Abstract
Sulfonated Sulfurol supported Fe3O4 (Fe3O4@SiO2-Pr-Sulfurol-SO3H) a new magnetic reusable nanocatalyst was prepared using chemical modification of magnetic nanoparticles (MNPs) surface with Sulfurol-SO3H. The Sulfurol-SO3H moieties on the surface of MNPs act as acidic catalytic sites for catalysis purposes. Fe3O4@SiO2-Pr-Sulfurol-SO3H was authenticated by usual analytical and spectroscopic techniques. The prepared Fe3O4@SiO2-Pr-Sulfurol-SO3H MNPs were applied to the preparation of novel Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives via the three-component condensation of isatins, dimedone and thioamides or thioureas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In catalytic studies, recovery and reusability of catalyst due to its adaptability to the environment are important features of the catalytic process that has received much attention in recent years [1, 2]. Due to the complex recovery process of homogeneous catalysts, the easy recovery and reusability of a homogeneous catalyst by its covalent anchoring to a recyclable support with keeping its original catalytic activity are important subjects, as such it is suggested to use heterogeneous catalysts in catalytic studies [3, 4]. Therefore, to overcome the problem of separation of homogeneous catalysts, they can be used as heterogeneous catalysts by grafting them on solid substrates [5,6,7,8,9,10,11]. Recently, heterogeneous catalysts, which consist of a hybrid organic–inorganic material, have received much attention due to both the advantages of homogeneous and heterogeneous catalysts [12, 13]. The integration of nanotechnology with green chemistry offers innovative prospects to meet current demands for ecological and economic sustainability [14]. Magnetic nanoparticles have received much attention due to their easy recycling ability in synthetic chemistry [15, 16]. However, magnetic nanoparticles have gained a lot of applications because of their high surface area, which enables high loading capacity of the catalysts [17, 18]. Magnetic nanoparticles, especially iron oxide nanoparticles, due to suitable properties for catalytic and environmental processes, were considered as a solid substrate for immobilization of catalysts [19,20,21]. Different organic catalysts can be easily functionalized on the surface of iron oxide nanoparticles, and due to the magnetic nature of the iron oxide nanoparticles, they are easily separated from the reaction medium and the problem of separation steps such as centrifugation, filtration and membrane eliminated [22, 23, 24]. Sulfonic acid-based catalysts were used in a variety of organic reactions. In order to resolve problems such as waste neutralization, difficult separations and the inability for reuse inhibit their applicability, the need for their heterogenization has arisen [25]. In recent years, function-based sulfonic acid is a significant branch of magnetic nanoparticles of application as catalyst in organic transformations [26,27,28,29,30,31,32].
Multicomponent reaction (MCR) consists of three or more of the easily accessible raw materials in an easy, safe, environmentally friendly process that has received much attention due to its wide range of applications in pharmaceutical chemistry [33, 34]. The thiazole ring is a constituent of natural compounds such as thiamine, thiamine pyrophosphate and a range of biological properties, including antimicrobial, bacteriostatic activities, antimalarial, anticancer, hypertension, inflammation and also applied in the development of medicines for the treatment of allergies [35,36,37,38,39,40,41,42]. Recently, isatin-based multicomponent reactions have been developed for the preparation of polycyclic heterocyclic compounds [43,44,45,46,47]. We aimed Sulfurol-SO3H-functionalized MNPs onto their surfaces as a catalyst system for preparation of new Spiro[acridine-9,5′-thiazole]-1,4′-dione compounds with condensation reaction to vigorous stirring of equimolar quantities of isatins, dimedone and thioamides or thioureas.
