Abstract
The excess emission of greenhouse gases (GHGs) such as CO2 and CH4 is posing an acute threat to the environment, and efficient ways are being sought to utilize GHGs to produce syngas (H2, CO) and lighter hydrocarbons (HCs). In this study, the dry reforming of methane (DRM) has been carried out at 700 °C using La2O3 co-supported Ni/MgAl2O4 nano-catalyst in a fixed bed thermal reactor. The catalyst is characterized using various techniques such as XRD, FESEM, EDX-mapping, CO2-TPD, H2-TPR and TGA. The modified MgAl2O4 shows the flake type structure after the addition of La2O3. The TPR and TPD analysis shows the highly dispersed metal and strong basic nature of the catalyst consequently enhances the conversion of CO2 and CH4. The highest conversion for CH4 is 87.3% while CO2 conversion is nearly 89.5% in 20 h of operation time. The selectivity of H2 and CO approached 50% making the H2/CO ratio above unity. In the longer time-on-stream (TOS) test, the catalyst shows elevated potential for longer runs showcasing better catalytic activity. The stability of the catalyst is indicated via a proposed reaction mechanism for DRM in operating conditions. Moreover, TGA indicates the lower weight loss of spent catalyst which ascribed the lower formation of carbon during TOS 20 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dry reforming of methane (DRM) (Eq. 1) is an important technique which has been used to convert the greenhouse gases such as CO2 and CH4 to syngas (H2, CO) and lighter hydrocarbons (C2-C3) [1]. The syngas can be used as feedstock for the well-known Fischer–Tropsch (FT) synthesis process in gas-to-liquid fuel technology [2]. The CH4 and CO2 have been successfully converted into fuels via DRM [3], however, the carbon deposition is a major concern identified in the recent studies [4, 5]. Mainly, the carbon is formed via two famous reactions which are methane cracking (Eq. 2) and Boudouard reaction (Eq. 3) [6]. Another issue is the H2/CO ratio, which is usually less than unity due to the reverse water gas shift reaction (RWGS) (Eq. 4) [7, 8].
The aim of developing an efficient and stable catalyst is to improve the conversion efficiency, stability and H2/CO ratio with lower carbon formation [9]. Various catalyst systems were employed to improve the conversion efficiency. The Ni-based catalyst with various supports such as Al2O3 [10], MgAl2O4 [11], SiO2 [12], Mesoporous silica molecular sieves (MCM) [13], MgO [14], zeolites [15] and ZrO2 [16] were reported for DRM [17]. Furthermore, co-supported catalysts were also extensively studied with multiple objectives such as CO2 adsorption and metal dispersion [14, 18]. The noble metals demonstrate high catalytic activity as well as inhibit the coke formation reported in the literature. However, the high cost makes them less suitable for the commercialization of the DRM process, extensively compiled by Pakhare & Spivey [19].
The Ni-based catalysts need further improvements to make them more viable for the DRM as the carbon growth on Ni sites is the most common issue with the single-supported catalyst. Recently, the co-support system with Ni as active metal is seeking remarkable attention such as Al2O3-MgO [20]. To achieve the communal effect of both Al and Mg, the Ni/MgAl2O4 spinel has been synthesized for DRM, and it resulted in a stable and enhanced performance than Ni/Al2O3. However, issues such as carbon formation and H2/CO ratio were not resolved [21, 22]. The catalyst performance in the DRM also depends on the physicochemical properties of the material. The physicochemical properties of catalyst can be modified by the preparation techniques and by introducing a co-support which will improve the basicity and metal dispersion on the catalyst support [23,24,25]. The basic nature of the catalyst is also important for carbon deposition and deactivation of the catalytic performance [26, 27]. Ni interface with MgAl2O4 is improved by the communal effect of MgAl2O4 and La2O3 as mixed matrix support [28]. In thermo-catalytic DRM, the combined effect of La2O3 and MgAl2O4 catalyst is barely reported [29].
Herein, we investigate the Ni/La-Mg catalyst for high-temperature DRM to analyse the catalytic activity, products distribution and stability during the long-term continuous operation. We prepared the mixed-matrix support nano-catalyst of 10 wt.% Ni/La2O3-MgAl2O4 and tested for DRM in a fixed bed thermal reactor. The catalyst material was characterized by X-ray diffraction (XRD), field emission electron microscopy (FESEM), H2 temperature-programmed reduction (H2-TPR), CO2 temperature-programmed desorption (CO2-TPD), N2 adsorption–desorption (BET) EDX-mapping and TGA. Finally, a probable reaction mechanism was proposed based on product distribution.
