Abstract
Inspired by the strong adhesion of mussels, a super-hydrophobic sponge was designed and prepared by a simple and inexpensive one-pot solution immersion method. The prepared superhydrophobic sponge can not only efficiently separate the oil–water mixture, more importantly, but also remove volatile organic compounds in the atmospheric environment. Polydopamine (PDA) enables polydivinylbenzene (PDVB) particles to be firmly and tightly attached to the melamine sponge skeleton, thereby making the hydrophilic sponge superhydrophobic and providing adsorption sites for volatile organic compounds in the air. The synergy enables the sponge/PDA/PDVB to quickly adsorb oils and organic substances, and it has high stability and capacity even after 20 cycles. In addition, superhydrophobic sponges can still perform outstanding adsorption performance even under highly acidic and alkaline environments. Meanwhile, the static adsorption capacity of the sponge/PDA/PDVB for gaseous toluene is 5.7 times that of activated carbon. Compared with pure PDVB, the super-hydrophobic sponge in the dynamic experiment has a penetration time increased from 6 to 390 min, which is 65 times longer than that of the PDVB, and the adsorption performance has been greatly improved. Therefore, our strategy may achieve a new effect, which can quickly and easily separate oil–water mixtures and remove volatile gaseous pollutants, and it can provide potential options for practical applications
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the development of industry, more organic materials are used, and the leakage of organic solvents and industrial emissions have become more and more serious [1,2,3]. It is estimated that approximately 2 million tons of oil enter the marine environment each year [4]. It causes serious environmental impact, including contamination of beaches, and the damage to the fishing industry. However, it is still difficult to efficiently separate oil from oil–water mixtures [5]. Adsorption has been considered as one of the most effective methods for oil/water separation due to its low cost, facile operation and minimum harmful effects on ecosystems [6,7,8]. However, the traditional adsorbent materials, such as activated carbon [9], textiles [10] and sponges [11], have the disadvantages of low adsorption capacity due to their simultaneous adsorption for oil and water [12].
Sponge as an inexpensive three-dimensional porous material with large surface area is a perfect substrate for oil–water separation, which has been reported by many researchers [13,14,15,16,17,18,19,20,21,22,23]. After hydrophobic modification, the sponge was applied for oil containing wastewater treatment. For example, Gu et al. prepared a MOF@rGO coated polyurethane sponge for oil/water separation [24]. Cao et al. prepared the polyurethane sponge functionalized with nanodiamond particles and perfluorodecanethiolby by dipping method [25]. However, these modification methods are relatively complex and costly, and often require the expensive chemicals as graphene or environmentally unfriendly chemicals as strong acids and bases.
On the other hand, the leakage of organic solvents will not only affect the water and soil environment, but also seriously harm the human body when they evaporate into the atmospheric environment. For example, high concentration of toluene can cause childhood acute leukemia, central nervous system poisoning, asthma, etc. Even low concentration of toluene can cause humans to suffer from various chronic diseases such as respiratory and cardio-cerebral vascular diseases after a certain period of exposure, and even can cause serious harms such as carcinogenesis, teratogenesis and mutagenesis. However, so far there is no report that can solve the problem of oil–water separation with high efficiency, and also involve the treatment of gaseous organic matter.
Inspired by the remarkable adhesive ability of polydopamine, we developed a superhydrophobic/superoleophilic melamine sponge coated with polydopamine (PDA) and polydivinylbenzene (PDVB) using a one-step solution immersion method, which was facile, eco-friendly and cost-effective. The superhydrophobic sponge shows high mechanical strength, great chemical stability and high reusability. It exhibits high selectivity, excellent adsorption capacity and recyclability for various organic solvents and oils. Furthermore, the as-prepared sponge displays excellent adsorption performance for volatile organic compounds (VOCs). The modified hydrophobic sponge with low cost and easy to manufacture has exhibited great potential in solving the problems of oily wastewater and VOCs.
Experimental
Materials
A melamine sponge was purchased in a local market. Dopamine hydrochloride (100%), tetrahydrofuran (C4H8O, ≥ 99.0%), divinylbenzene (C10H10 ≥ 80.0%), tris (hydroxymethyl) aminomethane (Tris, ≥ 99.8%), azobisisobutyronitrile (C8H12N4, ≥ 98.0%), and n-hexane were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Methylene blue (MB, 100%), oil red O (100%) and ethanol (EtOH, 96.5%) were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were used without further purification.
