Abstract
A series of 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives (8a–p) have been synthesized. The structure of the newly synthesized compounds was determined by spectral analysis. The title compounds were screened for their preliminary antitubercular activity against Mycobacterium tuberculosis H37Ra (MTB, ATCC 25177) and Mycobacterium bovis BCG (BCG, ATCC 35743). Further, the synthesized compounds were screened for antimicrobial activity against standard Gram-negative bacteria Escherichia coli (NCIM 2576) and Pseudomonas flurescence (NCIM 2059) and Gram-positive bacteria Staphylococcus aureus (NCIM 2602) and Bacillus subtilis (NCIM 2162). Among all the synthesized compounds, 8a–c, f–h, m exhibited good activity against dormant M. bovis BCG strain. Compounds 8h, j exhibited good activity against all tested bacterial strains. All active compounds were screened for cytotoxicity and found inactive. Their high potency and promising antimycobacterial activity suggest that these compounds could serve as good leads for further optimization and development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycobacterium tuberculosis (MTB) is among the most challenging bacterial infections. TB was one of the top 10 causes of death worldwide in 2015, responsible for more deaths than human immunodeficiency virus (HIV) and malaria [1]. In addition, M. bovis BCG is among the most commonly administered vaccines worldwide [2]. Due to emerging infectious diseases and the increasing number of multidrug-resistant microbial pathogens in recent decades, new classes of antimicrobial agents are required. The increase in antibiotic resistance due to multiple factors has encouraged the search for new compounds which are active against multidrug-resistant pathogens.
Synthesis of motifs containing more than one heterocycle ring has received much attention in recent years. Triazole and its derivatives are an important class of bioactive molecules, exhibiting significant pharmacological activities including antimicrobial [3, 4], antiinflammatory, anesthetic [5], antineoplastic [6], anticonvulsant [7], antiproliferative [8], anticancer [9], antimalarial [10], and antiviral activity [11]. Also, they show phosphodiesterase enzyme inhibition [12], anti-hepatitis C [13], β-lactamase inhibition [14], fungicidal [15], insecticidal [16], and plant growth inhibition [17] activity, as well as many more. Among heterocyclic derivatives, triazole compounds were reported to be the most promising candidates towards anti-TB activity [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33].
Thiazole and its derivatives are important structures in medicinal chemistry that could provide a rich spectrum of biological activities [34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59]. The structural diversity and biological importance of triazole and thiazoles have made them attractive targets for synthesis. 1,2,3-Triazole and thiazole rings present in the same molecule could represent a convenient model for investigation of their biological activity. Bearing in mind the biological significance of triazole and thiazole derivatives and in continuation of our study on synthesis and biological evaluation of various compounds [58, 59], we report herein synthesis of 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives 8a–p as potential antimycobacterial agents.
Experimental
All reactions were monitored and the purity of products checked by thin-layer chromatography (TLC) on Merck 60 F-254 silica gel plates with visualization by ultraviolet (UV) light. Melting points were determined in capillary tubes in silicone oil bath using a Veego melting point apparatus and are uncorrected. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on Bruker Avance II 500 NMR spectrometer (Bruker Instruments Inc., Billerica, MA, USA) at either 500 MHz (1H NMR) or 126 MHz (13C NMR). Chemical shifts are reported from internal tetramethylsilane standard and are given in δ units. Column chromatography was performed on silica gel (100–200 mesh) supplied by Acme Chemical Co. (Mumbai, Maharashtra, India). Starting (2-arylthiazol-4-yl)methanol compounds 3a–d were synthesized by known literature method [56]. Chemicals and solvents used were of laboratory grade and purified as per literature methods.
Synthesis of (2-phenylthiazol-4-yl)methyl methanesulfonate (5a)
To a mixture of (2-phenylthiazol-4-yl)methanol (3a, 2 g, 0.01 mol), triethylamine (2.02 mL, 0.02 mol) in dichloromethane (DCM, 20 mL) at 0 °C, and methanesulfonyl chloride (1.71 g, 0.015 mol) was added dropwise at 0 °C and the reaction mixture stirred for 3 h. After completion of reaction (TLC), the mixture was extracted with water. Organic layer was dried over sodium sulfate and evaporated on rotary evaporator to obtain (2-phenylthiazol-4-yl)methyl methanesulfonate (5a) as thick oil in yield of 2.42 g (90 %).
