiling point on the surface or in the pores of catalyst. These results indicate that the used SZr catalyst can be regenerated by a simple thermal treatment. It is worth mentioning that a mass loss stage of SZr samples is observed over 700 °C, which can be assigned to the decomposition of surface sulfate groups [33].
Figure 6 displays the nitrogen adsorption–desorption isotherms and pore diameter distribution curves of samples, and Table 3 summarizes their textural structure parameters. The results show that ZrO2 and SZr exhibit the type IV isotherms with type H2 hysteresis loop, which are the features of typical mesopores materials [34, 35]. As shown in Fig. 6a, the capillary condensation phenomenon at p/p0 = 0.4 was observed without obvious inflection point, indicating that the mesopores of catalysts are not uniform and relatively wide distribution (Fig. 6b). Moreover, the specific surface area of catalyst is obviously increased, while the pore diameter is decreased after sulfonating. Furthermore, the textural properties parameters of SZr sample were slightly reduced after five cycles. Particularly, the structural parameters of used SZr catalyst could be regenerated by calcination.
In order to further verify the metal or sulfur leaching of SZr catalyst in the reaction, the elemental contents of samples are determined by ICP (Table 3). The results demonstrated that Zr content (wt%) was basically unchanged, while the S content is only slightly reduced after SZr catalyst used for 5 times, indicating that a very small amount of sulfur was leached during the nitration reaction.
To identify the acidic sites of pure ZrO2 and SZr samples, the characterization of pyridine adsorbed FT-IR at around 1400–1650 cm−1 is performed, as shown in Fig. 7. Obviously, only two peaks at 1448 and 1600 cm−1 present in pure ZrO2, which belong to the vibrational mode of adsorbed protonated pyridium cation connected with Lewis-type acid sites [36]. However, another two characteristic peaks are also observed in SZr sample. The peak at 1545 cm−1 taken the indication of pyridine adsorbed with Brønsted acid sites [37] which mainly provides protons for the nitrification of aromatics. Moreover, the peak located at 1490 cm−1 is assigned to the pyridine adsorbed on either Lewis or Brønsted acid sites [38]. It can be seen that the pure ZrO2 has only Lewis acid sites, while SZr catalyst possesses both the Lewis acid sites and Brønsted acid sites.
Figure 8 exhibits the Raman spectrums of pure ZrO2 and fresh SZr samples. Normally, pure ZrO2 displays the characteristic Raman bands at around 100–800 cm−1 [39]. The Raman bands at 148, 268, 315, 462 and 642 cm−1 are identical to the reported spectra of pure tetragonal phase ZrO2 [40], which is in accordance with XRD results. However, the Raman band at 1031 cm−1 is detected in SZr sample, which is attributed to the symmetric and asymmetric vibrations of SO42− [41,42,43], further indicating that SO42− has been successfully bonded with ZrO2.
The possible nitration reaction mechanism
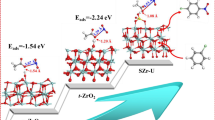
Combining the obtained experimental results in this work and studies [44,45,46], the possible nitration mechanism of 1-NN to 1,5-DNN or 1,8-DNN over SZr catalyst in Ac2O-O2 system is proposed in Scheme 2. Usually, liquid nitrogen dioxide presents in the form of NO2 and N2O4 which disproportionates to nitrosyl cations (NO+) and nitrate ion (NO3−). Meanwhile, the molecular oxygen easily oxidizes NO+ to electrophilic NO2+. The resulting NO2+·NO3− species reacted with Ac2O to generate AcONO2, and the formed AcONO2 was chemisorbed onto the surface of catalyst with acid sites [22]. Then, in the state of this chemical bond, the formed AcONO2+ species present high tendency to attack the 5-site than 8-site of 1-NN due to the steric hindrance effects [47], generating high proportion of 1,5-DNN. Next, the Wheland intermediate was formed by releasing acetic acid from the adsorbed AcONO2+. Finally, the SZr catalyst was recovered via the intermediate captured a proton, and simultaneously, the nitration products were obtained.
