Abstract
Core-crosslinked shelled-core microspheres of poly(styrene-co-methyl acrylic acid) (PS-co-PMAA), with cores rich in PS and the shell rich in PMAA, were synthesized by one-stage soap-free emulsion polymerization. A palladium (Pd)-iminodiacetic acid (IDA) complex catalyst is loaded on the shell of the PS-co-PMAA microsphere, which results in the advantage of high dispersion degree and, therefore, high activity. The resultant polymeric microspheres catalyst systems are then applied to catalyze the Suzuki reaction of aryl halides with phenylboronic acid in an ionic liquid of 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]). Our catalyst systems are proved to be efficient and active for both aryl bromides and aryl iodides. Compared to traditional Pd(Ph3)4 catalyst, the PS-co-PMAA-IDA-Pd catalyst used here affords higher yield of Suzuki reaction at even lower catalyst concentration. In addition, our polymeric-microsphere based catalytic system can be easily recycled at least four times with high activity in ionic [bmim][BF4] liquid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The palladium-catalyzed cross-coupling of aryl halides with arylboronic acids, known as the Suzuki reaction, represents a straightforward and highly effective method for carbon–carbon bond formation in synthesis of biaryl compounds [1,2,3]. Since their discovery in 1981, extensive efforts have been made to develop highly active and selective catalytic systems for Suzuki reactions [4,5,6]. The traditional Suzuki reaction usually proceeds using phosphine-based palladium catalysts [7,8,9,10]. The phosphine-based ligands are highly toxic and difficult to manipulate since they are air/moisture sensitive. Many attempts have been made to develop phosphine-free ligands. Alternative suitable ligands such as thioether–NHC [11] , nucleophilic carbene [12], carbapalladacycle [13], phosphanylferrocene ligand [14], mesoionic carbine [15] amide ligand [16] and meta-hydroxylated imine ligand [17] for palladium catalysts were reported. Moreover, various scaffolds such as silica [18, 19], ZrIV organophosphonate [20], functionalized grapheme [21], magnetic particles [22, 23], polymeric microspheres and microcapsules [24], and soluble single polymer chains [25] have been employed to immobilize palladium to enhance the reusability of the ligand-stabilized palladium catalyst.
A concern of the Suzuki reaction is extensive use of environmentally harmful organic solvents. Recently, there has been considerable interest in the use of ionic liquids as an environmentally benign reaction medium [26,27,28,29]. Numerous chemical reactions, including some enzymatic reactions [28], can be carried out in ionic liquids. Room temperature ionic liquids have also been widely explored as media for electrochemical technologies, chemical extractions, and other industrial processes. This is due to several intriguing properties of ionic liquids: high thermal and chemical stability, no measurable vapor pressure, inflammability. Ionic liquids, such as 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]), have also proved to be environmentally benign choices. Moreover, the products can be separated from the ionic liquid phase through simple extraction with ether or hexane, while the catalytic systems remain in the ionic liquid phase.
Many groups have focused on using polymeric colloid as a catalyst scaffold and found that a colloid-loaded palladium catalyst combines the merits of a readily recovered heterogeneous catalyst and a highly efficient homogeneous catalyst [30, 31]. Here, poly(styrene-co-methylacrylic acid) (PS-co-PMAA) shelled-core microspheres were synthesized by one-stage soap-free emulsion polymerization [32]. Then, the catalyst of palladium-iminodiacetic acid (Pd-IDA) complex was immobilized on the shell of the polymeric microsphere (Scheme 1). Finally, the activity of the resultant microsphere-loaded Pd-IDA catalyst was evaluated by the Suzuki reaction, performed in an ionic liquid of [bmim][BF4]. The catalysis demonstrates the efficiency of the shelled-core Pd-IDA complex-loaded PS-co-PMAA microspheres and that they are easily reused in ionic [bmim][BF4] liquid. By comparison, the catalyst presented here has three advantages. First, cheap and stable iminodiacetic acid, instead of a toxic and air/moisture sensitive phosphine-based ligand, is used. Second, the Pd-IDA catalyst complex is loaded on the outer shell of the core–shell microspheres, which is helpful for mass transition during the Suzuki reaction. Last, the 480-nm core–shell microsphere scaffold can be fully dispersed in the ionic [bmim][BF4] liquid, which results in the advantage of high dispersion degree and, therefore, high activity.