Materials and methods
Material synthesis and characterization
MgAl2O4 and La2O3 spinal was prepared by modifying the co-precipitation method followed by hydrothermal process reported elsewhere [30]. For MgAl2O4 the respective nitrate salts were added to ammonia solution with a ratio of 2:1 (Al: Mg). The required quantities of citric acid and dimethylformamide (DMF) were added to improve the metal dispersion and better crystal growth. The solution was kept at 160 °C for 24 h in an autoclave for the hydrothermal process. The slurry was then washed several times using ethanol and DI water, and the samples were dried in an oven. The same method is repeated for the synthesis of La2O3. For co-support, MgAl2O4 and La2O3 were taken as 4:1 (wt. ratio) as co-support and prepared by microemulsion technique [31]. The 10% wt. Ni as the active metal was impregnated by the modified incipient wetness impregnation method [32]. The catalyst was then calcined at 700 °C for 3 h in a muffle furnace. The material was characterized by XRD, FESEM, H2-TPR, CO2-TPD, EDX-mapping, BET and TGA. The characterization methods and equipment details were reported elsewhere [32]. The crystallography and morphology of the catalyst were analysed by XRD, FESEM and BET. The crystallite size was calculated using the Scherrer equation [32, 33]. The metal-support interaction and basicity of the materials were analysed by TPR and TPD, and TGA was used to explain the thermal stability of the synthesized material.
Experimental setup and calculations
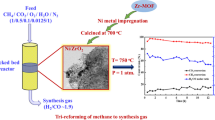
The experimental setup for the fixed-bed thermal reactor is presented in Fig. 1. Briefly, the feed gases CH4 (99.99%) and CO2 (99.99%) controlled by a mass flow controller (ALICAT) were provided to the fixed bed reactor. The fixed-bed reactor consists of a vertical furnace (Carbolite UK) integrated with a thermocouple. The catalyst is loaded in alumina tube (10 mm inner diameter) with the help of quartz wool. A condenser and silica-bed are used to separate the liquid and gaseous products, respectively, before sending the syngas to the gas analysis systems. The products gases were analysed using an online gas chromatograph (GC) (Agilent 6890 N) equipped with a thermal conductivity detector (TCD) and flame ionization detector (FID) [29].
The catalytic activity tests were conducted to analyse the performance of the DRM catalyst in a fixed-bed thermal reactor. The reactant conversion (X) and selectivity (S) were calculated according to Eqs. (5–9). When n represents the number of moles of the individual species. The experiments were repeated twice to determine the relative error.
Results and discussion
Physicochemical properties of the material
The crystallographic structure of the prepared catalysts was analysed by X-ray diffraction (XRD) as shown in Fig. 2a. The X-ray diffraction peaks for MgAl2O4 (PDF#72–6947) were analysed, and the cubical phase (hkl; 311) was confirmed at 38.5° with a space group of 227: Fd3m [24, 34]. The average crystallite size for MgAl2O4 was 10.5 nm. Similarly, La2O3 (71–5408) was detected in XRD analysis with a major peak at 30.3° (hkl;011) with an average crystallite size of 9.7 nm [35]. The NiO (PDF#44–1159) with major phase (101) was confirmed at 37.5° having an average crystallite size of 10.0 nm [32]. The major peak of NiAl2O4 (PDF #10–0339) was detected at 37.09° (311) with a crystallite size of 14.3 nm [36] and LaNiO3 peak (PDF #33–0710) at 23.08° (100) while the crystallite size is 13.8 [37] with rhombohedral structure.
The reduction behaviour of the developed DRM catalyst was analysed using H2-TPR technique depicted in Fig. 2b for the La2O3 co-supported Ni/MgAl2O4 calcined at 700 °C. The TPR shows the first major peak at 552 °C with the H2-uptake of 249.3 μmol g−1 while the second major peak was detected at 695 °C showing H2-uptake of 596.6 μmol g−1. The total H2-uptake of ~ 850 μmol g−1 which shows the 91% degree of reduction [38]. The higher H2 uptake at elevated temperature shows the good interaction of Ni/La2O3-MgAl2O4.From the intense TPR peak at 695 °C, it is evident that NiO has been reduced into Ni [39].
