Abstract
A novel, efficient, one-pot, catalyst-free grinding procedure for synthesis of 6-amino-3-methyl-4-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile is reported. The condensation of substituted benzaldehydes, 3-amino-5-methylpyrazole, and malononitrile according to a three-component reaction was investigated using density functional theory (DFT) at B3LYP/6-311G level to explore the reaction mechanism. All the routes were studied, the structure of the intermediates was optimized, and all the respective transition states were found. The results of the calculations show that the proposed mechanism relies on four intermediates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, pyrazolopyridines have attracted considerable attention owing to their high bioactivity [1,2,3]; For example, they are used as antagonists of angiotensin II and dopamine D3 receptor [4], inhibitors of cyclin-dependent kinase (CDK) [5], antileishmanial drugs [6], adenosine receptors [7], anxiolytic, antiherpetic and antitumor agents [8, 9], and antimicrobial and potent antitumor agents [10, 11]. The original procedure for preparation of this type of compounds involves one-pot condensation of benzaldehyde (R1), malononitrile (R2), and 3-amino-5-methylpyrazole (R3) [12] (Scheme 1).
Computational chemistry is an important field that studies the properties of compounds, their reactions, and the optimization of existing chemical methods using advanced and specialized software. One important area of such study is to analyze proposed mechanisms, which can be done using theoretical methods.
Multicomponent reactions (MCRs) represent one of the most efficient routes in synthetic organic chemistry, offering advantages such as simplicity, high speed, easy implementation, and high atom efficiency. They offer a model for diversity-oriented synthesis [13,14,15,16,17].
Experimental
Chemicals were purchased from Merck and Fluka. All solvents used were dried and distilled according to standard procedures. Thin-layer chromatography (TLC) was carried out on TLC silica gel 60 aluminum sheet from Merck. Melting points were measured on an Electrothermal 9100 apparatus. Infrared (IR) spectra were determined on a Shimadzu FT-IR 8600 spectrophotometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were determined on a Bruker 400 DRX Avance instrument at 500 and 125 MHz. Elemental analyses were carried out on a Carlo-Erba EA1110CNNO-S analyzer and agreed with the calculated values. For the ultrasound reactions, Astra 3D ultrasound apparatus (9.5 dm3, 45 kHz frequency, input power with heating, 305 W, number of transducers, 2) from TECNO-GAZ was used.
General procedure for synthesis of pyrazolo[3,4-b]pyridines 4a–o
A mixture containing benzaldehyde (1 mmol), malononitrile (1 mmol), and 5-amino-3-methylpyrazole (1 mmol) was ground at room temperature for the required reaction times. Reaction progress was monitored by TLC (EtOAc:petroleum ether 1:2). After reaction completion, EtOH (20 mL) was added, and the mixture was filtered. The solvent was removed under reduced pressure to afford the pure products. The solvent was recovered by rotary evaporator. All synthesized compounds were characterized by their physical constants, comparison with authentic samples, IR, 1H NMR, and 13C NMR spectroscopy, and elemental analysis.
6-Amino-3-methyl-4-(4-nitrophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4a)
Off-white solid, m.p. 309–311 °C, FT-IR (KBr, cm−1) νmax 3414, 3312, 2206, 1639, 1603, 1565, 1343, 1261 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.47 (s, 3H), 7.56 (d, J = 7.8 Hz, 2H), 7.81 (d, J = 7.8 Hz, 2H), 8.52 (s, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 31.6, 108.4, 119.3, 121.4, 129.5, 133.0, 141.3, 145.0, 149.3, 152.2, 154.8, 159.3 ppm. Anal. Calc. for C14H10N6O2: C, 57.14; H, 3.43; N, 28.56. Found: C, 57.16; H, 3.41; N, 28.59.
6-Amino-3-methyl-4-(3-nitrophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4b)
Off-white solid, m.p. 287–289 °C, FT-IR (KBr, cm−1) νmax 3372, 3305, 3232, 3094, 2917, 2180, 1603, 1505, 1354 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.45 (s, 3H), 7.44 (t, J = 7.8 Hz, 1H), 7.67 (d, J = 7.8 Hz, 1H), 8.02 (dd, J = 8.2 Hz, 2.4 Hz, 1H), 8.10 (t, J = 2.6 Hz, 1H), 8.54 (s, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 31.8, 109.5, 118.5, 120.4, 127.4, 129.6, 131.9, 133.4, 140.7, 143.6, 148.0, 151.3, 154.5, 159.7 ppm. Anal. Calc. for C14H10N6O2: C, 57.14; H, 3.43; N, 28.56. Found: C, 57.15; H, 3.45; N, 28.53.