Synthesis of polydivinylbenzene (PDVB)
Polydivinylbenzene (PDVB) was prepared through a modified solvothermal reaction [26]. 5.6 mL of divinylbenzene (DVB) was dissolved in 40 mL of tetrahydrofuran (THF) with 2 mL of deionized water, and then 0.15 g of azobisisobutyronitrile (AIBN) was added. After stirring for 4 h at room temperature, the solution was put into a stainless-steel reactor with a polytetrafluoroethylene liner and then treated at 100 °C for 24 h. The material was cooled to room temperature. PDVB was obtained after the evaporation of organic solvent and water and then was ground into powder.
Synthesis of the PDA/PDVB/sponge
The PDA/PDVB/sponge was synthesized via a one-pot solution immersion method. The pristine sponge was cut into 2.0 × 2.0 × 2.0 cm pieces, which were then ultrasonically cleaned in ethanol and distilled water for 20 min to remove stains and pigments, and then it was dried in an oven at 50 °C. The sponge was immersed in a Tris–HCl buffer solution (dopamine hydrochloride 0.12 g/H2O 50 mL/C2H5OH 50 mL, pH = 8.5) containing dopamine hydrochloride (0.15 g) and PDVB (2 g) for 24 h under stirring at room temperature. The as-prepared sponge was dried in an oven at 50 °C.
Characterization
The water contact angle (WCA) was measured using a contact angle meter (OCA15, Dataphysics, Germany) with 4 μL of water droplets at ambient temperature. The images of the morphology and structure of the samples were obtained from scanning electron microscopy (SEM, Nova Nano SEM 430, USA). Thermogravimetric analysis (TGA) was performed using a Netzsch TG 209 F1 Libra instrument at a nitrogen flow rate of 10 mL/min from 30 to 800 °C and a heating rate of 10 °C/min. The functionalized groups of the samples were characterized by Fourier-transformed infrared spectra (FT-IR, Nicolet-6700 FT-IR spectrometer) using KBr pellets.
Oil–water separation test
A PDA/PDVB/sponge was put in an oil–water mixture (50%, v/v), and then the oil adsorption capacity of the sponge was determined by weight measurements. The weight of the adsorbed oil was calculated using the following equation:
where m0 and m1 are the masses of a sponge before and after sorption of oils.
Adsorption of volatile organic compounds
Static adsorption and dynamic adsorption were used to evaluate the VOCs adsorption performance, such as toluene, acetone and methanol. Different organics were placed in the open beaker and a PDA/PDVB/sponge as an adsorbent was placed in another open container, and then the air in the closed container was evacuated to statically adsorb VOCs. Take out the adsorbent after 12 h and weigh the mass change to calculate the adsorption amount. Dynamic adsorption was carried out in a quartz tube (see the Supporting information, Figure S1). The mixture air stream (20 vol % O2/N2, 100 mL/min) containing about 40 ppm of toluene and water vapor (60% RH) was introduced into the quartz tube. The concentration of toluene was determined by a gas chromatography.
Results and discussion
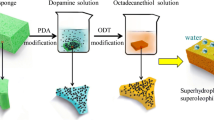
The mutual affinity between the original melamine sponge and PDVB was not sufficient to ensure that PDVB was firmly bound to the melamine sponge. Therefore, there was an urgent need for a universal molecular linker to attach PDVB to the surface of a melamine sponge. Fortunately, dopamine was used as the universal molecular linker in this work. The polydopamine (PDA) was prepared by oxidative polymerization of dopamine, because dopamine can undergo oxidation and spontaneous self-polymerization with oxygen as an oxidant under alkaline conditions. The polymerization process can follow the equilibrium pathway by forming reverse indole-like repeat units, which can lead to a crosslinked structure [27,28,29,30]. In this work, PDVB was ingeniously linked to the sponge backbone by dopamine via the one-pot solution immersion method (Fig. 1). By this method, the super-hydrophobic sponges with superior oil–water separation properties and reusability can be easily prepared.
