Abstract
In this study, an efficient and novel procedure has been reported for loading sulfanilic acid on the surface of magnetite Fe3O4 nanoparticles using tris(hydroxymethyl) aminomethane and 1,2-dichloroethane. Next, the synthesized nanocatalyst was fully characterized using FT-IR, XRD, TGA, VSM, SEM, and TEM. The results show that the synthesis of magnetic nanocatalyst has been successful with a range of 2–20 nm in size. Finally, the catalytic activity of this superparamagnetic nanocatalyst was explored for the synthesis of tetrahydrobenzo[b]pyran and 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives. The synthesized nanocatalyst has advantages such as non-toxicity, short reaction time, easy workup, cleaner reaction profiles under mild reaction conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, catalysis has received considerable attention because it has represented a new way to meet the challenges of sustainability and energy. Also, employed catalysts are often very expensive. Recovery and reusability are two noteworthy advantages of catalysts that make them economic in most of the modern catalytic processes [1,2,3,4,5,6]. Supported heterogeneous metal catalysts are widely used in industry such as environmental catalysis [7,8,9,10], electrocatalysis [11, 12], and organocatalysis [13, 14]. Also, they have been widely reported such as Fe@NC@Pd, CNT/PdFe/NC, and RGO@AC/Pd [15,16,17]. Among the various heterogeneous metal catalysts, magnetic nanoparticles (MNPs) received considerable attention due to their recyclable and localizing properties [18,19,20,21]. Magnetic nanoparticles (especially superparamagnetic Fe3O4 nanoparticles) have been widely employed in various applications in bioengineering and biomedical fields, such as separation of biomedical products, sorting and labeling of cells, magnetic resonance imaging (MRI), as well as cancer thermotherapy [22,23,24]. The most commonly applied MNPs in medical applications include maghemite (γ-Fe2O3), cobalt ferrite (Fe2CoO4), iron oxide (Fe3O4), and chromium dioxide (CrO2) [25]. The coating of Fe3O4 nanoparticles (NPs) prevents oxidation and aggregation and improves their chemical stability [26]. Due to significant characteristics of THAM such as non-corrosive, inert, and biodegradable [27], it can appear as a good coating agent for Fe3O4 NPs. THAM was widely applied in medicine, physiology, biology, and biochemistry, as a buffering agent capable of holding a pH in the physiological pH range [28]. Also, THAM has been used in the production of drugs as an excipient [27].
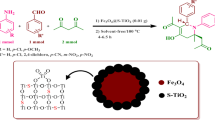
To continue the development of synthesis of MNPs as the green catalyst for the production of pharmaceutical compounds in one-pot synthesis, we wish to propose a novel magnetic nanocatalyst containing sulfuric acid-functionalized. Then, the synthesized nanocatalyst was used as a recyclable catalyst for one-pot, three-component synthesis of tetrahydrobenzo[b]pyran from reaction among aldehyde 1, malononitrile 2, and dimedone 3, and also efficient one-pot, three-component method for synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile using aldehyde 1 malononitrile 2, and 4-hydroxycoumarin 5 (Scheme 1).
Experimental
General
Melting points and FT-IR spectra of compounds were measured with an Electrothermal 9100 apparatus. The 1H NMR spectra were obtained on BRUKER DRX 300 and 400 MHz, using CDCl3 as a solvent. Ferrous chloride tetrahydrate (FeCl2·4H2O) and ferric chloride hexahydrate (FeCl3·6H2O) were obtained from Aldrich. Other reagents and solvents were obtained from Aldrich and Merck and were used without further purification.
Synthesis of the magnetic Fe3O4 nanoparticles (MNPs)
Fe3O4 nanoparticles were prepared by chemical coprecipitation method. First, FeCl3·6H2O (2.703 g, 10.0 mmol) and FeCl2·4H2O (0.994 g, 5.0 mmol) were dissolved in 100 mL deionized water under N2 atmosphere. Then, concentrated ammonia (25%) was immediately added to the mixture until the pH rise to 11 and a black suspension was formed. Then, the mixture was stirred strongly for 1 h at r.t. The resulting black precipitate was isolated and washed with distilled anhydrous ethanol and diethyl ether (several times) and finally dried in oven at 80 °C for 2 h.