Experimental details
Materials
An ionic liquid of 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) was synthesized as described elsewhere [33]. Monomers of styrene (St, > 98%, Tianjin Chemical Company, China) and methyl acrylic acid (MAA, > 99%, Tianjin Chemical Company) were distilled under vacuum before being used. Divinylbenzene (DVB, > 80%, Alfa Aesar) was washed in 5% aqueous sodium hydroxide solution and water, followed by drying with MgSO4. Iodobenzene (> 98%, Alfa Aesar), 4-iodophenol (> 99%, Shanghai Bangcheng Chemical Co., Ltd., China), 4-iodobenzoic acid (> 99%, Shanghai Bangcheng Chemical), 4-iodobenzaldehyde (> 99%, Shanghai Bangcheng Chemical), 4-iodoanisole (> 99%, Shanghai Bangcheng Chemical), bromobenzene (> 99%, China National Pharmaceutical Group Corporation), 4-bromoacetophenone (> 99%, Alfa Aesar), 3-bromotoluene (> 99%, Alfa Aesar), 4-bromophenol (> 99%, Tianjin Guangfu Fine Chemical Research Institute, China), 4-bromobenzoic acid (> 99%, Beijing Henye Fine Chemical Co., Ltd., China), 4-bromoanisole (> 99%, Tianjin Chemical Company, China), 4-chloronitrobenzene (> 99%, China National Pharmaceutical Group Corporation), PdCl2 (> 99.99%, Alfa Aesar), phenylboronic acid (> 99%, Beijing Wisdom Chemicals Co., Ltd., China), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl, > 99%, Shanghai Convachem Co., Ltd., China), N-hydroxysulfosuccin-imide sodium salt (sulfo-NHS, > 99%, Shanghai Convachem Co., Ltd.), iminodiacetic acid (IDA, > 99%, Tianjin Guangfu Fine Chemical Research Institute), tetrabutylammonium bromide (TBAB, > 99%, Tianjin Guangfu Fine Chemical Research Institute) and triethylamine (NEt3, > 99%, Tianjin Chemical Company) were all used as received. Double-distilled water was used in the following experiments. Other analytical reagents were used as received.
Synthesis of shelled-core PS-co-PMAA-IDA microspheres
The shelled-core PS-co-PMAA microspheres were synthesized from styrene and MAA by one-stage soap-free polymerization, and the synthetic route is similar to that introduced in a previous paper [32]. The resultant colloid was diluted and the carboxyl group concentration in the dispersion was calculated to be 0.12 mol/L. The functionalization method of PS-co-PMAA-IDA microspheres has been reported in our published article [34]. After the reaction was over, the dispersion was kept at room temperature for 36 h. Next, the dispersion was centrifuged and the precipitate phase was collected.
Synthesis of palladium-iminodiacetic-acid-complex-loaded PS-co-PMAA microspheres
After dispersing the collected precipitate in 100 mL of distilled water, 0.408 g PdCl2 was added and the mixture was allowed to stand for 12 h at room temperature. The dispersion was then centrifuged to concentrate the Pd2+ in the upper liquid phase, which was measured by atomic absorption spectrum (AAS), and the collected precipitate was again dispersed in 200 mL water. The resulting microsphere palladium-loading concentration was calculated to be 0.745 mg/mL.
General procedures for the Suzuki reaction
Aliquots of 1.0 mmol aryl halide, 3.0 mL of the catalytic dispersion and 3.0 mL [emim] [BF4] were added to a screw-capped vial with a side tube. The mixture was degassed under nitrogen purge for 10 min at room temperature, then slowly heated to 110 °C with vigorous stirring for 0.5 h to activate the reactants, as described elsewhere [35]. Next, the mixture was cooled to room temperature. Then, then 0.18 g phenylboronic acid (1.5 mmol) and, 0.40 mL NEt3 (3.0 mmol) were added. The mixture was again degassed under nitrogen purge for 10 min., after which the vial was bathed in preheated oil at a set temperature and magnetically stirred under nitrogen. After the reaction was complete, the mixture was cooled to room temperature rapidly. Using diethyl ether (3 × 20 mL), the product was extracted from the reaction mixture, then washed with water. The organic phase was collected, concentrated, and the subsequent product dried under vacuum at 40 °C, before weighing and analysis by 1HNMR.