6-Amino-3-methyl-4-(2-nitrophenyl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4c)
Brown solid, m.p. 288–290 °C, FT-IR (KBr, cm−1) νmax 3406, 3125, 2931, 2183, 1626, 1580, 1482, 1437 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.49 (s, 3H), 7.41 (t, J = 7.8 Hz, 1H), 7.66 (d, J = 8.2 Hz, 1H), 7.82 (t, J = 8.2 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 31.5, 108.3, 117.7, 119.8, 126.7, 128.9, 131.3, 133.2, 140.4, 142.0, 147.2, 150.6, 153.3, 157.9 ppm. Anal. Calc. for C14H10N6O2: C, 57.14; H, 3.43; N, 28.56. Found: C, 57.17; H, 3.45; N, 28.53.
6-Amino-4-(4-chlorophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4d)
Off-white solid, m.p. 266–268 °C, FT-IR (KBr, cm−1) νmax 3441, 3309, 3211, 3172, 2947, 1587, 1554, 1092 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.46 (s, 3H), 7.58 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 8.6 Hz, 2H), 8.84 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.1, 109.3, 118.5, 120.7, 127.8, 133.5, 141.4, 145.6, 148.6, 151.5, 154.5, 159.4 ppm. Anal. Calc. for C14H10ClN5: C, 59.27; H, 3.55; N, 24.68. Found: C, 59.25; H, 3.53; N, 24.70.
6-Amino-4-(3-chlorophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4e)
White solid, m.p. 261–263 °C, FT-IR (KBr, cm−1) νmax 3405, 3294, 3223, 3150, 2951, 1589, 1548, 1085 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.39 (s, 3H), 7.46 (t, J = 7.6 Hz, 1H), 7.63 (d, J = 7.6 Hz, 1H), 8.04 (dd, J = 8.2 Hz, 2.4 Hz, 1H), 8.13 (t, J = 2.4 Hz, 1H), 8.51 (s, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 31.2, 109.8, 118.3, 120.8, 126.5, 129.0, 131.2, 133.4, 139.3, 143.2, 147.3, 151.6, 153.8, 158.2 ppm.. Anal. Calc. for C14H10ClN5: C, 59.27; H, 3.55; N, 24.68. Found: C, 59.30; H, 3.57; N, 24.65.
6-Amino-4-(2-chlorophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4f)
White solid, m.p. 256–258 °C, FT-IR (KBr, cm−1) νmax 3306, 32824, 3209, 3138, 2957, 1586, 1538, 1082 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.53 (s, 3H), 7.45 (t, J = 7.6 Hz, 1H), 7.68 (d, J = 8.2 Hz, 1H), 7.84 (t, J = 8.4 Hz, 1H), 7.89 (d, J = 8.4 Hz, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.4, 108.7, 117.2, 119.5, 126.9, 129.5, 131.6, 133.5, 140.7, 142.4, 147.3, 150.8, 153.5, 158.3 ppm. Anal. Calc. for C14H10ClN5: C, 59.27; H, 3.55; N, 24.68. Found: C, 59.26; H, 3.53; N, 24.66.
6-Amino-4-(4-bromophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4g)
Off-white solid, m.p. 274–276 °C, FT-IR (KBr, cm−1) νmax 3614, 3108, 2904, 1605, 1574, 1466, 1114 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.48 (s, 3H), 8.07 (d, J = 7.8 Hz, 2H), 8.39 (d, J = 7.8 Hz, 2H), 8.74 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.6, 110.7, 118.9, 121.0, 126.9, 133.8, 141.6, 145.3, 147.8, 150.2, 154.2, 157.3 ppm. Anal. Calc. for C14H10BrN5: C, 51.24; H, 3.07; N, 21.34. Found: C, 51.22; H, 3.09; N, 21.31.