Experimental
Materials and methods
Chemicals were purchased from the Sigma-Aldrich and Merck in high purity. 1H-NMR (300 MHz) and 13C-NMR spectra were obtained on Bruker Avance 75 MHz instruments in DMSO-d6 as deuterated solvent. Melting point was measured in the open capillaries using a BUCHI 510 melting point apparatus. The progress of the reaction was monitored by thin-layer chromatography (TLC) using n-hexane/EtOAc as an eluent. The FT-IR spectra were recorded using KBr disks on a JASCO FT-IR 460 plus spectrophotometer. Elemental compositions were determined with a Leo 1450 VP scanning electron microscope equipped with an SC7620 energy-dispersive spectrometer (SEM–EDS) presenting a 133 eV resolution at 20 kV. Powder X-ray diffraction (XRD) was performed on a Bruker D8-advance X-ray diffractometer with Cu Kα (λ = 0.154 nm) radiation. The magnetic property of Fe3O4@SiO2-Pr-Sulfurol-SO3H was measured with VSM/AGFM. TGA was done on a thermal analyzer with a heating rate of 10 °C min−1 over a temperature range of 25–600 °C under flowing compressed N2.
Preparation of Fe3O4@SiO2-Pr-Sulfurol-SO3H
The synthesis of Fe3O4 followed a co-precipitation approach. FeCl2·4H2O (1.25 g) and FeCl3·6H2O (3.33 g) were dissolved in 80 mL water, and ammonium hydroxide solution (%25, 50 mL) was added dropwise to the stirring mixture at room temperature for 3 h and stirred continuously for 1 h; the black products were collected by an external magnet. Then, Fe3O4 MNPs were washed three times with water and ethanol and dried at 60 °C for 12 h (Scheme 1). Then the nanoparticles coated with silica, Fe3O4@SiO2 (0.5 g) were dispersed for 1 h in toluene; then, 3-(chloropropyl) trimethoxysilane (2 mmol) was added and reaction mixture refluxed at 110 °C for 36 h. The mixture was allowed to cool at room temperature, and the modified nano-Fe3O4@SiO2 (Fe3O4@SiO2-Pr-Cl) nanoparticles were washed with EtOH several times and dried under vacuum. In the next step, the Fe3O4@SiO2-Pr-Cl (0.5 g) were dispersed in toluene for 1 h. Then, 2-(4-methylthiazol-5-yl) ethan-1-ol (2 mmol) was added to this reaction mixture and stirred at reflux temperature for 24 h. Finally, Fe3O4@SiO2-Pr-Sulfurol-SO3H nanoparticles were obtained by reaction of Fe3O4@SiO2-Pr-Sulfurol with chlorosulfonic acid (Scheme 1).
General method for the synthesis of Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives (4a–l)
A mixture of isatins (1.0 mmol), thioamides or thioureas (1.0 mmol), dimedone (1.0 mmol) and Fe3O4@SiO2-Pr-Sulfurol-SO3H (7 mg, 0.43 mol%) was stirred at 80 °C in water:ethanol (3 mL). The progress of the reaction was monitored by TLC. After completion of the reaction, the solid crude product was separated by filtration. Sufficient ethanol was added to solid and heated to separate Fe3O4@SiO2-Pr-Sulfurol-SO3H by an external magnet. The warm ethanolic solution containing crude product was allowed to cool at room temperature until precipitated pure product was formed. The products were identified by FT-IR, 1H NMR, 13 C NMR and mass.
- 1.
2′-(4-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4a). yellow solid; mp: 289–290 °C. IR (KBr) (νmax/cm−1): 3269, 2940, 1709, 1601, 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.03(3H, s, CH3), 1.07(3H, s, CH3), 2.09–2.24 (2H, q, J = 15.94 Hz, CH2), 2.47–2.60 (2H, q, J = 12.93 Hz, CH2), 3.90(3H, s, OCH3), 6.85–7.29 (6H, H-Ar), 8.06–8.09(2H, dd, j1 = 6.00 Hz, j2 = 3.00 Hz),10.26(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.56, 28.52, 32.68, 50.41, 56.30, 66.50, 102.92, 115.17, 116.53, 122.78, 124.96, 127.51, 129.64, 130.92, 135.61, 154.49, 165.25, 191.51, 192.23, 194.78. Exact Mass (M)+: 418, Found: 418.
- 2.