The morphology of the materials was analyzed by SEM. As can be seen from Fig. 2, the addition of PDA and PDVB obviously changed the surface morphology of the pure melamine sponge. The sponge had an interconnected 3D network skeleton and smooth surface morphology (Fig. 2a). Compared with the pristine sponge, the network structure and porosity of the PDA/sponge did not change significantly, except for the formed PDA nanoparticles on the smooth skeleton surface (Fig. 2b), so the sponge surface became rougher. At the same time, the PDVB/sponge was sparsely covered with PDVB particles (Fig. 2c). As can be seen from Fig. 2d, PDA and PDVB particles were simultaneously loaded on the sponge skeleton, which increased the surface roughness of the sponge. For hydrophobic materials, surface roughness amplifies hydrophobicity [31, 32]. When water droplets contact the surface of the hydrophobic material, a water–air–solid three-phase interface is formed, and a large amount of air is filled in the rough pores on the surface of the hydrophobic material [33]. This feature is thought to capture a large number of air pockets on the surface of the sponge skeleton. It is well known that air pockets are necessary to achieve surface superhydrophobicity, because air is an effective hydrophobic medium [34, 35]. Thus, the increased surface roughness of the sponge by PDA and PDVB would further increase the hydrophobicity of the sponge. In addition, the porosity was investigated by the BET analysis (see Figure S2 and Table S1). The pore volume of the PODP/PDVB/sponge (1.94 cm3g−1) significantly increased compared to PDVB (1.35 cm3g−1).
TGA data showed that the PDA/PDVB/sponge had a weight loss of about 5.38% between 100 ~ 300 °C, which was due to the desorption of the residual organics during preparation. Starting from 300 °C, a significant mass loss occurred in the sample, indicating that the polymer began to decompose and convert to carbon dioxide [36]. After 370° C, the mass decreased in a cliff-like manner because the sponge skeleton starts to decompose [37]. Therefore, the PDA/PDVB/sponge had a good thermal stability below 300 °C (Fig. 3a). FT-IR spectra were performed to further confirm the formation of the PDA/PDVB/sponge (Fig. 3b). Compared with the original sponge, the new adsorption peak of the PDA/sponge appeared at 1630 cm−1 (–CN) [20], which proved that PDA was successfully loaded on the sponge. The new adsorption peaks of the PDVB/sponge appeared at 707 cm−1 (meta-substituted benzene ring) and 904 cm−1 (para-substituted benzene ring) [38, 39], which corroborated the existence of PDVB. The PDA/PDVB/sponge had the above several new adsorption peaks at the same time compared with the original sponge, proving that PDA and PDVB were loaded on the sponge simultaneously. At the same time, the peak intensity of the PDA/PDVB/sponge at 707 cm−1 and 904 cm−1 was significantly higher than that of the PDVB/sponge, indicating that more PDVB was loaded onto the sponge in the presence of PDA, leading to the increase of the characteristic peak strength.
After modification, the color of the sponge changed from white to dark brown. As shown in Fig. 4a, water droplet and oil droplet were quickly dropped on the original sponge and the PDA/PDVB/sponge, respectively. It can be seen that the water droplet deposited on the surface of the PDA/PDVB/sponge to form an almost perfect sphere, while the oil droplet was completely adsorbed by the PDA/PDVB/sponge. On the original sponge, both water and oil droplets were completely adsorbed. When the surface of the modified sponge was skimmed with a paper towel, it can be seen that the water droplets were completely adsorbed by the paper towel, leaving no traces on the modified sponge (Fig. 4b, c). This indicated that the modified sponge had excellent hydrophobic and lipophilic properties. Figure 4d, e shows the water contact angles of the original and modified sponges, respectively. For the original sponge, when water was dropped on it, contact angle was observed to be 0° due to the complete spreading of water into the sponge. After coating PDA/PDVB onto the sponge, it changed to be hydrophobic with a water contact angle of 141°. When the modified sponge was immersed in water by applying external force, air bubbles were trapped around the solid surface to prevent water from penetrating into the sponge, resulting in a silver mirror phenomenon [40] (Fig. 4f), which showed a good water repellency. Because when water droplets were placed on the surface of the composite material, the water droplets came into contact with the surface of the nanoparticles to form a water–air-solid three-phase interface. A large amount of air filled the pores on the surface of the composite material, making the real surface of the solid surface in contact with the water droplets far away less than the apparent area. According to the Cassie–Baxter equation [41], the area occupied by the air was 97.42% and the water droplets were almost completely repelled outside the rough surface. As for the original sponge, the water would completely occupy the inside of the sponge. The PDA/PDVB/sponge with advantages of high porosity, hydrophobicity and low density has great potential for the applications of oil/water separation.