Conclusions
In this work, a viable green, mild and highly efficient method for 1-NN nitration with pure liquid nitrogen dioxide to 1,5-DNN had been successfully developed employing SZr as catalyst in Ac2O-O2 system under low temperature conditions. The results showed that the synergistic effects of Ac2O, O2 and SZr played significant role in improving the 1-NN conversion and selectivity to 1,5-DNN. Furthermore, SZr catalyst showed an acceptable stability, and the spent catalyst could be regenerated by a simple and easy way of calcination method. This work will provide a promising method for the preparation of high proportion market demand 1,5-DNN from 1-DNN nitration with pure liquid nitrogen dioxide over solid superacid catalyst in O2-Ac2O system, which has potential application prospects.
References
J.P. Adams, J. Chem. Soc., Perkin Trans. 1(34), 2586 (2003)
D.M. Badgujar, M.B. Talawar, S.N. Asthana, P.P. Mahulikar, J. Hazard. Mater. 151, 289 (2008)
E.C. Prolingheuer, J.M. Barnes, R. Kopp, E.V. Seggern, J. Elastom. Plast. 24, 221 (1992)
P. Liu, W. Xiong, X. Wang, K. Huang, F. Hao, L. Wang, H. Luo, Res. Chem. Intermed. 41, 4533 (2015)
S.-Y. Hong, S.-M. Park, J. Electrochem. Soc. 150, 360 (2003)
C. Du, R. Xu, S. Han, J. Xu, L. Meng, J. Wang, H. Zhao, J. Chem. Thermodynamics 94, 24 (2016)
D.S. Ross, K.D. Moran, R. Malhotra, J. Org. Chem. 48, 2118 (1983)
X. Peng, N. Fukui, M. Mizuta, H. Suzuki, Org. Biomol. Chem. 1, 2326 (2003)
H.P. Schal, V.Y. Popkova, B.M. Laskin, A.S. Malin, S.B. Volkova, US 6992230 (2006)
S.K. Bharadwaj, P.K. Boruah, P.K. Gogoi, Catal. Commun. 57, 124 (2014)
B. Gigante, Á.O. Prazeres, M.J. Marcelo-Curto, A. Comélis, P. Laszlo, J. Org. Chem. 60, 3445 (1995)
M. Shi, S.-C. Cui, Adv. Synth. Catal. 345, 1329 (2003)
M. Brandt, S. Klein, G. Wegener, US 6737554 (2004)
G.L. Squadrito, F.R. Fronczek, D.F. Church, W.A. Pryor, J. Org. Chem. 54, 548 (1989)
H. Suzuki, T. Mori, J. Chem. Soc., Perkin Trans. 2(4), 677 (1996)
T. Mori, H. Suzuki, Synlett 26, 383 (1995)
H. Wang, X. Peng, C. Shi, X. Dong, Y. Tai, H. Liu, Res. Chem. Intermed. 40, 1495 (2014)
K. You, Z. Zhou, J. Jian, R. Deng, P. Liu, Q. Ai, H. Luo, Res. Chem. Intermed. 41, 8307 (2015)
R. Deng, K. You, F. Zhao, P. Liu, H. Luo, Can. J. Chem. Eng. 96, 2586 (2018)
G.X. Yan, A. Wang, I.E. Wachs, J. Baltrusaitis, Appl. Catal. A Gen. 572, 210 (2019)
B.M. Reddy, M.K. Patil, Chem. Rev. 109, 2185 (2009)
R. Deng, K. You, W. Ni, F. Zhao, P. Liu, H. Luo, Appl. Catal. A Gen. 594, 117468 (2020)
I. Sreedhar, M. Singh, K.V. Raghavan, Catal. Sci. Technol. 3, 2499 (2013)
M.M. Heravi, K. Bakhtiari, T. Benmorad, F.F. Bamoharram, H.A. Oskooie, M.H. Tehrani, Monatsh. Chem. 138, 449 (2007)
S. Bernasconi, G.D. Pirngruber, R. Prins, J. Catal. 224, 297 (2004)
D. Kong, Q. Peng, L. Shi, X. Wang, X. Meng, X. Hu, N. Liu, J. Chin. Chem. Soc. 67, 1644 (2020)