Synthesis of magnetite nanoparticles coated by tris(hydroxymethyl) aminomethane (Fe3O4@THAM)
Fe3O4 nanoparticles (1.0 g) were homogeneously dispersed in a mixture of deionized water and ethanol by ratio (40:60 ml) by ultrasonic bath for 30 min. Then, tris(hydroxymethyl) aminomethane [H2N–C–(CH2OH)3] (2.0 g, 16.5 mmol) was added, and the resulting mixture was regularly stirred under reflux condition and N2 atmosphere for 24 h. Finally, the resulting reaction mixture was cooled to room temperature and separated by an external magnet, washed several times with water and dried at 80 °C to give Fe3O4@tris(hydroxymethyl) aminomethane.
Synthesis of Fe3O4@THAM–CH2CH2–Cl
The Fe3O4@tris(hydroxymethyl) aminomethane (1.0 g) was dispersed in CH3CN (50 mL) using an ultrasonic bath for 15 min. Subsequently, dichloroethane (1 mL, 12.63 mmol) and triethylamine (0.1 mL) were added dropwise to the reaction mixture. The resulting mixture was regularly stirred under reflux condition and N2 atmosphere for 24 h. Finally, it was cooled to room temperature and washed with water/ethanol and dried at 80 °C.
Sulfanilic acid-functionalized tris(hydroxymethyl) aminomethane-coated magnetite nanoparticles (MNPs–PhSO3H)
The Fe3O4@THAM–CH2CH2–Cl (1.0 g) was dispersed in dry toluene (50 mL) using an ultrasonic bath for 30 min. Then, sulfanilic acid (2.0 g, 11.5 mmol) and triethylamine (1.6 mL, 11.5 mmol) as a proton scavenger were added. The resulting mixture was mechanically stirred under reflux condition and N2 atmosphere for 48 h. Finally, it was cooled to room temperature and was washed with dry toluene, dichloromethane, and ethanol and dried at 80 °C for 8 h.
General procedure for the synthesis of tetrahydrobenzo[b]pyran derivatives
MNPs–PhSO3H (0.01 g) was added to a mixture of dimedone (1.0 mmol), aromatic aldehydes (1.0 mmol) and malononitrile (1.0 mmol) in EtOH/H2O (1:1). The mixture was stirred at 100 °C for the appropriate time. After the completion of the reaction, as monitored with TLC, the resulting mixture was diluted with hot THF (10 mL), and the catalyst was easily separated by an external magnet and the crude products were recrystallized in ethanol to afford pure tetrahydrobenzo[b]pyran.
General procedure for the synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives
MNPs–PhSO3H (0.01 g) was added to a mixture of 4-hydroxycoumarin (1.0 mmol), aromatic aldehydes (1.0 mmol), and malononitrile (1.0 mmol) in EtOH/H2O (1:1). The mixture was stirred at 70 °C for the appropriate time. After the completion of the reaction, as monitored with TLC, the resulting mixture was diluted with hot THF (10 mL) and the catalyst was easily separated by an external magnet and the crude products were recrystallized in ethanol to afford pure 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile.
Characterization data of selected compounds
2-amino-5,6,7,8-tetrahydro-7,7-dimethyl-5-oxo-4-phenyl-4H-chromene-3-carbonitrile (4a)
M.p. 228–230 °C; 1H NMR (400 MHz, CDCl3) δ = 1.07 (s, 3H, CH3), 1.14 (s, 3H, CH3), 2.26 (dd, 2H, J = 22 Hz, J = 16.4 Hz), 2.49 (s, 2H, CH2), 4.44 (s, 1H, CH), 4.55 (s, 2H, NH2), 7.21-7.34 (m, 5H).
2-amino-5,6,7,8-tetrahydro-4-(4-nitrophenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (4b)
M.p. 179–180 °C; 1H NMR (300 MHz, DMSO-d6): δ = 0.95 (s, 3H, CH3), 1.027 (s, 3H, CH3), 2.11 (d, 1H, J = 16.0), 2.26 (d, 1H, J = 16.0), 2.51 (d, 2H J = 10.0), 4.36 (s, 1H, CH), 7.19 (s, 2H, NH2), 7.44 (d, 2H, Ar, J = 8.7), 8.17 (d,2H, Ar, J = 8.7).