2′-(4-chlorophenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4b). yellow solid; mp: 287–289 °C. IR (KBr) (νmax/cm−1): 3262, 2958, 1721, 1601. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.03(3H, s, CH3), 1.07(3H, s, CH3), 2.11–2.26 (2H, q, J = 15.04 Hz, CH2), 2.47–2.60 (2H, q, J = 6.01 Hz, CH2), 6.86–7.29 (4H, H-Ar), 7.67–8.12(4H, H-Ar),10.34(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.57, 28.49, 32.70, 50.29, 67.39, 102.78, 116.68, 122.24, 125.10, 127.55, 129.85, 129.98, 130.26, 131.35, 135.63, 140.18, 154.74, 191.58, 192.31, 194.82. Exact Mass (M + H)+: 423, Found: 423.
- 3.
7-chloro-2′-(4-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4c). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3262, 2957, 1708, 1600. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.03(3H, s, CH3), 1.05(3H, s, CH3), 2.10–2.29 (2H, m, CH2), 2.47–2.61 (2H, q, m, CH2), 3.68(3H, s, OCH3), 6.69–7.21 (4H, H-Ar), 7.34–7.38(1H, dd, J1 = 9.02 Hz, J2 = 3.00 Hz H-Ar), 8.09–8.11 (2H, H-Ar), 10.41(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.50, 28.49, 32.69, 50.35, 56.37, 65.86, 102.82, 115.28, 121.23, 124.46, 124.73, 126.57, 128.05, 129.83, 131.12, 134.77, 154.44, 165.49, 191.65, 192.34, 194.30. Exact Mass (M)+: 452, Found: 452.
- 4.
7-bromo-2′-(4-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4d). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3262, 2956, 1710, 1599. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.03(3H, s, CH3), 1.07(3H, s, CH3), 2.11–2.26 (2H, m, CH2), 2.55–2.61 (2H, m, CH2), 6.84–8.10 (7H, H-Ar), 10.41(1H, s, NH). Exact Mass (M + H)2+: 496, Found: 496.
- 5.
7-chloro-2′-(4-chlorophenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4e). yellow solid; mp: 282–284 °C. IR (KBr) (νmax/cm−1): 3261, 2959, 1721, 1600. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.02(3H, s, CH3), 1.06(3H, s, CH3), 2.17 (2H, d, J = 15.94, CH2), 2.55 (2H, d, J = 15.00, CH2) 6.79 (1H, d, J = 3.00, H-Ar), 7.10 (1H, d, J = 9.02, H-Ar), 7.33–8.13 (5H, H-Ar), 10.47(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.51, 28.46, 32.70, 50.24, 66.68, 102.71, 118.62, 123.88, 126.69, 128.25, 129.99, 130.41, 131.15, 134.78, 140.39, 154.65, 191.82, 192.40, 194.49. Exact Mass (M + H)2+: 458, Found: 458.
- 6.
7-bromo-2′-(phenylamino)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4f). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3261, 3172, 2962, 1742, 1603. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 0.66(3H, s, CH3), 0.99(3H, s, CH3), 2.08–2.27 (2H, m, CH2), 2.48–2.54 (2H, m, CH2), 6.56–7.65 (7H, H-Ar), 10.28(1H, s, NH), 12.29(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 26.91, 28.90, 32.24, 50.32, 70.58, 100.41, 115.90, 119.47, 121.75, 128.53, 128.61, 128.98, 129.35, 133.64, 135.53, 137.27, 155.14, 178.86, 180.96, 193.82. Exact Mass (M + H)+: 483, Found: 483.
- 7.
7-chloro-2′-phenyl-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4g). yellow solid; mp: 276–278 °C. IR (KBr) (νmax/cm−1): 3274, 2960, 1722, 1599. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.02(3H, s, CH3), 1.04(3H, s, CH3), 2.11–2.53 (4H, m, CH2), 6.78–8.13 (7H, H-Ar), 11.06(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.52, 28.27, 32.39, 50.27, 66.09, 102.77, 110.43, 118.59, 122.88, 125.17, 127.10, 128.68, 129.85, 133.36, 134.80, 142.55, 154.58, 178.19, 192.38, 194.60. Exact Mass (M)+: 422, Found: 422.