Synthesis of 4-(azidomethyl)-2-phenylthiazole (6a)
A mixture of (2-phenylthiazol-4-yl)methyl methanesulfonate (5a, 2.0 g, 0.007 mol) and sodium azide (0.58 g, 0.009 mol) in dimethyl sulfoxide (DMSO) was stirred for 3 h at 70 °C. After completion of reaction (TLC), the mixture was quenched in water and the product extracted by ethyl acetate (3 × 25 mL); organic layer was dried over sodium sulfate and evaporated under vacuum to give 4-(azidomethyl)-2-phenylthiazole (6a) as thick oil in yield of 1.3 g (86 %).
General procedure for synthesis of 2-phenyl-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole (8a)
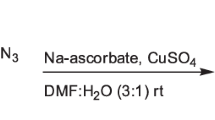
A reaction mixture of 4-(azidomethyl)-2-phenylthiazole (6a, 0.2 g, 0.0009 mol), ethynylbenzene (0.094 g, 0.0009 mol), copper sulfate (0.035 g, 0.00022 mol), and sodium ascorbate (0.045 g, 0.00022 mol) in dimethylformamide (DMF):water (6 mL, 3:1) was stirred overnight. After completion of reaction (TLC), the reaction mixture was quenched in water and extracted by ethyl acetate (3 × 15 mL). Organic layer was dried over sodium sulfate and evaporated on rotary evaporator. The crude product was purified by column chromatography (ethyl acetate:hexane) to furnish target compound 2-phenyl-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole (8a). Compounds 8b–p were synthesized by similar procedure.
2-Phenyl-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8a )
Yield: 70 %; m.p.: 138 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.76 (s, 2H), 7.25 (s, 1H), 7.37–7.43 (m, 3H), 7.45–7.51 (m, 3H), 7.85–7.87 (m, 2H), 7.99–7.93 (m, 2H), 8.02 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 117.7, 120.1, 125.8, 126.6, 128.2, 128.8, 129.1, 130.5, 130.6, 133.1, 148.1, 150.6, 169.5; HRMS: 319.1021 (M + H)+, 341.0840 (M + Na)+.
4-((4-(4-Fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-2-phenylthiazole ( 8b )
Yield: 76 %; m.p.: 146 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.75 (s, 2H), 7.11 (t, J = 8.7 Hz, 2H), 7.26 (s, 1H), 7.49–7.44 (m, 3H), 7.82 (dd, J = 8.8 and 3.3 Hz, 2H), 7.97–7.92 (m, 2H), 7.98 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 115.7, 115.9, 117.8, 119.9, 126.6, 126.8, 127.5, 127.5, 129.1, 130.6, 133.1, 147.3, 150.5, 161.7,163.7, 169.5; HRMS: 337.0930 (M + H)+, 359.0748 (M + Na)+.
4-((4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-2-phenylthiazole ( 8c )
Yield: 74 %; m.p.: 120 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.84 (s, 3H), 5.74 (d, J = 0.5 Hz, 2H), 6.98–6.93 (m, 2H), 7.23 (s, 1H), 7.48–7.43 (m, 3H), 7.80–7.74 (m, 2H), 7.93 (s, 1H), 7.95 (dd, J = 4.7 and 2.3 Hz, 2H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 55.3, 114.2, 117.6, 119.3, 123.3, 126.6, 127.1, 129.1, 130.5, 133.1, 148.0, 150.7, 159.6, 169.4; HRMS: 349.1124 (M + H)+, 371.0942 (M + Na)+.
4-((4-(3,4-Dimethoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-2-phenylthiazole ( 8d )
Yield: 80 %; m.p.: 138 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.90 (s, 3H), 3.95 (s, 3H), 5.74 (s, 2H), 6.90 (d, J = 8.3 Hz, 1H), 7.23 (s, 1H), 7.29 (dd, J = 8.1 and 1.8 Hz, 1H), 7.44–7.46 (m, 3H), 7.50 (d, J = 1.8 Hz, 1H), 7.94 (dd, J = 6.8 and 2.7 Hz, 3H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 56.0, 56.0, 109.0, 111.3, 117.6, 118.3, 119.5, 123.6, 126.6, 129.1, 130.5, 133.1, 148.0, 149.1, 149.3, 150.7, 169.4; HRMS: 379.1231 (M + H)+, 401.1050 (M + Na)+.