2-amino-5,6,7,8-tetrahydro-4-(4-methoxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (4i)
M.p. 202–204 °C; IR (KBr, ν, cm−1): 3465, 3320, 2955, 2190, 1676, 1247. 1H NMR (300 MHz, DMSO-d6): δ = 0.96 (s, 3H, CH3), 1.04 (s, 3H, CH3), 2.10 (d, 1H, J = 16.0), 2.25 (d, 1H, J = 16.0), 3.37 (d, 2H, J = 7), 3.72 (s, 3H, OCH3), 4.13(s, 1H, CH), 6.85 (d, 2H, Ar, J = 8.7 Hz), 6.98 (br, NH2), 7.06 (d, 2H, Ar, J = 8.4 Hz).
2-amino-5,6,7,8-tetrahydro-4-(4-hydroxyphenyl)-7,7-dimethyl-5-oxo-4H-chromene-3-carbonitrile (4f)
M.p. 268–270 °C; IR (KBr, ν, cm−1): 3285, 3160, 2960, 2185, 1675, 1209; 1H NMR (400 MHz, CDCl3): δ = 1.05 (s, 3H, CH3), 1.12 (s, 3H, CH3), 2.25 (dd, 2H J = 16.0 Hz, J = 20.0 Hz), 2.46 (dd, 2H, J = 16.0 Hz, J = 20.0 Hz), 4.35 (s, 1H, CH), 4.61(s, 2H, NH2), 6.05 (s, 1H, OH), 6.67–7.28 (m, 4H, Ar).
2-Amino-4,5-dihydro-4-(3-nitrophenyl)-5-oxopyrano[3,2-c]chromene-3-carbonitrile (6d)
M.p. 261-263 °C; IR (KBr, ν, cm−1): 3402, 3322, 2202, 1703, 1670, 1606, 1538, 1380. 1H NMR (400 MHz, DMSO-d6): δ = 4.75 (s, 1H, CH), 7.48-8.16 (m, 9H, Ar, NH2).
Results and discussion
Preparation and characterization of the catalyst
For the first time, we prepared the heterogeneous nanocatalyst (MNPs–PhSO3H) by following the method shown in Scheme 2. The magnetic Fe3O4 nanoparticles were simply synthesized by chemical coprecipitation procedure. Then, sulfanilic acid was coated on the surface of magnetite nanoparticles using tris(hydroxymethyl) aminomethane and 1,2-dichloroethane. The prepared nanocatalyst was fully characterized by elemental, Fourier transform infrared (FT-IR), X-ray diffraction (XRD), thermogravimetric (TGA), vibrating sample magnetometer (VSM), field emission scanning electron microscopy (FE-SEM), X-ray spectroscopy (EDS), and TEM analyses. The elemental analysis shows that nitrogen, hydrogen, and carbon contents of Fe3O4@THAM were 0.23, 0.6, and 1.27 (wt %), respectively. This analysis confirms the loading of THAM on MNPs.
FT–IR
The FT–IR spectra of the Fe3O4 NPs, Fe3O4@THAM–CH2CH2–Cl, and the MNPs–PhSO3H are shown in Fig. 1. The absorption peaks at around 582 and 631 cm−1 were attributed to Fe–O vibration bonds of uncoated Fe3O4 nanoparticles. Also, the peaks at 3374 and 1618 cm−1 can be attributed to the asymmetric stretching vibrations of O–H group and the surface-adsorbed water of Fe3O4 NPs, respectively. (curve a). Two peaks at 780 and 1200 cm−1 are attributed to C–Cl and CHCl vibrations, respectively (curve b). The spectrum of MNPs–PhSO3H (Fig. 1c) shows some bands at 3337 and 3423 cm−1, which are attributed to the stretching vibrations of two kinds of N–H bond of MNPs–PhSO3H. The absorption peaks at around 2923 and 3010 cm−1 were attributed to C-H stretching vibrations in the aliphatic and aromatic groups, respectively. Additionally, the presence of sulfanilic acid was affirmed by the S=O stretching vibration band at 1000–1350 cm−1 in curve c.