The basicity of MgAl2O4 and Ni/La2O3-MgAl2O4 is investigated using CO2-TPD and presented in Fig. 3 and Table 1. Three different peaks present at various regions from weak to strong basic nature of the prepared catalyst. The first two peaks for MgAl2O4 at 87 °C and 291 °C with CO2 uptake of 347 μmol g−1 and 122 μmol g−1, respectively, and indicating the weak basic sites [11]. While for Ni/ La2O3-MgAl2O4 sample, the first two peaks were detected at 95 °C and 361 °C with CO2 uptake of 206 μmol g−1 and 110 μmol g−1, ascribed to the weak and medium basic sites, respectively. The distant peak shift from weak region to the medium is due to the addition of La2O3. The strong basic sites for MgAl2O4 were detected at 542 °C having the CO2 uptake of 463 μmol g−1 [11]. In contrast, for Ni/La2O3-MgAl2O4 sample, the strong basic sites were present at 681 °C with the CO2 uptake of 683 μmol g−1. The higher CO2 uptake at the elevated temperature depicted the strong basic nature of the catalyst which is suitable for the DRM process and it is expected to exhibit better coke resistance during the long-term TOS tests [40].
The morphology of the prepared DRM catalysts was analysed by FESEM and depicted in Fig. 4. The MgAl2O4 sample shows agglomerated porous structure presented in Fig. 4a and La2O3 shows a uniform web-like structure is evident in the modified preparation method using DMF as a surfactant which also assists in the uniform crystal growth (Fig. 4b). La2O3 co-supported Ni/MgAl2O4 sample resulted in a nanoflake-type structure depicted in Fig. 4c. The irregular structure of MgAl2O4 is modified by web-like La2O3 infusing with the Ni particles. Furthermore, the thermal stability of the La2O3 co-supported Ni/MgAl2O4 presented in Fig. 4d. The total weight loss is less than 3.5% in the temperature range of 125 °C is ascribed to the removal of moisture [41].
The N2 adsorption–desorption isotherms, surface area (SBET), average pore volume (Vpore) and average pore radius is presented in Fig. 5. The samples exhibited type IV isotherm confirming the formation of mesoporous structure material for all the synthesized samples. The surface area of MgAl2O4 and La2O3 was 102 m2 g−1and 41.3 m2 g−1, respectively. While the addition of Ni into La2O3-MgAl2O4 reduces the surface area to 90 m2 g−1. It is ascribed to the infusion of Ni particle on the surface of the catalyst support. The average pore radius of the MgAl2O4, La2O3 and Ni/La2O3-MgAl2O4 is 8.6 nm, 5.7 nm and 8.1 nm, respectively.
Catalyst performance analysis
The DRM activity test has been carried outing using the developed catalyst shown in Fig. 6. At first, the DRM was carried without a catalyst which shows very low catalytic activity. The conversion of CH4 and CO2 is just below 10% at 700 °C. The selectivity of H2 and CO is found to be less than 6%. In contrast, adding MgAl2O4 catalyst in the fixed bed, the increment in the conversion and selectivity of the product is observed. The conversion of CH4 and CO2 is 35% and 32%, respectively. The selectivity H2, CO and C2H6 is less than 12%, 15% and 3.5% respectively. The Ni impregnation in MgAl2O4 further improves the catalytic activity with the increase in the conversion of CH4 and CO2 to 65 and 63%. Ni /MgAl2O4 also substantially improves the selectivity of H2, CO and C2H6 to 32%, 35% and 7.5% as depicted in Fig. 6. Whereas, incorporating 20% La2O3 as co-support into 10%Ni/MgAl2O4 enhances the conversion of CH4 and CO2 to 87.3% and 89.5%, respectively. The selectivity of the H2 improves from 32 to 51% for the composite La2O3 co-supported Ni/MgAl2O4 catalyst. CO selectivity is recorded 46% which is less than the selectivity of H2. In contrast, C2H6 selectivity decrease to 4.5% in the La2O3 co-supported Ni/MgAl2O4. This might be due to the higher yield and inhibition of methyl radical recombination [23].