6-Amino-4-(3-bromophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4h)
Off-white solid, m.p. 268–270 °C, FT-IR (KBr, cm−1) νmax 3532, 3246, 2932, 1611, 1571, 1462, 1109 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.49 (s, 3H), 7.43–7.62 (m, 2H), 7.92 (dd, J = 7.8 Hz, 2.2 Hz, 1H), 8.12 (t, J = 2.2 Hz, 1H), 8.39 (s, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 32.6, 110.1, 118.2, 120.5, 127.6, 129.7, 131.3, 133.0, 139.4, 142.4, 148.4, 150.5, 154.1, 158.2 ppm. Anal. Calc. for C14H10BrN5: C, 51.24; H, 3.07; N, 21.34. Found: C, 51.23; H, 3.10; N, 21.36.
6-Amino-4-(4-iodophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4i)
Yellow solid, m.p. 283–285 °C, FT-IR (KBr, cm−1) νmax 3418, 3120, 2918, 1631, 1583, 1472, 786 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.38 (s, 3H), 8.11 (d, J = 7.6 Hz, 2H), 8.41 (d, J = 7.6 Hz, 2H), 8.69 (s, 1H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.8, 111.4, 119.4, 120.5, 127.0, 133.5, 140.7, 145.9, 145.8, 150.9, 154.4, 158.9 ppm. Anal. Calc. for C18H18IN5: C, 50.13; H, 4.21; N, 16.24. Found: C, 50.10; H, 4.18; N, 16.26.
6-Amino-4-(4-fluorophenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4j)
White solid, m.p. 261–263 °C, FT-IR (KBr, cm−1) νmax 3371, 3126, 2971, 1617, 1564, 1432, 848 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.47 (s, 3H), 7.96 (d, J = 7.8, 5.3 Hz, 2H), 8.23 (dd, J = 19.2, 7.8 Hz, 2H), 8.76 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.0, 109.3, 118.5, 120.4, 125.3, 133.1, 142.4, 144.7, 148.9, 150.0, 154.5, 158.4 ppm. Anal. Calc. for C18H18FN5: C, 66.86; H, 5.61; N, 21.66. Found: C, 66.84; H, 5.58; N, 21.69.
6-Amino-3-methyl-4-phenyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4k)
White solid, m.p. 275–277 °C, FT-IR (KBr, cm−1) νmax 3463, 3161, 2983, 1627, 1559, 1446 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.48 (s, 3H), 8.01–8.09 (m, 2H), 8.39 (m, 2H), 8.46–8.72 (s, 3H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.8, 109.7, 119.6, 121.6, 125.5, 133.3, 142.0, 144.9, 148.0, 149.7, 154.3, 158.5 ppm. Anal. Calc. for C14H11N5: C, 67.46; H, 4.45; N, 28.10. Found: C, C, 67.48; H, 4.43; N, 28.07.
6-Amino-3-methyl-4-p-tolyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4l)
White solid, m.p. 272–274 °C, FT-IR (KBr, cm−1) νmax 3543, 3194, 2940, 1638, 1549, 1460, 1182 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.43 (s, 3H), 2.58 (s, 3H), 7.63 (d, J = 7.8 Hz, 2H), 8.04 (d, J = 7.8 Hz, 2H), 8.77 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.8, 42.6, 109.5, 119.4, 120.6, 125.4, 133.9, 142.7, 145.4, 148.4, 149.0, 154.5, 157.4 ppm. Anal. Calc. for C15H13N5: C, 68.42; H, 4.98; N, 26.60. Found: C, 68.40; H, 4.96; N, 26.63.
6-Amino-4-(4-ethylphenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4m)
White solid, m.p. 271–273 °C, FT-IR (KBr, cm−1) νmax 3482, 3164, 2971, 1640, 1562, 1467, 1148 cm−1. 1H NMR (500 MHz, DMSO): δH; 1.04 (s, br, 3H), 2.48 (s, 3H), 2.64 (s, br, 2H), 7.83 (d, J = 7.6 Hz, 2H), 8.19 (d, J = 7.6 Hz, 2H), 8.48 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 19.8, 33.8, 46.5, 109.5, 118.5, 121.7, 125.9, 132.6, 141.6, 145.0, 147.6, 148.6 154.6, 159.3 ppm. Anal. Calc. for C16H15N5: C, 69.29; H, 5.45; N, 25.25. Found: C, 69.31; H, 5.43; N, 25.22.