XRD
Figure 2 shows the crystalline structure of MNPs and MNPs–PhSO3H nanoparticles, which identified with the XRD technique. Diffraction peaks at 2θ = 30.04°, 35.68°, 43.36°, 53.85°, 57.35°, 62.79°, and 74.43°, which could be indexed to (2 0 2), (3 1 1), (4 0 0), (4 2 2), (5 1 1), (4 4 0), and (6 6 0) planes, are easily identified in the XRD pattern. The results indicate that the crystalline phase of Fe3O4 is increased by the organic layer coating.
TGA and DTG
The stability of the MNPs–PhSO3H catalyst is determined through derivative thermogravimetry (DTG) and thermogravimetric analysis (TGA) analysis (Fig. 3). The catalyst’s TGA curve shows three weight loss steps over the temperature range. The first step, including weight loss of 0.07% up to 100 °C, because of the elimination of surface hydroxyl groups and physically adsorbed solvent, the second weight loss of 12.51% at about 130 °C to nearly 220 °C is ascribed to the decomposition of SO3H groups, and the third weight loss of 39.75% between temperatures of 300–400 °C is ascribed to the breakdown of the decomposition of the coating organic layer in the nanocatalyst. Due to this mass loss, it was calculated that 1.5 mmol (125.19 mg) of SO3H groups and around 1.5 mmol (397.43 mg) of the organic layer were coated on 1 g of MNPs– PhSO3H. In addition, the DTG curve shows that the decomposition of the organic structure mainly happened at 200 °C. So, the MNPs–PhSO3H is stable below 150 °C.
VSM
The magnetic properties of Fe3O4 NPs and MNPs–PhSO3H were studied by VSM at room temperature (Fig. 4). The saturation magnetization value (Ms) of Fe3O4 NPs and MNPs–PhSO3H was found to be 116.6 and 20.4 emu g−1. The decrease in saturation magnetization value of MNPs–PhSO3H can be attributed to the organic layer coating on the surface of the Fe3O4 NPs.
FE-SEM and EDS
As shown in Fig. 5, the FE-SEM image shows morphology and size of MNPs–PhSO3H catalyst. The FE-SEM images confirmed the spherical morphology of MNPs–PhSO3H. The average size of nanoparticles was determined from 9 to 23 nm (Fig. 5). Also, the FE-SEM images show that the size and morphology of nanoparticles are uniform in both Fe3O4 (a) and MNPs–PhSO3H (d). The EDS results of MNPs–PhSO3H are shown in Fig. 6. The existing elemental composition in MNPs–PhSO3H (C, N, O, S, Fe) is clearly displayed.
TEM
The spherical morphology of MNPs–PhSO3H was confirmed by TEM (Fig. 7). The average size of MNPs–PhSO3H was measured using the histogram curve, and it is about 9 nm (Fig. 8), which confirmed the achieved SEM data.
Catalytic application of MNPs–PhSO3H
After the characterization of MNPs–PhSO3H, its catalytic activity was studied for the synthesis of tetrahydrobenzo[b]pyran and 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives (Scheme 1).
For the optimization of the reaction conditions in the synthesis of tetrahydrobenzo[b]pyran, the reaction of benzaldehyde (1), malononitrile (2), and dimedone (3) was selected as a model. Initially, the model reaction was optimized in several solvents as well as under solvent-free conditions, and the results are summarized in Table 1. The best result was achieved in H2O/EtOH (1:1) in the presence of 0.01 g of MNPs–PhSO3H catalyst at 70 °C. In these conditions, the desired product was obtained at 76% yields (Table 1, entry 2). Next, the amount of catalyst was investigated that the highest yield of product was obtained using 0.01 g of catalyst (Table 2, entry 1). Finally, the effect of temperature was investigated, and the results are shown in Table 2. It was found that conventional heating at 100 °C in the presence of 0.01 g of MNPs–PhSO3H is an efficient condition (Table 2, entry 8).

After optimizing the reaction conditions, in order to investigate the catalytic activity of MNPs–PhSO3H, various types of aldehyde carrying either electron-withdrawing or electron-donating groups were reacted with malononitrile (2) and dimedone (3) under reflux condition at 100 °C with 0.01 g of MNPs–PhSO3H in H2O/EtOH (1:1) to produce tetrahydrobenzo[b]pyran derivatives. The respective results are summarized in Table 3. All the desired products were obtained in short reaction times and good-to-excellent yields (65–95%). The fact that the electronic character of substituents of the aromatic aldehyde has no clear effect on the reaction is significant.