- 8.
2′-(3-methyl-2-methyl-imino)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4h). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3259, 2954, 1748, 1625. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.07(6H, s, CH3), 2.20 (2H, s, CH2), 2.52 (2H, s, CH2), 2.53 (3H, s, CH3), 3.26 (3H, s, CH3), 6.88–7.42 (4H, H-Ar), 10.44(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.83, 28.44, 28.54, 30.25, 32.55, 50.42, 66.97, 99.48, 117.05, 118.06, 124.91, 126.84, 130.52, 136.36, 155.46, 176.02, 180.69, 193.48. Exact Mass (M)+: 355, Found: 355.
- 9.
7-chloro-2′-(3-methyl-2-methyl-imino)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4i). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3279, 2958, 1744, 1639. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.07(6H, s, CH3), 2.21 (2H, s, CH2), 2.62 (2H, s, CH2), 2.75 (3H, s, CH3), 3.66 (3H, s, CH3), 6.88 (1H, d, J = 3.00, H-Ar), 7.19 (1H, d, J = 3.00, H-Ar), 7.45–7.49(1H, dd, J1 = 12.03 Hz, J2 = 3.00 Hz H-Ar), 10.57(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 27.80, 28.37, 28.70, 30.28, 32.54, 50.38, 66.65, 99.46, 119.06, 119.66, 126.15, 128.21, 130.81, 135.47, 155.40, 175.60, 180.90, 193.61. Exact Mass (M + H)+: 391, Found: 391.
- 10.
7-chloro-2′-(phenylamino)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4j). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3261, 3164, 2964, 1742, 1603. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 0.64(3H, s, CH3), 0.99(3H, s, CH3), 2.09–2.28 (2H, m, CH2), 2.49–2.54 (2H, m, CH2), 6.59–6.63 (2H, H-Ar), 7.71–7.28(5H, H-Ar), 7.50–7.54(1H, dd, J1 = 12.03 Hz, J2 = 3.00 Hz H-Ar), 10.29(1H, s, NH), 12.30(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 26.91, 28.90, 32.24, 50.33, 70.67, 102.25, 119.21, 121.35, 126.06, 128.22, 128.54, 128.61, 129.35, 130.87, 135.18, 137.24, 155.17, 175.86, 180.98, 193.83. Exact Mass (M + H)2+: 439, Found: 439.
- 11.
7-chloro-2′-methyl-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4k). yellow solid; mp: 262–264 °C. IR (KBr) (νmax/cm−1): 3285, 2959, 2688, 1659, 1612. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.01(3H, s, CH3), 1.04(3H, s, CH3), 2.14–2.48 (7H, CH3, CH2), 7.25–8.32 (4H, H-Ar), 10.37(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 22.12, 27.81, 28.27, 32.24, 52.54, 66.88, 100.66, 110.433, 114.30, 120.71, 122.88, 128.89, 134.83, 142.54, 149.66, 178.20, 181.55, 190.41. Exact Mass (M)+: 360, Found: 360.
- 12.
2′-phenylamino-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione(4l). yellow solid; mp: > 295 °C. IR (KBr) (νmax/cm−1): 3260, 3130, 2960, 1741, 1605. 1H NMR (300 MHz, DMSO-d6): δH (ppm) 0.65(3H, s, CH3), 1.00(3H, s, CH3), 2.08–2.53 (4H, m, CH2), 6.57–7.43 (9H, H-Ar), 10.13(1H, s, NH), 12.18(1H, s, NH). 13C NMR (75 MHz, DMSO-d6): δC (ppm) 26.97, 28.96, 32.24, 50.43, 71.09, 100.33, 117.10, 119.75, 124.98, 127.04, 128.42, 128.59, 129.14, 130.61, 136.08, 137.54, 155.14, 176.30, 180.87, 193.71. Exact Mass (M)+: 403, Found: 403.