6-Amino-4-(4-methoxyphenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4n)
White solid, m.p. 279–281 °C, FT-IR (KBr, cm−1) νmax 3548, 3121, 2904, 1624, 1548, 1460, 1238 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.43 (s, 3H), 3.57 (s, 3H), 7.85 (d, J = 7.8 Hz, 2H), 8.05 (d, J = 7.8 Hz, 2H), 8.82 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 33.8, 60.7, 109.4, 118.9, 121.8, 125.7, 131.9, 141.7, 143.6, 147.8, 149.9, 153.6, 159.2 ppm. Anal. Calc. for C15H13N5O: C, 64.51; H, 4.69; N, 25.07. Found: C, 64.48; H, 4.72; N, 25.09.
6-Amino-4-(3-methoxyphenyl)-3-methyl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (4o)
White solid, m.p. 271–273 °C, FT-IR (KBr, cm−1) νmax 3424, 3184, 2962, 1617, 1573, 1464, 1262 cm−1. 1H NMR (500 MHz, DMSO): δH; 2.48 (s, 3H), 3.61 (s, 3H), 7.41–7.60 (m, 2H), 7.83 (dd, J = 7.6 Hz, 2.4 Hz, 1H), 8.09 (t, J = 2.4 Hz, 1H), 8.41 (s, 2H) ppm. 13C NMR (125 MHz, DMSO): δC; 34.3, 63.4, 110.4, 118.5, 121.5, 125.6, 129.6, 131.7, 133.8, 138.7, 142.6, 150.6, 151.6, 154.7, 158.8 ppm. Anal. Calc. for C15H13N5O: C, 64.51; H, 4.69; N, 25.07. Found: C, 64.53; H, 4.66; N, 25.05.
Results and discussion
In continuation of our studies on synthesis of heterocyclic and pharmaceutical compounds using mild and practical protocols and theoretical study of heterocyclic compounds [18,19,20,21,22,23,24], we report herein experimental results on synthesis of 6-amino-3-methyl-4-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles, using various benzaldehydes, 3-amino-5-methylpyrazole, and malononitrile under grinding at room temperature (Scheme 1).
Table 1 compares the efficiency of the grinding method with some available methods previously reported for synthesis of pyrazolo[3,4-b]pyridines. It is clear from Table 1 that our method is more efficient, less time consuming, and simpler for synthesis of pyrazolo[3,4-b]pyridine derivatives (Table 1; entry 11).
On the other hand, solvent-free grinding is more efficient than use of classical Lewis acids such as ZnCl2, K10, and nano-Fe3O4 or ionic liquids.
To expand the scope of the efficient grinding reaction, we synthesized aldehydes with various substituents in this reaction condition (Table 2). According to Table 2, the rate of reaction and reaction time were improved for aldehydes with electron-withdrawing rather than electron-donating groups, although considerable yield improvement was not observed. It was found that the grinding procedure in this case can increase the reaction rate and thereby reduce energy consumption. All the synthesized compounds in Table 2 were characterized by spectroscopic methods (IR, 1H NMR, and 13C NMR) and elemental analysis.
Calculation method
The main purpose of this research is to carry out density functional theory (DFT) calculations at B3LYP level of the reactants, intermediates, transition states, and products in this reaction. First, all reactants, intermediates, and products were optimized using Gaussian 2009 software, and the total energy (Hartrees/particles) of the target compounds was evaluated. Calculations to characterize the obtained structures as minima or transition states on the TS (QST2) were carried out (Table 3).
Method of analysis
All theoretical calculations were performed using Gaussian 09 W program package [25] without any constraint on the geometry. All possible structures of reactants, intermediates, and products were fully optimized at B3LYP level using the 6-311G** basis set.
Quantum-chemistry methods are widely used for elucidating the mechanisms [26,27,28,29] of chemical reactions. The mechanism of the one-pot reaction for synthesis of some pyrazolo[3,4-b]pyridines was investigated using the DFT method here.