A proposed mechanism for the synthesis of tetrahydrobenzo[b] pyran is provided in Scheme 3. In the first step, the electrophilicity of the carbonyl group of aldehyde (1) was increased by the proton from MNPs–PhSO3H. Next, the Knoevenagel condensation of malononitrile (2) with activated aldehyde generates intermediate (A) that with dehydration, give 2-benzylidenemalononitrile intermediate (B). In the second step, the dimedone (3) was converted to the enolizable dimedone (C) in the presence of MNPs–PhSO3H. Subsequently, Michael addition of the enolizable dimedone (C) to 2-benzylidene malononitrile intermediate (B), followed by continuous intramolecular cyclization happen to give the intermediate (E). Finally, tautomerization affords the corresponding tetrahydrobenzo[b]pyran.
In another study for the synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives, we selected the reaction of benzaldehyde (1), malononitrile (2), and 4-hydroxycoumarin (5) as a model reaction. Initially, the model reaction was optimized in several solvents as well as under solvent-free conditions, and the results are summarized in Table 4. The best result was achieved in H2O/EtOH (1:1). In these conditions, the desired product was obtained at 87% yields (Table 4, entry 2). Next, the amount of catalyst was investigated that the highest yield of product was obtained using 0.01 g of catalyst (Table 5, entry 1). Finally, the effect of temperature was investigated, and the results are shown in Table 5. It was found that conventional heating at 70 °C in the presence of 0.01 g of MNPs–PhSO3H is an efficient condition (Table 5, entry 1).

After optimizing the reaction conditions, in order to investigate the catalytic activity of MNPs–PhSO3H, various types of aldehyde carrying either electron-withdrawing or electron-donating groups were reacted with malononitrile (2) and 4-hydroxycoumarin (5) under reflux condition at 70 °C with 0.01 g of MNPs–PhSO3H in H2O/EtOH (1:1) to produce 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives. The respective results are summarized in Table 6. All the desired products were obtained in short reaction times and good-to-excellent yields (79–98%). The fact that the electronic character of substituents of the aromatic aldehyde has no clear effect on the reaction is significant.
A proposed mechanism for the synthesis of tetrahydrobenzo[b] pyran is provided in Scheme 4. In the first step, the electrophilicity of the carbonyl group of aldehyde (1) was increased by the proton from MNPs–PhSO3H. Next, the Knoevenagel condensation of malononitrile (2) with activated aldehyde generates intermediate (A) that with dehydration, gives 2-benzylidene malononitrile intermediate (B). In the second step, Michael addition of the active methylene of 4-hydroxycoumarin reacts to 2-benzylidene malononitrile intermediate (B), followed by continuous intramolecular cyclization happen to give the intermediate (D). Finally, the corresponding product (6) is afforded by tautomerization.
Comparison of the catalytic activity of MNPs–PhSO3H with some reported catalytic systems
In order to show the catalytic activity of MNPs–PhSO3H for the synthesis of tetrahydrobenzo[b]pyran and 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives, this system was compared with the known data from the other literature. For comparison, the derivatives 4a and 6a were selected, as shown in Table 7. It is clearly demonstrated that short reaction times and high yields make this route as a useful protocol. Also, the new catalyst is comparable in non‐toxicity, stability, and easy separation.
Catalyst recovery and reuse
One of the most important advantages of this superparamagnetic nanocatalyst is its reusability and recovery that makes it valuable in commercial applications. Thus, the recovery and reusability of the MNPs–PhSO3H were analyzed in the synthesis of tetrahydrobenzo[b]pyran via the reaction of dimedone (1.0 mmol), benzaldehyde (1.0 mmol), and malononitrile (1.0 mmol) in EtOH/H2O (1:1) at 100 °C and synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene 3-carbonitrile via the reaction of 4-hydroxycoumarin (1.0 mmol), benzaldehyde (1.0 mmol), and malononitrile (1.0 mmol) in EtOH/H2O (1:1) at 70 °C. The catalyst was reused for at least six runs in the synthesis of tetrahydrobenzo[b]pyran and synthesis of 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene 3-carbonitrile, without any decrease in its catalytic activity (Fig. 9). This small decrease can be attributed to the loss of catalyst after every recycling.