Result and discussion
Characterization of catalyst
Fe3O4@Si-Pr-Sulfurol-SO3H nanoparticles were characterized by infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), EDX, field emission scanning electron microscopy (FE-SEM), thermogravimetry analysis (TGA) and vibrating sample magnetometer (VSM).
XRD analysis
At XRD, six diffraction peaks at 2θ = 30.29°,35.63°, 43.33°, 53.86°, 57.26°, 63.07° attributed to the crystal structure of the Fe3O4. As shown in Fig. 1b, no remarkable changes occur in the number of peaks. These results indicate that the crystal structure of Fe3O4 core after immobilization retains unchanged (Fig. 1).
SEM–EDS analysis
FE-SEM images of Fe3O4@Si-Pr-Sulfurol-SO3H show surface morphology and average size for nanoparticles (Fig. 2). The diameter of particles is about 20 nm in a spherical shape and morphology of the catalyst observed points to crystalline phase.
The EDX spectrum, which is shown in Fig. 3, shows the presence of Fe, C, N, O, Cl and S atoms in the catalyst.
TGA analysis
The thermal behavior of the synthesized Fe3O4@Si-Pr-Sulfurol-SO3H was investigated by thermogravimetric analysis from room temperature to 600 °C (TGA) (Fig. 4). The TGA curves of the catalyst show a weight loss of 2% below 180 which is due to the loss of the adsorbed solvent. The TGA curve of the Fe3O4@Si-Pr-Sulfurol-SO3H substrate shows 18% weight loss (180–480 °C) because of the loss of the grafted organic moiety (Fig. 4).
VSM analysis
The VSM diagram shows the magnetic properties of synthesis catalyst (Fig. 5). The hysteresis loops of Fe3O4@SiO2-Pr-Sulfurol-SO3H were measured. Magnetization (emu g−1) as a function of applied field (Oe) is depicted in Fig. 5 with the confined field from − 10,000 to + 10,000 Oe. The superparamagnetic behavior of Fe3O4@Si-Pr-Sulfurol-SO3H illustrates small particle size. As it can be observed, the saturation magnetization value of Fe3O4@SiO2-Pr-Sulfurol-SO3H is 35 amu g−1.
The FT-IR spectrum of the Fe3O4@SiO2-Pr-Sulfurol-SO3H catalyst is presented in Fig. 6. The Fe–O stretching vibration was observed 550–650 cm−1 and Si–O-Si stretching vibration was observed 1000–1350 cm−1. The functionalization of –SO3H groups on Fe3O4@SiO2 was approved by the absorption of S–O and S=O stretching bands of the –SO3H moiety at 990–1230 cm-1 in FT-IR spectrum.
The content of –SO3H in the described catalyst can be determined by a back-titration method. The Fe3O4@SiO2-Pr-Sulfurol-SO3H nanocatalyst (0.05 g) was added to 5 mL of 0.1 N sodium hydroxide solution. An excess extent of base was added that reacted with all of the catalyst, some of which was left. Two drops of phenolphthalein solution were then added to the remaining solution. The back titration of unreacted base began with adding HCl 0.1 N and ended with the disappearance of the purple solution. This sequence was repeated for three times. The acid amount of the heterogeneous acidic catalyst was found to be 0.62 mmol/g−1.
Reaction optimization
After characterization of the synthesized Fe3O4@ SiO2-Pr-Sulfurol-SO3H nanocatalyst, we have focused to evaluate its catalytic acting in three-component reaction for the preparation of Spiro[acridine-9,5′-thiazole]-1,4′-diones (Table 1).