The overall catalytic activity of the reported samples is in such order: MgAl2O4 < Ni/MgAl2O4 < La2O3 co-supported Ni/MgAl2O4. The non-co-supported catalyst activity is lower than that of Co-supported catalyst, which ascribes the occurrence of reverse water gas shift (RWGS) reaction is limited [23]. The La2O3 co-supported catalyst resists the progress of RWGS and H2 selectivity is improved. Furthermore, the possible formation of La2O2CO3 inhibit the carbon formation and improves the catalyst activity [23]. The improvement in the CH4 conversion is due to the good formation of active sites as depicted in H2-TPR results. The bulk formation of active sites activates CH4 and resist the CxHx recombination by a further breakdown.
The comparison with the literature is drawn in Table 2 for the reference. As we can see that the majority of the reports shows the H2/CO ratio below 1.0 except for Ni/h-BNNs catalyst reported by [42]. The higher H2/CO ratio depicts the CH4 decomposition or Boudouard reaction. These reactions usually occur on Ni active sites and block them, however, if α-C is formed, it can be easily gasified or reacts with La2O3 to form La2O2CO3[23, 42]. The addition of La2O3 also assists the chemisorption of CO2 and regeneration of active metal and La2O3 along with major support MgAl2O4 [23, 24].
Catalyst stability and reaction mechanism
The catalyst stability is one of the most important parameters after the fundamental catalytic performance. Herein, the La2O3 co-supported Ni/MgAl2O4 was tested for 20 h of time on stream (TOS) test keeping the process parameters constant. The conversion of CH4 is shown in Fig. 7a, a stable trend having only 03% reduction in 20 h of TOS observed. Similarly, CO2 also shows the same trend in the same TOS and experimental conditions. The stable conversion in the reported TOS for both reactants is encouraging for the reported catalyst. The selectivity of the H2 and CO partly declined during the 20 h TOS presented in Fig. 7b. The selectivity of the C2H6 is slightly higher in the 20 h TOS. Figure 8 shows that the H2/CO ratio is above unity during the 20 h TOS indicating the low carbon formation over the catalyst and enhanced stability.
The conversion stability of the developed catalyst is associated with better metal-support interaction (MSI high active sites and high basicity due to the addition of La2O3. It also supports methane activation as well as CO2 adsorption due to its basic nature [46]. The CH4 activation is due to the Ni and formed Ni-C and 2H2 (Eq. 10). The formation of La2O2CO3 intermediate carbonate during the adsorption of CO2 (Eq. 11) and after reaction with C-Ni to regenerate the La2O3, Ni and CO resist the carbon deposition on the catalyst surface (Eq. 12) [47]. The schematic representation of the reaction mechanism is proposed in Fig. 9.
Characterization of the spent catalyst
After 20 h of TOS, the spent catalyst has been characterized by EDX mapping and TGA. Figure 10a presented elemental mapping shows the formation of carbon over the surface of the catalyst. The mapping indicates the formation of carbon is not in the bulk form (Fig. 10b). The inset FESEM shows the formation of carbon nanofibres on the surface of the spent catalyst. The carbon nanofibres can easily reduce in the gasification process and formed CO or CO2.
The TGA profile of the spent La2O3 co-supported Ni/MgAl2O4 after 20 h of TOS shows the total weight loss of 9–10%, which confirms the formation of a lower amount of carbon. The moisture and volatile matter removal are at 200 °C [11, 48] presented in Fig. 11. The weight loss in between 200–500 °C is associated with the fibrous carbon which is referred as β-carbon, as well as the decomposition of La-hydroxide intermediate phase which is usually formed under the moist conditions due to RWGS [29]. The total of 3% weight loss between 500–900 °C (Column III-IV) which confirm the low formation of carbon after 20 h TOS. This reduction is also ascribed to the dissociation of La2O2CO3 [49]. The carbon formed above 700 °C is ascribed to filamentous carbon (γ-C).
Conclusions
The synthesized La2O3 co-supported Ni/MgAl2O4 has been characterized using various techniques and employed for the DRM in thermal fixed bed reactor. The catalyst shows enhanced performance and higher H2/CO ratio, much suitable feedstock for downstream chemicals. The enhanced performance is ascribed to the suitable physicochemical properties of a catalyst such as metal-support interaction and the strong basic nature with well-structured morphology. While testing for longer runs, the catalyst shows stability for 20 h with less than 3% declined in the DRM activity and TGA of spent catalyst confirms the lower formation of carbon. This stability suggests the potential of the upgradation of the developed catalyst for the DRM process for industrial-scale production of syngas.