2-(4-Bromophenyl)-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8e )
Yield: 72 %; m.p.: 122 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.76 (s, 2H), 7.29 (s, 1H), 7.35 (d, J = 7.6 Hz, 2H), 7.62 - 7.56 (m, 3H), 7.89–7.84 (m, 2H), 7.91 (d, J = 7.9 Hz, 2H), 8.13 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 118.3, 120.1, 125.2, 125.8, 128.0, 128.9, 129.4, 130.6, 132.3, 133.4, 148.2, 150.9, 167.6; HRMS: 397.0124 (M + H)+, 418.9942 (M + Na)+.
2-(4-Bromophenyl)-4-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8f )
Yield: 75 %; m.p.: 98 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.76 (s, 2H), 7.12 (t, J = 8.6 Hz, 2H), 7.32 (s, 1H), 7.35 (d, J = 8.7 Hz, 2H), 7.75–7.78 (m, 2H), 7.89 (d, J = 8.7 Hz, 2H), 8.13 (s, 6H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 115.8, 115.9, 118.4, 119.9, 125.2, 127.5, 127.6, 129.4, 130.6, 132.3, 133.4, 147.4, 150.8, 161.7, 163.7, 167.6; HRMS: 415.0030 (M + H)+, 436.9848 (M + Na)+.
2-(4-Bromophenyl)-4-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8g )
Yield: 80 %; m.p.: 98 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.84 (s, 3H), 5.74 (s, 2H), 6.96 (d, J = 8.6 Hz, 2H), 7.27 (s, 1H), 7.33 (d, J = 7.9 Hz, 2H), 7.79 (d, J = 7.9, Hz, 2H), 7.91 (d, J = 7.9 Hz, 2H), 8.12 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 55.3, 114.3, 118.2, 119.3, 123.2, 123.2, 125.2, 127.1, 129.4, 130.6, 133.3, 148.1, 151.0, 159.7, 167.5; HRMS: 427.0226 (M + H)+, 449.0041 (M + Na)+.
2-(4-Bromophenyl)-4-((4-(p-tolyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8h )
Yield: 68 %; m.p.: 110 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 2.84 (s, 3H), 5.74 (s, 2H), 7.15 (d, J = 8.6 Hz, 2H), 7.24 (s, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.77 (d, J = 8.6 Hz, 2H), 7.87 (d, J = 8.1 Hz, 2H), 7.92 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 21.3, 49.9, 118.2, 119.7, 125.2, 125.7, 129.5, 130.6, 132.3, 133.3, 134.9, 138.1, 148.3, 151.0, 167.5; HRMS: 411.0269 (M + H)+, 433.0083 (M + Na)+.
2-(4-Chlorophenyl)-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8i )
Yield: 72 %; m.p.: 138 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.75 (s, 2H), 7.26 (s, 1H), 7.34 (d, J = 7.3 Hz, 2H), 7.42-7.44 (m, 3H), 7.85 (d, J = 8.0 Hz, 2H), 7.88 (d, J = 7.3 Hz, 2H), 8.00 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 117.9, 120.1, 125.8, 127.8, 128.2, 128.9, 129.3, 130.5, 131.6, 136.5, 148.2, 150.8, 168.1; HRMS: 353.0630 (M + H)+, 375.0448 (M + Na)+.
2-(4-Chlorophenyl)-4-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8j )
Yield: 66 %; m.p.: 120 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.73 (s, 2H), 7.08 (t, J = 8.6 Hz, 2H), 7.25 (s, 1H), 7.34 (d, J = 8.7 Hz, 2H), 7.76–7.78 (m, 2H), 7.88 (d, J = 8.7 Hz, 2H), 7.91 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0,115.7, 115.9, 118.0, 119.8, 126.8, 127.5, 127.5, 127.8, 129.3, 131.5, 136.6, 147.3, 150.7, 161.7, 163.7, 168.1; HRMS: 371.0534 (M + H)+, 393.0352 (M + Na)+.