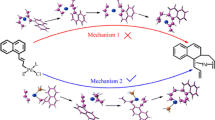
The progress of the reaction can be divided into three routes. The first route for synthesis of these pyrazolo[3,4-b]pyridines consists of five steps. Structures of four intermediates were optimized, and five transition states were found. The imaginary total energy (Hartrees/particles) of the five transition states are − 570.38, − 570.52, − 814.77, − 814.78, and − 815.52, respectively. The first step in this condensation is the reaction of R1 with R2 to produce IM1 via transition state TS1. A four-member ring is formed in TS1. Next, IM1 is expected to undergo rapid dehydration to α,β-unsaturated compound IM2. This step can be regarded as Michael addition of R3 to IM3. Then, IM3 converts to IM4 by intramolecular nucleophilic attack of amino group to nitryl group. This step is followed by tautomerization in intermediate IM4, producing compound PP. Finally, PP is changed to the final product P via a dehydration reaction (Scheme 2).
In the second pathway, there are three intermediates and four transition states for synthesis of these pyrazolo[3,4-b]pyridines. The imaginary total energy (Hartrees/particles) values of the four transition states are − 658.5 (TS6), − 844.4 (TS7), − 814.78 (TS4), and − 815.52 (TS5), respectively. The first step in this condensation is reaction of R1 with R3 to produce IM5 via TS6 as a four-member ring transition state. Next, IM5 and R2 undergo a dehydration reaction via TS7. The other steps are in accordance with the mechanism in the first route as shown in Scheme 3.
The third route consists of three steps (Scheme 4). The structures of the two intermediates were optimized, and three transition states were found. The total energy (Hartrees/particles) values of the transition states are − 662.02, − 661.81, and − 809.56, respectively. The first step in this condensation is reaction of R1 with R3 to produce IM7 via transition state TS8. Next, IM7 is expected to undergo rapid dehydration to compound IM8. This step can be regarded as nucleophilic addition of R2 to IM8. Finally, BP is produced as a byproduct in this proposed route.
The first route is the best proposed mechanistic pathway because the transition states in this mechanism have the lowest energy level. The rate-determining step is the third step of this route, including TS3 (Fig. 1).
Some studies have related the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap to the stability of a structure. However, the HOMO and LUMO values are completely dependent on the chemical structure of the compound. The HOMO and LUMO values were thus determined by DFT calculations, then the HOMO–LUMO gaps of all the intermediates were also considered. The results indicated that the HOMO–LUMO gap of IM1 was maximum, revealing that IM1 is the most stable form among the intermediates. These results confirm that the first route for synthesis of pyrazolo[3,4-b]pyridine 4k in Scheme 2 is correct (Fig. 2).
Conclusions
This is the first report of synthesis of pyrazolo[3,4-b]pyridines by multicomponent reaction between benzaldehydes, 3-amino-5-methylpyrazole, and malononitrile under grinding at room temperature as a green and low-cost method with high yield in very short time.
According to the calculated mechanisms, the reaction starts with Knoevenagel condensation between the benzaldehyde and malononitrile, followed by rapid C–C bond formation. Next, a synthesized α,β-unsaturated compound is produced by rapid dehydration. Then, Michael addition of 3-amino-5-methylpyrazole with intermediate, as the rate-limiting step, occurs. The final product is then produced by intramolecular nucleophilic attack of amino group to nitryl group, tautomerization in the related intermediate, and a dehydration reaction.