In order to optimize the reaction conditions, the reaction of isatin (1 mmol), dimedone (1 mmol) and 4-methoxythiobenzamide (1 mmol) was examined to production of 2′-(4-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione (4a) as a model compound. This model reaction with various solvents, temperatures and catalysts was studied to find the best conditions. As shown in Table 1, optimization of the reaction started with different types of catalysts. The reaction did not carry out without catalyst even after 15 h (Table 1, entries 10, 11). The reaction also was investigated with different catalysts, utilizing a variety of solvents including EtOH, CH3CN and H2O (Table 1, entries 1–12). Catalysts such as p-toluenesulfonic acid (p-TSA), DABCO and AcOH were tested in two different solvents and the moderate yields of products were obtained (Table 1, entries 1–4, 8). Catalysts such as dodecyl sulfate (SDS) and β-cyclodextrin exhibited efficient yield (Table 1, entries 5–7). The reaction in the presence of nano-Fe3O4 as catalysts produced desired product to moderate yields (Table 1, entry 9). Based on these promising results from Table 1, Fe3O4@SiO2-Pr-Sulfurol-SO3H improved the efficiency of the reaction (Table 1, entries 12, 13, 15). Evaluation of reaction under kind of solvents indicated higher activity of Fe3O4@SiO2-Pr-Sulfurol-SO3H was achieved in mixture EtOH: H2O (1:1) at 80 °C and desired product obtained with 93% yield within 3 h (Table 1, entry 15). In order to assess the effect of temperature on the reaction, we carried out the reactions to various temperatures as shown in Table 1(entries 15, 17–19). In order to show the merit of the Fe3O4@SiO2-Pr-Sulfurol-SO3H in comparison with the use of Fe3O4@SiO2-Pr-Sulfurol catalyst for the synthesis of 2′-(4-methoxyphenyl)-3,3-dimethyl-3,4-dihydro-2H-4′H-Spiro[acridine-9,5′-thiazole]-1,4′-dione in Table 1(entries 15, 16), the results demonstrate that Fe3O4@SiO2-Pr-Sulfurol-SO3H promote the reaction more effectively in terms of short reaction time.
Based on the criteria such as reaction times, greener approach, cost-effectiveness and excellent yields, EtOH: H2O (1:1) mixture solvent proved to be the best solvent candidate for the present procedure and was used for all further reactions of substituted Spiro[acridine-9,5′-thiazole]-1,4′-diones.
Effects of variation in catalyst amounts to yield of products were studied in model reaction. The summarized results (Table 2, entries 1–4) indicate that an increase in mass of catalyst from 3 to 8 mg causes increased yield from 73 to 93% and also a decrease in reaction time. A further increase in amount of catalyst had no further positive effect on product yield (Table 2, entries 4–5). Hence, 7 mg of catalyst was used for the additional reactions.
In this research, we have continued to use Fe3O4@SiO2-Pr-Sulfurol-SO3H as the catalyst in the synthesis of a variety of Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives (Table 3). As shown in Table 3, investigation of the substrate scope shows that both aromatic and aliphatic thioamides are effective in this reaction.
Thiobenzamides bearing different substituents, such as 4-Cl and 4-OMe, were found to be useful in this reaction to synthesize Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives. Nevertheless, aliphatic thioamides such as thioacetamide also provided the corresponding products under similar conditions with good yields. Next, we tested both aromatic and aliphatic thiourea derivatives in this reaction. In all cases, the corresponding Spiro[acridine-9,5′-thiazole]-1,4′-diones were obtained in good yield [6, 8,9,10, 12]. Similarly, expanding the scope of this method, isatins were also varied. Other isatins, such as 5-Cl and 5-Br, also provide the desired products in good yields, and the results are summarized in Table 3.
On the basis of our results, we propose a mechanism for Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives catalyzed by Fe3O4@SiO2-Pr-Sulfurol-SO3H as shown in Scheme 2. First, the reaction of dimedone with isatins was formed intermediate (I) and by removing water convert to intermediate (II). In the next step, Michael addition to intermediate (II) by a thiol group of thioamides presumably formed an intermediate (III). In the following, intermediate (IV) was formed by the nucleophilic attack of amino group of thioamides to the carbonyl group of isatins. Finally, nucleophilic attack of NH2 group of isatins to the carbonyl group dimedone in intermediate (IV) formed an intermediate (V). The desired final products were formed by removing water intermediate (V). The catalyst is released back into the catalytic cycle (Scheme 2).