2-(4-Chlorophenyl)-4-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8k )
Yield: 76 %; m.p.: 122 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.83 (s, 3H), 5.74 (s, 2H), 6.96 (d, J = 8.9 Hz, 2H), 7.25 (s, 1H), 7.3 (d, J = 8.1 Hz, 2H), 7.77 (d, J = 8.9 Hz, 2H), 7.88 (d, J = 8.1 Hz, 2H), 7.91 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 55.3, 114.3, 117.8, 119.3, 123.2, 127.1, 127.8, 129.3, 131.6, 136.5, 148.0, 150.9, 159.7, 168.0; HRMS: 383.0735 (M + H)+, 405.0553 (M + Na)+.
2-(4-Chlorophenyl)-4-((4-(3,4-dimethoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8l )
Yield: 80 %; m.p.: 120 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.89 (s, 3H), 3.94 (s, 3H), 5.72 (s, 2H), 6.88 (d, J = 8.3 Hz, 1H), 7.25 (s, 1H),7.27 (dd, J = 8.3 Hz and 1.5 Hz, 2H), 7.40 (d, J = 8.5 Hz, 2H), 7.47 (d, J = 1.5 Hz, 1H), 7.85 (d, J = 8.5 Hz, 2H), 7.92 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 55.9, 56.0, 109.0, 111.3, 117.9, 118.2, 119.5, 123.5, 127.8, 129.3, 131.5, 136.5, 148.0, 149.1, 149.3, 150.8, 168.0; HRMS: 413.0840 (M + H)+, 435.0658 (M + Na)+.
2-(4-Fluorophenyl)-4-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8m )
Yield: 70 %; m.p.: 96 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.75 (s, 2H),7.16 (dd, J = 12.6 and 4.7 Hz, 2H), 7.24 (s, 1H), 7.37–7.31 (m, 1H), 7.43 (m, 2H), 7.85 (m, 2H), 7.97–7.91 (m, 2H), 8.01 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 116.1, 116.3, 117.6, 120.1, 125.8, 128.2, 128.5, 128.6, 128.9, 129.5, 130.5, 148.2, 150.6,163.1, 165.1, 168.2; HRMS: 373.0930(M + H)+, 359.0748 (M + Na)+.
2-(4-Fluorophenyl)-4-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8n )
Yield: 66 %; m.p.: 154 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 5.74 (s, 2H), 7.13–7.15 (m, 4H), 7.26 (s, 1H), 7.84–7.78 (m, 2H), 7.95–7.91 (m, 2H), 7.96 (s, 1H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 115.7, 115.9, 116.1, 116.3, 117.7, 119.8, 126.8, 127.5, 127.5, 128.5, 128.6, 129.4, 147.3, 150.5, 161.7, 163.1, 163.7,165.1, 168.3; HRMS: 355.0831 (M + H)+, 377.0649 (M + Na)+.
2-(4-Fluorophenyl)-4-((4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)thiazole ( 8o )
Yield: 74 %; m.p.: 118 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.84 (s, 3H), 5.73 (s, 2H), 6.98–6.94 (m, 2H), 7.15 (t, J = 8.6 Hz, 2H), 7.23 (s, 1H), 7.79–7.75 (m, 2H), 7.91 (s, 1H), 7.96–7.92 (m, 2H); 13C NMR (126 MHz, CDCl3): δ C ppm = 49.9, 55.3, 114.2, 116.1, 116.3, 117.6, 119.3, 123.3, 127.1, 128.5, 128.6, 129.5, 148.0, 150.8, 159.64, 163.1, 165.1, 168.2; HRMS: 367.1031 (M + H)+, 389.0849 (M + Na)+.