In order to study the removal of organic matter in water, the PDA/PDVB/sponge was used for the oil/water separation test. As shown in Fig. 5a, the PDA/PDVB/sponge was placed in a mixed liquid of the oil phase (dyed with oil red) and the water phase (dyed with methylene blue). Once the modified sponge contacted the oil phase, all the oil was quickly adsorbed and the clear water phase was left, and the liquid level of water did not drop. In contrast, the original sponge adsorbed both the oil and water, but cannot completely adsorb the oil phase, while the water level was significantly reduced (Fig. 5b). In addition to light oils, heavy oils (chloroform dyed with Oil Red O) can also be removed from water using the PDA/PDVP sponges (Fig. 5c and Movie 1). When contacted with the modified sponge, the heavy oil at the bottom of the water penetrated into the sponge immediately.
The adsorbed oil in the modified sponge can be recovered again through extrusion, and the modified sponge still floated on the water after extrusion, indicating the good oil resistance and recyclability, which can be used for the next oil/water separation. Moreover, the modified sponges were used to adsorb various solvents and oils (Fig. 6a). The PDA/PDVB/sponges had a wide range of adsorption capacities for different solvents and oils from 24 to 56 g/g. The pure sponges and PDA/sponges cannot perform effective oil/water separation because they were not hydrophobic and would adsorb oil and water at the same time. The effects of PDA and PDVB content on the adsorption capacity of the modified sponges were studied, as shown in Figures S3 and S4. The results suggested that proper PDA and PDVB content increased the adsorption capacity of the sponges. After 20 cycles of adsorption of toluene on the modified sponge, the adsorption capacity nearly remained unchanged (Fig. 6b), indicating its excellent recyclability. Compared with the reported oil–water separation materials, the adsorption performance and stability of the PDA/PDVB/sponge was competitive and even better than most adsorption materials (see Table S2 for a comparison). Unlike many reported adsorbents, which involve complex synthesis procedure, the preparation method of this work is simpler and more convenient. In general, the PDA/PDVB/sponges have obvious advantages of large adsorption capacity and easy preparation in oil–water separation materials.
The mechanical stability of the modified sponge was tested by repeated compression and relaxation through over 250 cycles (Fig. 7a). Obviously, the water contact angle of the PDA/PDVB/sponge remained at 140° during the 250 compressions, while that of the PDVB/sponge without dopamine decreased from 130° to 93°, indicating that the mechanical stability of the modified sponge was significantly improved after adding dopamine. Under different pH values and salt concentrations, the adsorption capacity of the modified sponge remained unchanged (Fig. 7b, c). When the PDA/PDVB/sponge was immersed in ethanol for 3 days, its water contact angle remained basically unchanged, indicating its high chemical stability (Fig. 7d).
The PDA/PDVB/sponge can not only separate oil and water but also adsorb gaseous organics in air, which expands its application range. The adsorption tests were performed in static and dynamic environments, respectively. In a closed container, different organics were placed in the open beaker and the sponge as the adsorbent was placed in another open container. Then, the air in the closed container was evacuated, and the VOCs were adsorbed statically by the sponge adsorbent for 12 h. The adsorption capacity was obtained by weighing the weight changes before and after adsorption of VOCs. As shown in Fig. 8a, toluene, methanol and acetone were selected as probe molecules, and the static adsorption capacity of the PDA/PDVB/sponge for toluene, acetone and methanol reached 1.71, 1.87, and 1.30 g/g. In order to compare the adsorption performance, taking toluene as the target pollutant, PDVB and the primitive sponge were selected for comparison (Fig. 8b). The adsorption capacity of PDA is very small [42] so it was not tested. The static adsorption capacity of the PDA/PDVB/sponge was 1.4 and 19.1 times higher than that of PDVB and the primitive sponge, respectively. Moreover, for the common porous adsorbents, the PDA/PDVB/sponge possessed the highest adsorptive capacity which was 5.7, 5.4, 3.2 and 14.3 times higher than that of activated carbon, MCM-41, SBA-15 and ZSM-5, respectively. These results indicate that an efficient removal of VOCs pollutants is achieved on the PDA/PDVB/sponge.