Recycling and reusing of the catalyst
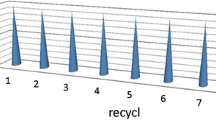
The reusability of the Fe3O4@SiO2-Pr-Sulfurol-SO3H was studied by repeating the model study using the recovered catalyst under optimized reactions. It was concluded that the Fe3O4@SiO2-Pr-Sulfurol-SO3H nanocatalyst could be reused six times. The catalyst kept its stability without any loss of any significant activity (Fig. 7).
Conclusion
We have developed a simple, facile, green and efficient protocol for multicomponent synthesis of novel Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives by the reaction of thioamides, dimedone and isatins in the presence of Fe3O4@SiO2-Pr-Sulfurol-SO3H as catalyst in water/ethanol. The products were obtained in excellent yields within short reaction times. The nanocatalyst can be conveniently separated and recovered from the reaction system by magnet and can be reused for six times without detectable loss in catalytic activity.
References
T. Tamoradi, M. Ghadermazi, A. Ghorbani-Choghamarani, Appl. Organometal. Chem. 32, e3974 (2018)
P. Kumar, K. Kadyan, M. Duhan, J. Sindhu, K. Hussain, S. Lal, Chem. Pap. 73, 1153 (2019)
D.A. Alonso, A. Baeza, R. Chinchilla, C. Gómez, G. Guillena, I.M. Pastor, D.J. Ramón, Catalysts 8, 202 (2018)
A. Corma, H. Garci, Adv. Synth. Catal. 348, 1391 (2006)
S. Ko, J. Jang, Angew. Chem. Int. Ed. 45, 7564 (2006)
A. Ghorbani-Choghamarani, B. Tahmasbi, P. Moradi, N. Havasi, Appl. Organometal. Chem. 30, 619 (2016)
J. Davarpanah, A.R. Kiasat, Catal. Commun. 42, 98 (2013)
D. Saberi, M. Sheykhan, K. Niknam, A. Heydari, Catal. Sci. Technol. 3, 2025 (2013)
A.R. Kiasat, J. Davarpanah, Catal. Commun. 69, 179 (2015)
M. Gawande, P. Branco, R. Varma, Chem. Soc. Rev. 42, 3371 (2013)
M. Nikoorazm, M. Ghobadi, Silicon 11, 983 (2019)
A.R. Sardarian, M. Kazemnejadi, M. Esmaeilpour, Dalton Trans. 48, 3132 (2019)
J. Rakhtshah, B. Shaabani, S. Salehzadeh, N. Hosseinpour Moghadam, Appl. Organomet. Chem. 33, e4754 (2019)
J.B.M. de Resende Filho, G.P. Pires, J.M.G. de Oliveira Ferreira, E.E.S. Teotonio, J.A. Vale, Catal. Lett. 147, 167 (2017)
B. MirzaHedayat, M. Noorisepehr, E. Dehghanifard, A. Esrafili, R. Norozi, J. Mol. Liq. 264, 571 (2018)
N. Zohreh, S.H. Hosseini, M. Tavakolizadeh, C. Busuioc, R. Negrea, J. Mol. Liq. 266, 393 (2018)
S.N. Vajekar, G.S. Shankarling, ChemistrySelect 3, 5848 (2018)
J. Rafique, S. Saba, T.E.A. Frizon, A.L. Braga, ChemistrySelect 3, 328 (2018)
E. Akceylan, A. Uyanik, S. Eymur, O. Sahin, M. Yilmaz, Appl. Catal. A 499, 205 (2015)
B. Karimi, F. Mansouri, H.M. Mirzaei, ChemCatChem 7, 1736 (2015)