Authors Contibution
Asif Hussain Khoja and Nor Aishah Saidina Amin develop the conceptualization of the work and drafted the manuscript. Arslan Mazhar assisted in material synthesis and in conducting the experiments. Mustafa Anwar and Sehar Shakir workout the material characterization analysis. Muhammad Taqi Mehran assisting in the result analysis of GC, drafting and revising the manuscript.
References
J.A. Frankel, Greenhouse Gas Emissions (Brookings Institution, Washington, D.C, 1999)
A. Rafiee, K. Rajab Khalilpour, D. Milani, M. Panahi, J. Environ. Chem. Eng. 6(5), (2018)
R. Dębek, K. Zubek, M. Motak, P. Da Costa, T. Grzybek, Res. Chem. Intermed. 41(12), (2015)
A.H. Khoja, M. Tahir, N.A.S. Amin, Energy Convers. Manag. 183, (2019)
M.A. Gerber, Review of Novel Catalysts for Biomass Tar Cracking and Methane Reforming (Pacific Northwest National Laboratory Richland, WA, USA, 2007)
Y.H. Hu, E. Ruckenstein, Adv. Catal. 48(49), (2004)
B. Abdullah, N.A.A. Ghani, D.V.N. Vo, J. Clean. Prod. 162, (2017)
E. Horvath, K. Baan, E. Varga, A. Oszko, A. Vago, M. Toro, A. Erdohelyi, Catal. Today 281, (2017)
M. Usman, W.M.A.W. Daud, H.F. Abbas, Renew. Sustain. Energy Rev. 45, (2015)
K. Selvarajah, N.H.H. Phuc, B. Abdullah, F. Alenazey, D.-V.N. Vo, Res. Chem. Intermed. 42(1), (2016)
I.H. Son, S. Kwon, J.H. Park, S.J. Lee, Nano Energy 19, Supplement C (2016)
S. Tomiyama, R. Takahashi, S. Sato, T. Sodesawa, S. Yoshida, Appl. Catal. A Gen. 241(1–2), (2003)
H. Arbag, S. Yasyerli, N. Yasyerli, G. Dogu, Int. J. Hydrog. Energy 35(6), (2010)
Y.J.O. Asencios, E.M. Assaf, Fuel Process. Technol. 106, (2013)
P. Frontera, A. Aloise, A. Macario, F. Crea, P.L. Antonucci, G. Giordano, J.B. Nagy, Res. Chem. Intermed. 37(2), (2011)
N. Rahemi, M. Haghighi, A.A. Babaluo, M.F. Jafari, P. Estifaee, J. Ind. Eng. Chem. 19(5), (2013)
V. Sadykov, V. Rogov, E. Ermakova, D. Arendarsky, N. Mezentseva, G. Alikina, N. Sazonova, A. Bobin, S. Pavlova, Y. Schuurman C. Mirodatos, Thermochim. Acta 567, (2013)
S.H. Zeng, L. Zhang, X.H. Zhang, Y. Wang, H. Pan, H.Q. Su, Int. J. Hydrog. Energy 37(13), (2012)
D. Pakhare, J. Spivey, Chem. Soc. Rev. 43(22), (2014)
L. Zhang, Q. Zhang, Y. Liu, Y. Zhang, Appl. Surf. Sci. 389, Supplement C (2016)
S. Dash, R.K. Sahoo, A. Das, S. Bajpai, D. Debasish, S.K. Singh, J. Alloys Compd. 726, Supplement C (2017)
H.-J. Kim, E.-H. Yang, Y.S. Noh, G.H. Hong, J.I. Park, S.A. Shin, K.-Y. Lee, D.J. Moon, Res. Chem. Intermed. 44(2), (2017)
X.Y. Li, D. Li, H. Tian, L. Zeng, Z.J. Zhao, J.L. Gong, Appl. Catal. B-Environ. 202, (2017)
A.S. Al-Fatesh, M.A. Naeem, A.H. Fakeeha, A.E. Abasaeed, Chin. J. Chem. Eng. 22(1), (2014)
M.A. Uzair, A. Waqas, A.H. Khoja, N. Ahmed, Energy Source Part A 38(24), (2016)