Conclusions
In this study, we were able to prepare a new nanocatalyst using a very easy and inexpensive method. The synthesized nanocatalyst has characterized by FT-IR, TGA, XRD, SEM, VSM, and TEM analyses. This nanocatalyst has been successfully used in the synthesis of tetrahydrobenzo[b] pyran and 2-amino-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitrile derivatives that all the derivatives have fully synthesized in good-to-excellent yields. The nanocatalyst was simply separated using an external magnetic and reused several times without a considerable decrease in its catalytic activity.
References
L. Chen, A. Noory Fajer, Z. Yessimbekov, M. Kazemi, M. Mohammadi, J. Sulfur Chem. 40, 451 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, Z. Taherinia, J. Iran. Chem. Soc. 16, 411 (2019)
A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi, M. Ghadermazi, Polyhedron 158, 25 (2019)
M. Kazemi, M. Ghobadi, A. Mirzaie, Nanotechnol. Rev. 7, 43 (2018)
M. Schulz‐Dobrick, I. Schnell, Open Chem. 3, SI1 (2007)
A. Ghorbani-Choghamarani, M. Mohammadi, R.H.E. Hudson, T. Tamoradi, Appl. Organomet. Chem. 33, e4977 (2019)
F. Chen, S. Xie, X. Huang, X. Qiu, J. Hazard. Mater. 322, 152 (2017)
J. Ke, J. Liu, H. Sun, H. Zhang, X. Duan, P. Liang, X. Li, M.O. Tade, S. Liu, S. Wang, Appl. Catal. B-Environ. 200, 47 (2017)
Z. Wen, Y. Zhang, Y. Wang, L. Li, R. Chen, Chem. Eng. J. 312, 39 (2017)
F. He, J. Luo, S. Liu, Chem. Eng. J. 294, 362 (2016)
Z.W. Seh, J. Kibsgaard, C.F. Dickens, I. Chorkendorff, J.K. Nørskov, T.F. Jaramillo, Science 355, eaad4998 (2017)
J. Shi, Chem. Rev. 113, 2139 (2013)
J. Wang, R. Nie, L. Xu, X. Lyu, X. Lu, Green Chem. 21, 314 (2019)
G. Li, H. Yang, H. Zhang, Z. Qi, M. Chen, W. Hu, L. Tian, R. Nie, W. Huang, ACS Catal. 8, 8396 (2018)
X. Duan, J. Liu, J. Hao, L. Wu, B. He, Y. Qiu, J. Zhang, Z. He, J. Xi, S. Wang, Carbon 130, 806 (2018)
D. Wang, J. Liu, J. Xi, J. Jiang, Z. Bai, Appl. Surf. Sci 489, 477 (2019)
J. Xi, H. Sun, D. Wang, Z. Zhang, X. Duan, J. Xiao, F. Xiao, L. Liu, S. Wang, Appl. Catal. B-Environ. 225, 291 (2018)
A. Tokarev, J. Yatvin, O. Trotsenko, J. Locklin, S. Minko, Adv. Funct. Mater. 26, 3761 (2016)
S.Z. Li, W. Zhang, M.H. So, C.M. Che, R.M. Wang, R. Chen, J. Mol. Catal. A-Chem. 359, 81 (2012)
W.T. Hu, B.C. Liu, Q. Wang, Y. Liu, Y.X. Liu, P. Jing, S.L. Yu, L.X. Liu, J. Zhang, Chem. Commun. 49, 7596 (2013)
Z.P. Wen, Y.L. Zhang, Y. Wang, L.N. Li, R. Chen, Chem. Eng. J. 312, 39 (2017)
A. Abo Markeb, A. Alonso, A.D. Dorado, A. Sànchez, X. Font, Environ. Technol. 37, 2099 (2016)
M. Seifan, A. Ebrahiminezhad, Y. Ghasemi, A.K. Samani, A. Berenjian, Appl. Microbiol. Biotechnol. 102, 175 (2018)
P. Biehl, M. Von der Lühe, S. Dutz, F.H. Schacher, Polymer 10, 91 (2018)