P.B. Bhat, B.R. Bhat, New J. Chem. 39, 273 (2015)
S.E. Garcia-Garrido, J. Francos, V. Cadierno, J.M. Basset, V. Polshettiwar, Chemsuschem 4, 104 (2011)
Y. Zhou, S. Wang, B. Ding, Z. Yang, Chem. Eng. J. 138, 578 (2008)
M.B. Gawande, Y. Monga, R. Zboril, R. Sharma, Coord. Chem. Rev. 288, 118 (2015)
C.S. Gill, B.A. Price, C.W. Jones, J. Catal. 251, 145 (2007)
M. Farahi, B. Karami, R. Keshavarz, F. Khosravian, RSC Adv. 7, 46644 (2017)
H. Mohammadi, H.R. Shaterian, Res. Chem. Intermed. 44, 7519 (2018)
M. Khaleghi-Abbasabad, D. Azarifar, Res. Chem. Intermed. 45, 2095 (2019)
M.A. Ghasemzadeh, M. Azimi-Nasrabad, Res. Chem. Intermed. 42, 1057 (2016)
A. Kiasat, J. Davarpanah, Res. Chem. Intermed. 41, 2991 (2015)
V.N. Mahire, G.P. Patil, A.B. Deore, P.G. Chavan, H.D. Jirimali, P.P. Mahulikar, Res. Chem. Intermed. 44, 5801 (2018)
A. Kasprzak, M. Bystrzejewski, M. Poplawska, Dalton Trans. 47, 6314 (2018)
T.E. Glotova, M.Y. Dvorko, A.I. Albanov, O.N. Kazheva, G.V. Shilov, O.A. D’yachenko, Russ. J. Org. Chem. 44, 1532 (2008)
C. Hulme, V. Gore, Curr. Med. Chem. 10, 51 (2003)
H. Anaraki-Ardakani, B. Mirza, N. Mokhtarian, Appl. Chem. Res. 9, 103 (2015)
R.K. Yadlapalli, O.P. Chourasia, M.P. Jogi, A.R. Podile, R.S. Perali, Des. Med Chem Res. 22, 2975 (2013)
J. Banothu, K. Vaarla, R. Bavantula, P.A. Crooks, Chin. Chem. Lett. 25, 172 (2014)
K.A. Milinkevich, L. Ye, M. Kurth, ACS Comb. Sci. 10, 521 (2008)
M.A. Gouda, M.A. Berghot, G.E. Abd El-Ghani, A.M. Khalil, Eur. J. Med. Chem. 45, 1338 (2010)
P.C. Lv, K.R. Wang, Y. Yang, Bioorganic Med. Chem. Lett. 19, 6750 (2009)
A. Zablotskaya, I. Segal, A. Geronikaki, Eur. J. Med. Chem. 70, 846 (2013)
L. Yurttaş, Y. Özkay, H.K. Gençer, U. Acar, J. Chem. (2015)
A.V. Chate, S.P. Kamdi, A.N. Bhagat, J.N. Sangshetti, C.H. Gill, Synth. Commun. 48, 1701 (2018)
D.L. Kong, G.P. Lu, M.S. Wu, Z.F. Shi, Q. Lin, ACS Sustain. Chem. Eng. 5, 3465 (2017)
K. Debnath, K. Singha, A. Pramanik, RSC Adv. 5, 31866 (2015)
C.H. Gill, A.V. Chate, G.Y. Shinde, A.P. Sarkate, S.V. Tiwari, Res. Chem. Intermed. 44, 4029 (2018)
K. Meena, S. Kumari, J.M. Khurana, A. Malik, C. Sharma, H. Panwar, Chin. Chem. Lett. 28, 136 (2017)
Acknowledgements
We gratefully appreciate the University of Sistan and Baluchestan Research Councils for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Shaterian, H.R. Sulfonated magnetic nanocatalyst and application for synthesis of novel Spiro[acridine-9,5′-thiazole]-1,4′-dione derivatives. Res Chem Intermed 46, 1109–1125 (2020). https://doi.org/10.1007/s11164-019-04022-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04022-9