A.H. Khoja, M. Tahir, N.A.S. Amin, A. Javed, M.T. Mehran, Int. J. Hydrog. Energy 45(22), (2020)
R. Pereñiguez, V.M. Gonzalez-delaCruz, A. Caballero, J.P. Holgado, Appl. Catal. B: Environ. 123, (2012)
D.X. Li, P. Pirouz, A.H. Heuer, S. Yadavalli, C.P. Flynn, Philos. Mag. A 65(2), (1992)
A.H. Khoja, M. Tahir, N.A.S. Amin, Energy Convers. Manag. 144, (2017)
A. Samad, K.Y. Lau, I.A. Khan, A.H. Khoja, M.M. Jaffar, M. Tahir, J. Phys. Chem. Solids 120, (2018)
M. Usman, W.M.A.W. Daud, RSC Adv. 6(44), (2016)
A.H. Khoja, M. Tahir, N.A.S. Amin, Fuel Process. Technol. 178, (2018)
A.H. Khoja, M. Tahir, N.A. Saidina Amin, Energy Fuels 33(11), (2019)
S. Sokolov, E.V. Kondratenko, M.M. Pohl, A. Barkschat, U. Rodemerck, Appl. Catal. B-Environ. 113, (2012)
D. Ağaoğulları, İ. Duman, M.L. Öveçoğlu, Ceramics Int. 38(8), (2012)
N. Sahli, C. Petit, A.C. Roger, A. Kiennemann, S. Libs, M.M. Bettahar, Catal. Today 113(3), (2006)
X. Song, X. Dong, S. Yin, M. Wang, M. Li, H. Wang, Appl. Catal. A Gen. 526, (2016)
A. Bordoloi, S. Das, R. Goyal, R.K. Singha, C.R. Pendem, S.K.L. Narayan, R. Bal, V.V.D.N. Prasad, N.N. Botcha, M. Kumar, (Google Patents, 2017)
J. Shen, A.A.C. Reule, N. Semagina, Int. J. Hydrog. Energy 44(10), (2019)
S. Das, M. Sengupta, J. Patel, A. Bordoloi, Appl. Catal. a-Gen. 545, Supplement C (2017)
J. Guo, H. Lou, H. Zhao, X. Zheng, React. Kinet. Catal. Lett. 84(1), (2005)
Y. Cao, P. Maitarad, M. Gao, T. Taketsugu, H.R. Li, T.T. Yan, L.Y. Shi, D.S. Zhang, Appl. Catal. B-Environ. 238, (2018)
R.O. da Fonseca, R.C. Rabelo-Neto, R.C.C. Simões, L.V. Mattos, F.B. Noronha, Int. J. Hydrog. Energy 45(8), (2020)
U. Guharoy, E. Le Sache, Q. Cai, T.R. Reina, S. Gu, J. CO2 Util. 27, (2018)
T. Zhang, Z. Liu, Y.-A. Zhu, Z. Liu, Z. Sui, K. Zhu, X. Zhou, Appl. Catal. B: Environ. 264, (2020)
L. Zhang, X. Wang, C. Chen, X. Zou, X. Shang, W. Ding, X. Lu, RSC Adv. 7(53), (2017)
J.K. Xu, W. Zhou, J.H. Wang, Z.J. Li, J.X. Ma, Chin. J. Catal. 30(11), (2009)
C.C. Chong, Y.W. Cheng, H.D. Setiabudi, N. Ainirazali, D.-V.N. Vo, B. Abdullah, Int. J. Hydrog. Energy 45(15), (2020)
E.P. Komarala, I. Komissarov, B.A. Rosen, Catalysts 10(1), (2019)
Acknowledgements
The authors would like to extend their deepest appreciation to the Universiti Teknologi Malaysia for the financial support of this research under RUG (Research University Grant, Vot13H35) and FRGS-MRSA grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khoja, A.H., Anwar, M., Shakir, S. et al. Thermal dry reforming of methane over La2O3 co-supported Ni/MgAl2O4 catalyst for hydrogen-rich syngas production. Res Chem Intermed 46, 3817–3833 (2020). https://doi.org/10.1007/s11164-020-04174-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04174-z