In addition to adsorption activity, recyclability is another important factor that should be considered for most adsorbents. After the adsorption was completed, the entire sponge can be taken out for desorption in a vacuum environment at 105 °C. Figure 8c, d shows the cyclic adsorption-vacuum desorption data of the PDA/PDVB/sponge. The PDA/PDVB/sponge was subjected to adsorption-vacuum desorption for 10 times, and the adsorption capacity was maintained at 1.7 g/g each time. From the desorption efficiency, it can be found that the regeneration performance of the PDA/PDVB/sponge is excellent, reaching 100% almost each time. The results suggest that the reuse of the PDA/PDVB/sponge has high stability and reliability.
Figure 9a shows the toluene penetration curves of PDVB, the primitive sponge, and the modified sponge under dynamic conditions. The concentration of gaseous toluene was 40 ppm and the gas flow rate was 100 mL/min. Taking C/C0 = 5% as the penetration point, it can be found that the penetration time of the modified sponge was increased from 6 to 390 min, and the adsorption performance was greatly improved. The PDA/PDVB/sponge shows higher adsorption capacity than pure PDVB. Based on these results, this composite shows great potential application in atmospheric VOCs treatment.
Conclusions
In summary, a robust and low-cost PDA/PDVB/sponge was developed by a one-pot solution immersion method. With the help of polydopamine, the strong adhesion of PDVB to the original melamine sponge ensured the durable structural stability of the sponge. The successful integration of the PDVB and the melamine sponge can combine their superior properties into the PDA/PDVB/sponge, such as large surface area, good flexibility and low density of the melamine sponge and high hydrophobicity and affinity for organic matter. Due to its excellent properties, the prepared composites have superior oil/water separation properties, mechanical stability and recyclability. At the same time, the PDA/PDVB/sponge can also effectively adsorb VOCs in the atmospheric environment. Therefore, the PDA/PDVB/sponge with low cost and easy to manufacture shows great potential application value in solving the problems of oily wastewater and VOCs.
References
H. Zhu, S. Qiu, W. Jiang, D. Wu, C. Zhang, Environ. Sci. Technol. 45, 4527 (2011)
H. Adib, A. Raisi, B. Salari, Res. Chem. Intermed. 45, 5725 (2019)
M.A. Shannon, P.W. Bohn, M. Elimelech, J.G. Georgiadis, B.J. Marinas, A.M. Mayes, Nature 452, 301 (2008)
I.B. Ivshina, M.S. Kuyukina, A.V. Krivoruchko, A.A. Elkin, S.O. Makarov, C.J. Cunningham, T.A. Peshkur, R.M. Atlas, J.C. Philp, Environ. Sci. Process Impacts 17, 1201 (2015)
H. Adib, A. Raisi, Res. Chem. Intermed. 46, 3227 (2020)
S. Shi, M.S. Sadullah, M.A. Gondal, Y. Sui, S. Liu, Z.H. Yamani, K. Shen, Q. Xu, J. Mao, Res. Chem. Intermed. 41, 8019 (2015)
X. Zhang, Z. Li, K. Liu, L. Jiang, Adv. Funct. Mater 23, 2881 (2013)
R.K. Gupta, G.J. Dunderdale, M.W. England, A. Hozumi, J. Mater. Chem. A 5, 16025 (2017)
A. Bayat, S.F. Aghamiri, A. Moheb, G.R. Vakili-Nezhaad, Chem. Eng. Technol. 28, 1525 (2005)