4-((4-(3,4-Dimethoxyphenyl)-1H-1,2,3-triazol-1-yl)methyl)-2-(4-fluorophenyl)thiazole ( 8p )
Yield: 76 %; m.p.: 136 °C; 1H NMR (500 MHz, CDCl3): δ H ppm = 3.91 (s, 3H) 3.96 (s, 3H), 5.74 (s, 2H), 6.91 (d, J = 8.3 Hz, 1H), 7.15 (t, J = 8.6 Hz, 2H), 7.23 (s, 1H), 7.29 (t, J = 4.1 Hz, 2H), 7.50 (d, J = 1.8 Hz, 1H), 7.97–7.90 (m, 2H); 13C NMR (126 MHz, CDCl3): δ C ppm = 50.0, 55.9, 56.0, 109.0, 111.3, 116.1, 116.3, 117.6, 118.2, 119.5, 123.5, 128.5, 128.6, 129.4, 148.1, 149.1, 149.3, 150.7, 163.1, 165.1, 168.2; HRMS: 397.1136 (M + H)+, 419.0954 (M + Na)+.
Experimental protocol for biological activity
Antitubercular activity
All synthesized compounds were screened for their in vitro activity against M. tuberculosis H37Ra (ATCC 25177) and M. bovis BCG (ATCC 35743) using twofold dilution technique to determine the actual minimum inhibitory concentration (MIC). Activity against MTB was determined through XTT reduction menadione assay (XRMA), reading absorbance at 470 nm as per the protocol described in literature [60,61,62,63,64]. Nitrate reductase (NR) assay was performed to estimate inhibition of M. bovis BCG [60,61,62,63,64], measuring absorbance at 540 nm. In vitro activity against MTB and M. bovis BCG at dormant (12 days) stage was performed using the XRMA and NR assay, respectively, as described above. Percentage inhibition was calculated using the following formula:
where “control” is the activity of mycobacteria without compounds, “CMP” is the activity of mycobacteria in presence of compound, and “blank” is the activity of culture medium without mycobacteria.
Cytotoxicity
To check the selectivity, selected 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives (8a–p) were assayed for their cytotoxic effects in two different cell lines (HeLa, HCT) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [65, 66]. The cell lines were maintained under standard cell culture conditions under 5 % CO2 at 37 °C in 95 % air humidified environment. Each concentration was tested in duplicates in a single experiment.
Antibacterial activity
All bacterial cultures were first grown in Luria–Bertani medium at 37 °C at 180 rpm. Once the culture reached 1 OD, it was used for antibacterial assay. Bacterial strains Escherichia coli (NCIM 2576), Pseudomonas flurescence (NCIM 2059) as Gram-negative and Staphylococcus aureus (NCIM 2602) and Bacillus subtilis (NCIM 2162) as Gram-positive were obtained from NCIM (NCL, Pune) and grown in Luria–Bertani medium from HiMedia, India. The assay was performed in 96-well plates after 8 and 12 h for Gram-negative and Gram-positive bacteria, respectively. Screening was carried out using 0.1 % of 1-OD culture at 620 nm. Inoculated culture was added into each well of 96-well plate containing the compounds to be tested. Optical density for each plate was measured at 620 nm after 8 h for Gram-negative bacteria and after 12 h for Gram-positive bacteria.
Results and discussion
A series of 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives (8a–p) were synthesized according to Scheme 1. Ethyl 3-bromo-2-oxopropanoate 2 on cyclocondensation reaction with aryl thioamides 1a–d gave ethyl 2-arylthiazole-4-carboxylates 3a–d. Ester 3a–d on reduction with lithium aluminum hydride in tetrahydrofuran (THF) gave (2-arylthiazol-4-yl)methanol 4a–d; subsequent reaction with methanesulfonyl chloride and triethylamine in DCM furnished (2-phenylthiazol-4-yl)methyl methanesulfonate 5a–d, which on reaction with sodium azide in DMSO gave 4-(azidomethyl)-2-arylthiazole 6a–d. Azide 6a–d, on click reaction with substituted alkynes 7a–d, furnished target compounds 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole 8a–p.