H. Wang, Y. Li, F. Yu, Q. Wang, B. Xing, D. Li, R. Li, Chem. Eng. J. 364, 111 (2019)
S. Wang, J. Pu, J. Wu, H. Liu, H. Xu, X. Li, H. Wang, ACS Omega 5, 30139 (2020)
Y. Zhang, T. Chen, G. Zhang, G. Wang, H. Zhang, Appl. Catal. A Gen. 562, 258 (2018)
C. García-Sancho, J.A. Cecilia, A. Moreno-Ruiz, J.M. Mérida-Robles, J. Santamaría-González, R. Moreno-Tost, P. Maireles-Torres, Appl. Catal. B Environ. 179, 139 (2015)
C.D.M. Miranda, A.E.S. Ramírez, S.G. Jurado, C.R. Vera, J. Mol. Catal. A Chem. 398, 325 (2015)
X.H. Lin, X.J. Yin, J.Y. Liu, S.F.Y. Li, Appl. Catal. B Environ. 203, 731 (2017)
J. Gardy, E. Nourafkan, A. Osatiashtiani, A.F. Lee, K. Wilson, A. Hassanpour, X. Lai, Appl. Catal. B Environ. 259, 118093 (2019)
B. Tyagi, M.K. Mishra, R.V. Jasra, Catal. Commun. 7, 52 (2006)
Y.-Y. Huang, T.J. Mccarthy, W.M.H. Sachtler, Appl. Catal. A Gen. 148, 135 (1996)
B.H. Davis, R.A. Keogh, S. Alerasool, D.J. Zalewski, D.E. Day, P.K. Doolin, J. Catal. 183, 45 (1999)
K. Föttinger, G. Kinger, H. Vinek, Appl. Catal. A Gen. 266, 195 (2004)
F. Babou, G. Coudurier, J.C. Vedrine, J. Catal. 152, 341 (1995)
S. Kuba, P.C. Heydorn, R.K. Grasselli, B.C. Gates, M. Che, H. Knözinger, Phys. Chem. Chem. Phys. 3, 146 (2001)
B.M. Reddy, P.M. Sreekanth, V.R. Reddy, J. Mol. Catal. A: Chem. 225, 71 (2005)
C. Li, M. Li, J. Raman Spectrosc. 33, 301 (2002)
S. Kanitkar, M.A. Abedin, S. Bhattar, J.J. Spivey, Appl. Catal. A Gen. 575, 25 (2019)
T. Yamamoto, T. Tanaka, S. Takenaka, S. Yoshida, T. Onari, Y. Takahashi, T. Kosaka, S. Hasegawa, M. Kudo, J. Phys. Chem. B 103, 2385 (1999)
S. Zhou, K. You, Z. Yi, P. Liu, H.A. Luo, Front. Chem. Sci. Eng. 11, 205 (2017)
Y. Jiao, M. Zhu, R. Deng, J. Jian, Y. Yin, K. You, Res. Chem. Intermed. 43, 3961 (2017)
S. Zhou, K. You, H. Gao, R. Deng, F. Zhao, P. Liu, Q. Ai, H. Luo, Mol. Catal. 433, 91 (2017)
S. Bernasconi, G.D. Pirngruber, A. Kogelbauer, R. Prins, J. Catal. 219, 231 (2003)
Acknowledgements
The authors are grateful for the financial support of the National Natural Science Foundation of China (21676226), Key Research and Development Program in Hunan Province (2019GK2041), the Postgraduate Scientific Research Innovation Project of Hunan Province (CX20200637), Innovation and Entrepreneurship Training Program for College Students in Hunan Province (S201910530024) and Hunan Key Laboratory of Environment Friendly Chemical Process Integrated Technology.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, J., Ni, W., You, K. et al. Highly selective catalytic nitration of 1-nitronaphthalene with NO2 to 1,5-dinitronaphthalene over solid superacid SO42−/ZrO2 promoted by molecular oxygen and acetic anhydride under mild conditions. Res Chem Intermed 47, 3569–3582 (2021). https://doi.org/10.1007/s11164-021-04502-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04502-x