Z. Wang, G. Liu, S. Huang, Angew. Chem. Int. Edn. 55, 14610 (2016)
Y.S. Lee, B.K. Kaang, N. Han, H.-J. Leeb, W.S. Choi, J. Mater. Chem. A 6, 16371 (2018)
S. Basak, J. Nanda, A. Banerjee, J. Mater. Chem. 22, 11658 (2012)
Q. Zhu, Q. Pan, F. Liu, J. Phys. Chem. C 115, 17464 (2011)
D.D. Nguyen, N.-H. Tai, S.-B. Leea, W.-S. Kuob, Energy Environ. Sci. 5, 7908 (2012)
L. Wu, L. Li, B. Li, J. Zhang, A. Wang, A.C.S. Appl, Mater. Interfaces 7, 4936 (2015)
X. Chen, J.A. Weibel, S.V. Garimella, Ind. Eng. Chem. Res. 55, 3596 (2016)
C. Ruan, K. Ai, X. Li, L. Lu, Angew. Chem. 126, 5556 (2014)
G. Jiang, R. Hu, X. Xi, X. Wang, R. Wang, J. Mater. Res. 28, 651 (2012)
H. Wang, E. Wang, Z. Liu, D. Gao, R. Yuan, L. Sun, Y. Zhu, J. Mater. Chem. A. 3, 266 (2015)
Y. Lu, W. Yuan, A.C.S. Appl, Mater. Interfaces 9, 29167 (2017)
Z. Cheng, C. Li, H. Lai, H.L.Y. Du, M. Liu, K. Sun, N.Z.L. Jin, N. Zhang, L. Jiang, Adv. Mater. Interfaces 3, 1600370 (2016)
N. Lv, X. Wang, S. Peng, L. Luo, R. Zhou, RSC Adv. 8, 30257 (2018)
B. Liu, L. Zhang, H. Wang, Z. Bian, Ind. Eng. Chem. Res. 56, 5795 (2017)
J. Gu, H. Fan, C. Li, J. Caro, H. Meng, Angew. Chem. 58, 5297 (2019)
N. Cao, B. Yang, A. Barras, S. Szunerits, R. Boukherroub, Chem. Eng. J. 307, 319 (2016)
Y. Zhang, S. Wei, F. Liu, Y. Du, S. Liu, Y. Ji, T. Yokoi, T. Tatsumi, F. Xiao, Nano Today 4, 135 (2009)
J. Yang, M.A.C. Stuart, M. Kamperman, Jack of all trades: versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 43, 8271 (2014)
J.H. Ryu, P.B. Messersmith, H. Lee, A.C.S. Appl, Mater. Interfaces 10, 7523 (2018)
B.H. Kim, D.H. Lee, J.Y. Kim, D.O. Shin, H.Y. Jeong, S. Hong, J.M. Yun, C.M. Koo, H. Lee, S.O. Kim, Adv. Mater. 23, 5618 (2011)
F. Guo, Q. Wen, Y. Peng, Z. Guo, J. Mater. Chem. A 5, 21866 (2017)
W.B. Neinhuis, Planta 202, 1 (1997)
R.D. Hazlett, J. Colloid Interface Sci. 137, 527 (1990)
N.A. Patankar, Transition between superhydrophobic states on rough surfaces. Langmuir 20, 7097 (2004)
J. Bico, C. Marzolin, D. Quéré, Europhys. Lett. 47, 743 (1999)
B. He, N.A. Patankar, J. Lee, Langmuir 19, 4999 (2003)
S.R. Churipard, K.S. Kanakikodi, D.A. Rambhia, C.S.K. Raju, A.B. Halgeri, N.V. Choudary, G.S. Ganesh, R. Ravishankar, S.P. Maradur, Chem. Eng. J. 380, 122481 (2020)
S. Qiu, B. Jiang, X. Zheng, J. Zheng, C. Zhu, M. Wu, Carbon 84, 551 (2015)
J. Zhao, M. Wang, K.K. Gleason, Adv. Mater. Interfaces 4, 1700270 (2017)
C.D. Petruczok, R. Yang, K.K. Gleason, Macromolecules 46, 1832 (2013)
Q. Pan, J. Liu, Q. Zhu, A.C.S. Appl, Mater. Interfaces 2, 2026 (2010)
A.B.D. Cassie, S. Baxter, Faraday Soc 40, 546 (1944)
Y. Liu, K. Ai, L. Lu, Chem. Rev. 114, 5057 (2014)
Acknowledgements
This work was supported by Scientific Research Project of Guangzhou City (201804020026) and National Natural Science Foundation of China (21777047).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, J., Zhang, J., Zhang, X. et al. Durable multifunctional superhydrophobic sponge for oil/water separation and adsorption of volatile organic compounds. Res Chem Intermed 46, 4297–4309 (2020). https://doi.org/10.1007/s11164-020-04207-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04207-7