The structure of the title compounds 8a–p was confirmed by NMR and high-resolution mass spectrometry (HRMS). As a representative analysis of compound 4-((4-(4-fluorophenyl)-1H-1,2,3-triazol-1-yl)methyl)-2-phenylthiazole (8b), the 1H NMR spectrum of compound 8b displayed one singlet in aliphatic region at δ 5.75 (thiazole-CH2-triazole). A triplet at δ 7.11 and multiplet at δ 7.80–7.83 were attributed to protons of fluoro-substituted phenyl ring, while multiplet at δ 7.44–7.49 and δ 7.92–7.97 corresponds to protons of phenyl ring. Thiazole and triazole protons resonated as singlet at δ 7.26 and 7.98, respectively. The 13C NMR spectrum of compound 8b showed one signal of thiazole-CH2-N carbon at δ 50.0. Aromatic carbons of fluoro-substituted phenyl showed typical fluoro-coupling [C 1–F δ 163.66, 161.69 (1 J = 248 Hz), C 2-F δ 115.89, 115.72 (2 J = 21.42 Hz), C 3-F δ 127.54, 127.48 (3 J = 7.56 Hz)]. Structure of compound 8b was further confirmed by HRMS, which showed molecular ion peak at m/z 337.0930 (M + H)+, 359.0748 (M + Na)+. Structures of all derivatives were ascertained similarly.
Biological evaluation
Antitubercular activity
The antitubercular activity of each synthesized compound was determined by measuring inhibition of growth against a virulent dormant-stage strain of M. tuberculosis H37Ra (MTB, ATCC 25177) and M. bovis (BCG, ATCC 35743) in liquid medium. In preliminary screening, the antimycobacterial activity of these compounds was assessed at concentrations of 30, 10, and 3 μg/mL using first-line antitubercular drug rifampicin as reference standard. In vitro activity studies against dormant stage of MTB and M. bovis BCG were performed using XRMA [60,61,62,63] and NR assay [60,61,62,63], respectively. The results of antitubercular activity are reported in Table 1.
The in vitro antitubercular assay against M. tuberculosis H37Ra and M. bovis BCG revealed that compounds 8a–c, f–h, m (MIC 0.03–2.47 µg/mL) exhibited good activity against dormant-stage BCG strain. The preliminary structure–activity relationship study revealed that replacement of hydrogen atom of phenyl ring A and B (Fig. 1) by substituent groups such as Br, Cl, F, OMe, and CH3 affected the antitubercular activity.
Further, it was also noted that, among the compounds 8a–d with unsubstituted phenyl ring A and substituted phenyl ring B, compound 8a (R1 = H) showed good activity against M. tuberculosis H37Ra (MIC 9.28 µg/mL) and M. bovis (MIC 0.88 µg/mL), compound 8b (R1 = 4-F) exhibited good activity against H37Ra (MIC 8.99 µg/mL) and M. bovis (MIC 1.69 µg/mL), whereas compound 8c (R1 = 4-OMe) showed good activity against M. bovis (MIC 1.37 µg/mL). Among the compounds 8e–h with 4-bromo-substituted phenyl ring A and substituted phenyl ring B, compound 8f (R1 = 4-F) and 8h (R1 = 3,4-OMe) showed excellent activity against M. bovis (MIC 0367 and 0.35 µg/mL respectively) and 8g (R1 = 4-OMe) exhibited good activity with MIC of 2.25 µg/mL Among the compounds 8i–l with 4-chloro-substituted phenyl ring A and substituted phenyl ring B, all compounds were found less active. From the compounds 8m–p with 4-fluoro-substituted phenyl ring A and substituted phenyl ring B, compound 8m exhibited good activity against M. bovis (MIC 2.47 µg/mL), whereas compounds 8n–p were found less active. It was notable that compounds with unsubstituted or 4-bromo-substituted phenyl ring A and substituted phenyl ring B showed good antitubercular activity against BCG.
Compounds 8a–p were further evaluated against two human cancer cell lines (HeLa and HCT116) and against primary human umbilical vein endothelial cells (HUVECs) to check the toxicity of these compounds (Table S-2). All active compounds were relatively nontoxic up to 100 μg/mL against HUVECs; the results of cytotoxicity screening are presented in Table 2.
According to the study on drug susceptibility of TB, the antimycobacterial activity was considered to be specific for selectivity index >10. The selectivity index (SI) was calculated by dividing GI50 for cell lines (HeLa, HCT 116, and HUVEC) by the MIC for in vitro activity against dormant MTB and M. bovis BCG. For dormant state of M. bovis BCG, all compounds showed SI higher than >40 against primary HUVEC cells (Table 2).