Characterization
Transmission electron microscopy (TEM) was employed to observe the morphology of the microsphere and the polymeric-microsphere-supported catalyst. A small drop of the sample was deposited onto a carbon-coated copper grid and dried at room temperature under atmospheric pressure, then TEM was conducted using a Philips T20ST electron microscope at an acceleration voltage of 200 kV. The IR measurements were performed on a Bio-Rad FTS-6000 IR spectrometer. Using CDCl3 or DMSO as solvent, 1H NMR spectra was applied and recorded on a UNITY PLUS-400 spectrometer to measure the yield of the reaction. The Pd2+ concentration in aqueous solution was measured by atomic absorption spectrum (AAS), using a Solaar AAS 2 atomic absorption spectrometer.
Results and discussion
Synthesis and characterization of shelled-core, palladium-catalyst-loaded PS-co-PMAA microspheres
By one-stage soap-free polymerization with styrene (St) and methyl acrylic acid (MAA) in water, the shelled-core PS-co-PMAA microspheres containing a PS-rich core and a PMAA-rich shell were fabricated. A 5 wt% cross-linker of DVB was added in order to enhance the stability of the polymeric scaffold. As shown in Fig. 1, the cross-linked PS-co-PMAA microspheres with a shelled-core structure can be clearly distinguished. The average diameter of the cross-linked PS-co-PMAA is 480 nm and the diameter of the core is about 350 nm. Before loading the iminodiacetic acid (IDA) ligand onto the thin shell of PMAA to form IDA-functionalized shelled-core microspheres (PS-co-PMAA-IDA), part of the carboxyl groups in the PMAA shell were pre-activated with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl). This intermediate product was followed by treatment with N-hydroxysulfosuccin-imide sodium salt (sulfo-NHS) [34]. The IR spectra of the shelled-core PS-co-PMAA microspheres before and after being functionalized (Figure S1) indicate the characteristic absorption at 1650 cm−1 and 860 cm−1 is due to C=O stretching-vibration of the acylamide group in the pendant IDA. Thus, the formation of functionalized microspheres of PS-co-PMAA-IDA is confirmed.
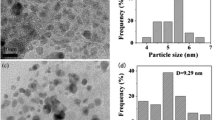
It is well known that IDA can strongly coordinate with Pd2+ [36], so the IDA-Pd-complex-loaded PS-co-PMAA microsphere (PS-co-PMAA-IDA-Pd) catalyst forms when the functionalized microspheres of PS-co-PMAA-IDA are dispersed in PdCl2 aqueous solution. To further confirm loading of IDA-Pd complex on the shell of the PS-co-PMAA microspheres, excess NaBH4 aqueous solution was added to the dispersion of the IDA-Pd-complex-loaded PS-co-PMAA microspheres to reduce the ions of Pd2+ to Pd(0). As shown in Fig. 2, the 3-nm nanoparticles synthesized in situ are mainly embedded in the shell of the shelled-core PS-co-PMAA microspheres where IDA was mainly distributed.
As introduced in the Experimental section, the molar ratio of PdCl2 to PMAA was 1:1. The added PdCl2 is excessive for IDA, because the PMAA segment on the surface of the PS-co-PMAA microspheres cannot completely react with IDA. In the experimental process, the concentration of Pd2+ loaded onto the functioned microspheres of PS-co-PMAA-IDA is 0.85 mmol/g, which is calculated based on the Pd2+ concentration in the centrifugal aqueous solution as measured by atomic absorption spectrum (AAS). AAS suggests that only 60% carboxyl groups in the shell-forming PMAA segment of the PS-co-PMAA microspheres are modified by IDA. The unmodified carboxyl groups in the shell-forming PMAA segment are useful to disperse the IDA-Pd catalyst in polar solvents, such as water (especially in basic aqueous solution), ethanol and in ionic [bmim][BF4] liquid, forming a stable colloidal dispersion. In fact, the concentration of the functionalized PS-co-PMAA-IDA microspheres in colloidal dispersion of [bmim][BF4]/H2O mix (1:1 by volume) is as high as 25 mg/mL at room temperature.