W. Fu, H. Yang, S. Liu, M. Li, G. Zou, Mater. Lett. 60, 1728 (2006)
S. Taheri, H. Veisi, M. Hekmati, New J. Chem. 41, 5075 (2017)
K.S. Pandit, P.V. Chavan, U.V. Desai, M.A. Kulkarni, P.P. Wadgaonkar, New J. Chem. 39, 4452 (2015)
W.A. Bubb, H.A. Berthon, P.W. Kuchel, Bioorg. Chem. 23, 119 (1995)
T.S. Jin, A.Q. Wang, X. Wang, J.S. Zhang, T.S. Li, Synlett 05, 0871 (2004)
A. Jamshidi, B. Maleki, F.M. Zonoz, R. Tayebee, Mater. Chem. Phys. 209, 46 (2018)
B. Maleki, M. Baghayeri, S.A.J. Abadi, R. Tayebee, A. Khojastehnezhad, RSC Adv. 6, 96644 (2016)
S.F. Hojati, N. MoeiniEghbali, S. Mohamadi, T. Ghorbani, Org. Prep. Proced. Int. 50, 408 (2018)
H. Sharma, S. Srivastava, RSC Adv. 8, 38974 (2018)
M. Hajjami, F. Gholamian, R.H. Hudson, A.M. Sanati, Catal. Lett. 149, 228 (2019)
H. Alinezhad, M. Tarahomi, B. Maleki, A. Amiri, Appl. Organomet. Chem. 33, e4661 (2019)
K.K. Krishnan, V.V. Dabholkar, A. Gopinathan, R. Jaiswar, J. Chem. Chem. Sci. 8, 66 (2018)
M. Norouzi, D. Elhamifar, R. Mirbagheri, Z. Ramazani, J. Taiwan Inst. Chem. Eng. 89, 234 (2018)
M. Norouzi, D. Elhamifar, Catal. Lett. 149, 619 (2019)
M.A. Shaikh, M. Farooqui, S. Abed, Res. Chem. Intermed. 45, 1595 (2019)
M. Gholamhosseini-Nazari, S. Esmati, K.D. Safa, A. Khataee, R. Teimuri-Mofrad, Res. Chem. Intermed. 45, 1841 (2019)
M. Esmaeilpour, J. Javidi, F. Dehghani, F.N. Dodeji, RSC Adv. 5, 26625 (2015)
B. Shitole, N. Shitole, M. Shingare, G. Kakde, Curr. Chem. Lett. 5, 137 (2016)
D.S. Patel, J.R. Avalani, D.K. Raval, J. Saudi Chem. Soc. 20, S401 (2016)
S.S. Sajadikhah, M.T. Maghsoodlou, N. Hazeri, M. Norouzi, M. Moein, Res. Chem. Intermed. 41, 8665 (2015)
D. Elhamifar, Z. Ramazani, M. Norouzi, R. Mirbagheri, J. Colloid Interface Sci. 511, 392 (2018)
J. Yang, S. Liu, H. Hu, S. Ren, A. Ying, Chin. J. Chem. Eng. 23, 1416 (2015)
H. Hu, F. Qiu, A. Ying, J. Yang, H. Meng, Int. J. Mol. Sci. 15, 6897 (2014)
J. Davarpanah, A.R. Kiasat, S. Noorizadeh, M. Ghahremani, J. Mol. Catal. A-Chem. 376, 78 (2013)
F. Adibian, A.R. Pourali, B. Maleki, M. Baghayeri, A. Amiri, Polyhedron 175, 114179 (2019)
F. Ataie, A. Davoodnia, A. Khojastehnezhad, Polycycl. Aromat. Compd. 1 (2019)
E. Mollashahi, M. Nikraftar, J. Saudi Chem. Soc 22, 42 (2018)
B. Maleki, Org. Prep. Proced. Int. 48, 3 (2016)
Acknowledgements
The authors gratefully appreciate the partial support from the Research Council of University of Sistan and Baluchestan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faroughi Niya, H., Hazeri, N., Rezaie Kahkhaie, M. et al. Preparation and characterization of MNPs–PhSO3H as a heterogeneous catalyst for the synthesis of benzo[b]pyran and pyrano[3,2-c]chromenes. Res Chem Intermed 46, 1685–1704 (2020). https://doi.org/10.1007/s11164-019-04056-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04056-z