References
A.M. Li, Y. Ouyang, Z.Y. Wang, Y.Y. Cao, X.Y. Liu, L. Ran, C. Li, L. Li, L. Zhang, K. Qiao, J. Med. Chem. 56, 3593 (2013)
A.H. Shamroukh, A.E. Rashad, H.H. Sayed, J. Phosphorus Sulfur Silicon Rel. Elem. 180, 2347 (2005)
L. Commeiras, S.C. Woodcock, J.E. Baldwin, R.M. Adlington, A.R. Cowley, P. Wilkinson, Tetrahedron 60, 933 (2004)
A. Cappelli, C. Nannicini, A. Gallelli, G. Giuliani, S. Valenti, G.P. Mohr, M. Anzini, L. Mennuni, F. Ferrari, G. Caselli, A. Giordani, W. Pereis, F. Makovec, G. Giorgi, S. Vomero, J. Med. Chem. 51, 2137 (2008)
R. Lin, P.J. Connolly, Y. Lu, G. Chin, S. Li, Y. Yu, S. Huang, X. Li, S.L. Emanuel, S.A. Middleton, R.H. Gruninger, M. Adams, A.R. Fuentes-Pesquera, L.M. Greenberger, J. Bioorg. Med. Chem. Lett. 17, 4557 (2007)
H. de Mello, A. Echevarria, A.M. Bernardino, M. CantoCavalheiro, L.L. Leon, J. Med. Chem. 47, 5427 (2004)
F. Manetti, S. Schenone, F. Bondavalli, C. Brullo, O. Bruno, A. Ranise, L. Mosti, G. Menozzi, P. Fossa, M.L. Trincavelli, C. Martini, A. Martinelli, C. Tintori, M. Botta, J. Med. Chem. 48, 7172 (2005)
B.A. Johns, K.S. Gudmundsson, E.M. Turner, S.H. Allen, V.A. Samano, J.A. Ray, G.A. Freeman, F.L. Boyd, C.J. Sexton, D.W. Selleseth, K.L. Creech, K.R. Moniri, J. Bioorg. Med. Chem. 13, 2397 (2005)
K.S. Gudmundsson, B.A. Johns, Z. Wang, E.M. Turner, S.H. Allen, G.A. Freeman, F.L.B. Jr, C.J. Sexton, D.W. Selleseth, K.R. Monirib, K.L. Creech, J. Bioorg. Med. Chem. 13, 5346 (2005)
F.E. Goda, A.A.M. Abdel-Aziz, O.A. Attef, J. Bioorg. Med. Chem. Lett. 12, 1845 (2004)
N.M. Parekh, K.C. Maheria, J. Res. Chem. Intermed. 38, 885 (2012)
L. Zare, N.O. Mahmoodi, A. Yahyazadeh, M. Mamaghani, Syn. Commun. 41, 2323 (2011)
P.A. Wender, J. Nat. Prod. Rep. 31, 433 (2014)
A.Z. Halimehjani, I.N. Namboothiri, S.E. Hooshmand, RSC Adv. 4, 48022 (2014)
A.Z. Halimehjani, I.N. Namboothiri, S.E. Hooshmand, RSC Adv. 4, 51794 (2014)
V. Estévez, M. Villacampa, J.C. Menéndez, J. Chem. Soc. Rev. 43, 4633 (2014)
A. Domling, W. Wang, K. Wang, J. Chem. Rev. 112, 3083 (2012)
M. Nikpassand, L. Zare Fekri, M. Nabatzadeh, Comb. Chem. High Throughput Screen. 20, 533 (2017)
L. Zare Fekri, M. Nikpassand, J. Chil. Chem. Soc. 57, 1415 (2012)
M. Nikpassand, L. ZareFekri, S. Sanagou, Dyes Pigm. 136, 140 (2017)
H. Taherkhorsand, M. Nikpassand, Comb. Chem. High Throughput Screen. 21, 65 (2018)
L. Zare Fekri, M. Nikpassand, M. Goldoost, Russ. J. Gen. Chem. 83, 2352 (2013)
L. Zare Fekri, M. Nikpassand, Russ. J. Gen. Chem. 83, 2395 (2013)
M. Nikpassand, L. Zare Fekri, K. Hematinezhad, J. Polycycl. Arom. Comp. in press (2018)
M.J. Frisch, et al., GAUSSIAN09, Gaussian, Inc., Revision B. 05, Pittsburgh PA (2009)
J.G. Ma, J.M. Zhang, H.H. Jiang, W.Y. Ma, J.H. Zhou, Chin. Chem. Lett. 19, 375 (2008)
S. Karmakar, A. Datta, J. Org. Chem. 82, 1558 (2017)
S. Karmakar, A. Datta, J. Phys. Chem. B 121, 7621 (2017)
K. Bhattacharyya, S. Karmakar, A. Datta, Phys. Chem. Chem. Phys. 19, 22482 (2017)
Acknowledgements
Financial support from the Research Council of Islamic Azad University, Rasht Branch is sincerely acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikpassand, M., Fekri, L.Z. & Rahro, P.N. Catalyst-free grinding method: a new avenue for synthesis of 6-amino-3-methyl-4-aryl-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile and DFT studies on the mechanistic pathway of this category of compounds. Res Chem Intermed 45, 1707–1719 (2019). https://doi.org/10.1007/s11164-018-3701-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3701-9