Suzuki reaction via the PS-co-PMAA-IDA-Pd catalyst in [bmim][BF4]/H2O mixture
As a model system in preliminary catalytic studies, we observed the Suzuki reaction of 4-bromoacetophenone and phenylboronic acid in a mixture of [bmim][BF4]/H2O (1:1 by volume) at temperatures ranging 80–110 °C, in the presence of 1.0 mol% PS-co-PMAA-IDA-Pd catalyst and 3 equiv. of various bases [Na2CO3, NaOH and triethylamine (NEt3)]. Here, it should be pointed out that the mixture of [bmim][BF4]/H2O instead of the pure ionic liquid of [bmim][BF4] is used as the reaction medium since the PS-co-PMAA-IDA-Pd catalyst is dispersed in aqueous solution. We found that the PS-co-PMAA-IDA-Pd catalyst can be “homogenously” dispersed in the [bmin][BF4]/water solvent mix just as a homogenous catalyst does at or above room temperature. By contrast, the substrates of aryl halides and the resultant biaryls cannot be dissolved in [bmin][BF4]/water solvent mix at room temperature. At or above 80 °C, the aryl halides and the resultant biaryls become soluble. The great difference in the solubility between the aryl halides and biaryls with the PS-co-PMAA-IDA-Pd catalyst is very useful in separating the resultant product from the reaction mixture, and reuse of the catalyst. The catalysis results are recorded in Table 1, and show that the bases used have little effect on the biaryl yields. Of the bases tested, NaOH and NEt3 resulted in highest coupling yields after 4 h (Table 1, entries 1–3). Compared with the bases, temperature has much greater effect on biaryl yields (Table 1, entries 4–6). For example, the yield of 4-acetylbiphenyl is just 58% at 80 °C in 1 h. (Table 1, entry 4), which increases to 99% at 110 °C in 30 min (Table 1, entry 6).
In following experiment, unless otherwise specified, the Suzuki reactions were performed in the [bmim][BF4]/H2O mix at 110 °C in the presence of 1.0 mol % PS-co-PMAA-IDA-Pd catalyst and 3 equiv. NEt3.
With the optimized conditions in hand, the Suzuki coupling reaction of phenylboronic acid was tested with a wide range of substrates, such as iodinated, brominated and chlorinated aromatic compounds. The results are summarized in Table 2 and indicate that the catalytic system is highly active for both aryl bromides and aryl iodides, especially aryl iodides. Corresponding biaryl yields are greater than 95% between 0.5 and 4 h (Table 2, entries 1–11). Furthermore, we found the catalytic activity of PS-co-PMAA-IDA-Pd is almost as efficient as the homogeneous IDA-Pd catalyst (data not shown). However, respective yields for the aryl chlorides of 4-chloronitrobenzene and 4-chloroacetophenone (Table 2, entries 12 and 13), are only 68% and 13%, despite adding one equiv. of TBAB and prolonging reaction time to 12 h.
As shown in Table 2, the activity of the aryl halides decreases in the order of I > Br > Cl. Moreover, the substituting groups of aryl halides have significant influence on their activity. The electron-deficient aryl halide is generally more active than the electron-rich one, excepting 4-iodobenzoic acid and 4-bromobenzoic acid (Table 2, entries 3 and 8). A possible cause is electrostatic repulsion between the electronegative substrates and the catalyst scaffold of the PS-co-PMAA microspheres in basic conditions.
Catalysis results were compared with those obtained by Mathews et al. using a homogeneous Pd(Ph3)4 catalyst under very similar conditions. The PS-co-PMAA-IDA-Pd catalyst used here affords slightly higher yield than the Pd(Ph3)4 catalyst of Mathews et al. [35] and does so at lower Pd catalyst concentration (1.0 mol% vs. 1.2 mol%). When iodobenzene (Table 2, entry 1), bromobenzene (Table 2, entry 6) and 4-bromoacetophenone (Table 2, entry 7) were used as substrate, the novel catalyst also yielded much more quickly (0.5 h vs.. 3 h). When 4-bromoanisole is substrate, PS-co-PMAA-IDA-Pd and Pd(Ph3)4 catalyst activity is similar (Table 2, entry 11). Compared with PdCl2 catalyst [37], the PS-co-PMAA-IDA-Pd catalyst have higher activity. For example, when m-bromomthyl benzene (Table 2, entry 9) were used as substrate, The PS-co-PMAA-IDA-Pd catalyst had the same yield at lower Pd catalyst concentration (1.0 mol % vs. 5.0 mol %) and shorter time (2 h vs. 8 h). As anticipated, the nature of PS-co-PMAA-IDA markedly influences the reaction outcome. As the well-accepted mechanism of the Suzuki reaction [38], the first key step is the formation of a PdII(aryl)(halide) species. The IDA-Pd catalyst dispersed on the surface of the PS-co-PMAA microspheres is favorable for the formation of PdII species which are stabilized in the ILs. Yield efficiency and activity results suggest that this novel PS-co-PMAA-IDA-Pd catalyst is a promising alternative.
For heterogeneous catalysts, an essential insight is whether the active metal migrates from solid to liquid phase, since thus-leached Pd will become significantly responsible for the extent of catalytic activity. To explore possible catalyst leaching, a Suzuki reaction on 4-bromoacetophenone with benzeneboronic acid was carried out in [bmim][BF4]/H2O mix (1:1 by volume), in the presence of 0.10 mol % of PS-co-PMAA-IDA-Pd catalyst until conversion reached 64% at 1 h. At that point, the PS-co-PMAA-IDA-Pd catalyst solid was filtered out at reaction temperature. The remaining liquid phase was allowed to react again, with conversion slightly increasing from 64 to 71% in 2 h, as shown in Fig. 3. These observations indicate that minimal Pd catalyst leaches into the liquid phase of [bmim][BF4]/H2O. Herein, it should be pointed out that a relatively low PS-co-PMAA-IDA-Pd catalyst content of 0.10 mol% was used to perform the Suzuki reaction of 4-bromoacetophenone with benzeneboronic acid, in sustained steady conditions over 3 h, as shown in Fig. 3. When a high PS-co-PMAA-IDA-Pd catalyst content of 1.0 mol % was used, the relative catalyst leached was negligible.
Last, we transfer our attention to recycling of the PS-co-PMAA-IDA-Pd catalyst. As discussed, the catalyst of PS-co-PMAA-IDA-Pd exists as a colloid in the [bmin][BF4]/water mix at room temperature, while most of aryl halides and the synthesized biaryls are not at all soluble, which makes reuse of the catalyst easy. After the catalytic reaction was stopped, the reaction system was cooled to room temperature, and extraction was performed with diethyl ether. The residual aryl halides and the synthesized biaryls transfer to ether phase, while the PS-co-PMAA-IDA-Pd catalyst remains in the [bmin][BF4]/water mix. To test the reusability of the PS-co-PMAA-IDA-Pd catalyst in [bmim][BF4], a Suzuki reaction on 4-bromoacetophenone by phenylboronic acid was chosen as a typical example. Catalysis results for this reaction appear in Table 3, where the isolated yield of 4-acetylbiphenyl is as high as 96% in the fourth cycle, confirming high reusability for PS-co-PMAA-IDA-Pd catalyst in [bmin][BF4]/water mix.
Conclusions
In summary, shelled-core PS-co-PMAA microspheres were prepared via one-stage soap-free polymerization method. Through modification of the shelled-core PS-co-PMAA microspheres, an IDA-Pd complex was loaded on the microsphere shell to form a PS-co-PMAA-IDA-Pd-loaded microsphere catalyst. This catalytic system can be used as a highly efficient and easily recycled catalyst for Suzuki reaction performed in ionic 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim][BF4]) liquid.
References
N. Miyaura, T. Yanagigand, A. Suzuki, Synth. Commun. 11, 513 (1981)
A. Suzuki, J. Organomet. Chem. 576, 147 (1999)
J. Hassan, M. Sevignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 102, 1359 (2002)
L. Yin, J. Liebscher, Chem. Rev. 107, 133 (2007)
Z.C. Xiong, N.D. Wang, M. Dai, A. Li, J.H. Chen, Z. Yang, Org. Lett. 6, 3337 (2004)
L.L. Zhang, Y.L. Guo, A. Iqbal, B. Li, M. Deng, D.Y. Gong, W.S. Liu, W.W. Qin, J. Nanopart. Res. 19, 150 (2017)
R.B. Bedford, S.J.C. Cazin, S.L. Hazelwood, Angew. Chem. Int. Ed. 114, 4294 (2002)
J.P. Stambuli, R. Kuwano, J.F. Hartwig, Angew. Chem. Int. Ed. 41, 4746 (2002)
R.B. DeVasher, L.R. Moore, K.H. Shaughnessy, J. Org. Chem. 69, 7919 (2004)
J.H. Kirchhoff, C. Dai, G.C. Fu, Angew. Chem. Int. Ed. 114, 2025 (2002)
K.N. Sharma, N. Satrawala, R.K. Joshi, Eur. J. Inorg. Chem. 16, 1743 (2018)
M. Ibrahim, I. Malik, W. Mansour, M. Sharif, M. Fettouhi, B.E. Ali, J. Organomet. Chem. 859, 44 (2018)
O. Navarro, R.A. Kelly, S.P. Nolan, J. Am. Chem. Soc. 125, 16194 (2003)
O. Bárta, I. Císařová, P. Štěpnička, Eur. J. Inorg. Chem. 2, 489 (2016)
R. Maity, A. Verma, M. van der Meer, S. Hohloch, B. Sarkar, Eur. J. Inorg. Chem. 1, 111 (2016)
H.Y. Liu, X.S. Li, F. Liu, Y. Tan, Y.Y. Jiang, J. Organomet. Chem. 794, 27 (2015)
A. Avila-Sorrosa, H.A. Jiménez-Vázquez, A. Reyes-Arellanoa, J.R. Pioquinto-Mendoza, R.A. Toscano, L. González-Sebastiánb, D. Morales-Morales, J. Organomet. Chem. 819, 69 (2016)
M. Khajehzadeh, M. Moghadam, J. Organomet. Chem. 863, 60 (2018)
H.H. Zhang, J. Han, F. Tian, Q.Z. Chen, C.Z. Wang, H. Jin, G.Y. Bai, Res. Chem. Intermed. 41, 6731 (2014)
S. Borah, S. Mishra, L. Cardenas, G. Nayanmoni, J. Inorg. Chem. 6, 751 (2018)
Y.L. Huang, Q. Wei, Y.Y. Wang, L.Y. Dai, Carbon 136, 150 (2018)
S. RoyKula, K.K. Senapati, P. Phukan, Res. Chem. Intermed. 41, 5753 (2014)
C. Biglione, A.L. Cappelletti, M.C. Strumia, S.E. MartínPaula, M. Uberman, J. Nanopart. Res. 20, 127 (2018)
J. Chen, J. Zhang, D.J. Zhu, T. Li, J. Porous Mater. 24, 847 (2017)
X. Liu, X.H. Zhao, M. Lu, J. Organomet. Chem. 768, 23 (2014)
B.H. Zhang, Y.G. Xue, A.N. Jiang, Z.M. Xue, Z.H. Li, J.C. Hao, A.C.S. Appl, Mater. Interfaces 9, 7217 (2017)
Y.C. Hu, N. Li, G.Y. Li, A.Q. Wang, Y. Cong, X.D. Wang, T. Zhang, ACS Catal. 7, 2576 (2017)
T. Itoh, Chem. Rev. 117, 10567 (2017)
A. Mondal, A. Das, B. Adhikary, D.K. Mukherjee, J. Nanopart. Res. 16, 2366 (2014)
P.W. Zheng, W.Q. Zhang, J. Catal. 250, 324 (2007)
X.W. Jiang, G.W. Wei, X. Zhang, W.Q. Zhang, P.W. Zheng, F. Wen, L.Q. Shi, J. Mol. Catal. A: Chem. 277, 102 (2007)
X.W. Jiang, Y. Wang, W.Q. Zhang, P.W. Zheng, L.Q. Shi, Macromol. Rapid Commun. 27, 1833 (2006)
S.T. Handy, X. Zhang, Org. Lett. 3, 233 (2001)
J.Z. Zhang, W.Q. Zhang, Y. Wang, M.C. Zhang, Adv. Synth. Catal. 350, 2065 (2008)
C.J. Mathews, P.J. Smith, T. Welton, Chem. Commum. 1249 (2000)
M. Pesavento, R. Biesuz, M. Gallorini, A. Profumo, Anal. Chem. 65, 2522 (1993)
C. Pan, M. Liu, L. Zhang, H. Wu, J. Ding, J. Cheng, Catal. Commun. 9, 508 (2008)
A. Fihri, M. Bouhrara, B. Nekoueishahraki, J.-M. Basset, V. Polshottiwar, Chem. Soc. Rev. 40, 5181 (2011)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 21706002), Natural Science Foundation of Anhui Province (1808085QB53), and the Research Fund of School of Chemistry and Chemical Engineering (Anhui University).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, J., Chen, J., Zhang, Q. et al. Polymeric microsphere-loaded palladium-iminodiacetic acid complex as an efficient and easily recycled catalyst for Suzuki reaction in ionic liquid. Res Chem Intermed 45, 2503–2514 (2019). https://doi.org/10.1007/s11164-019-03738-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-03738-y