Cytotoxic activity evaluation
The results of this evaluation are presented in Table 2.
Antibacterial activity
The antibacterial activity of synthesized compounds was determined against standard Gram-negative bacteria E. coli (NCIM 2576) and P. flurescence (NCIM 2059) and Gram-positive bacteria S. aureus (NCIM 2602) and B. subtilis (NCIM 2162). Ampicillin served as positive control for antibacterial activity [64]. The in vitro preliminary screening values (% inhibition) against the tested microorganisms are summarized in Table S-2. The antibacterial activity results are reported as minimum inhibitory concentration in Table 3.
Analysis of the antibacterial activity results presented in Table 3 provides some lead molecules with good antibacterial activity. Among the compounds 8a–p tested, it was observed that the synthesized compounds showed moderate to good activity. It is worthwhile to mention that compounds 8h (R = Br, R1 = CH3) and 8j (R = Cl, R1 = F) exhibited good activity with MIC values of 4.5 to 20.2 μg/mL against all tested strains. Compounds 8l (R = Cl, R1 = CH3) and 8o (R = F, R1 = OCH3) showed good antibacterial activity against S. aureus with MIC of 25.6 and 28.5 μg/mL, respectively, while compound 8o also exhibited good activity against E. coli with MIC of 23.3 μg/mL. Thus, it is concluded that compounds with R = Br, R1 = CH3 and R = Cl, R1 = F group showed good antibacterial activity.
Conclusions
We describe synthesis and biological screening of 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives 8a–p. Most of the synthesized compounds with unsubstituted or 4-bromo-substituted phenyl ring at 2-position of thiazole and substituted phenyl ring at 4-position of 1,2,3-triazole showed good antitubercular activity against M. bovis. Most of the synthesized compounds exhibited good antibacterial activity. These results warrant synthesis of similar libraries with other substituents to confirm the trend described herein.
References
World Health Organization, Tuberculosis Fact Sheet (2016), http://www.who.int/tb/publications/global_report/en/
Q. Wang, F. Song, X. Xiao, P. Huang, L. Li, A. Monte, W.M. Abdel-Mageed, J. Wang, H. Guo, W. He, F. Xie, H. Dai, M. Liu, C. Chen, H. Xu, M. Liu, A.M. Piggott, X. Liu, R.J. Capon, L. Zhang, Angew. Chem. Int. Ed. 52, 1231–1234 (2013)
R. Ramesh, R.D. Shingare, V. Kumar, A. Anand, B. Swetha, S. Veeraraghavan, S. Viswanadha, R. Ummanni, R. Gokhale, D.S. Reddy, Eur. J. Med. Chem. 122, 723–730 (2016)
V.U. Jeankumar, S.R. Rudraraju, R. Vats, R. Janupally, S. Saxena, P. Yogeeswari, D. Sriram, Eur. J. Med. Chem. 122, 216–231 (2016)
M.D. Chen, S.J. Lu, G.P. Yuag, S.Y. Yang, X.L. Du, Heterocycl. Commun. 6, 421–426 (2000)
B.S. Holla, M. Mahalinga, M.S. Karthikeyen, B. Poojary, P.M. Akberali, N.S. Kumari, Eur. J. Med. Chem. 40, 1173–1178 (2005)
H.N. Hafez, H.A. Abbas, A.R. El-Gazzar, Acta Pharm. 58, 359–378 (2008)
A. Passannanti, P. Diana, P. Barraja, F. Mingooia, A. Lauria, G. Cirrincine, Heterocycles 48, 1229–1235 (1998)
L.P. Guan, Q.H. Jin, G.R. Tian, K.Y. Chai, Z.S. Quan, J. Pharm. Sci. 10, 254–262 (2007)
S. Manfredini, C.B. Vicentini, M. Manfrini, N. Bianchi, C. Rustigliano, C. Mischiati, R. Gambari, Bioorg. Med. Chem. 8, 2343–2346 (2000)
V. Dmitry, A.V. Demchuk, N.B. Samet, V.I. Chernysheva, G.A. Ushkarov, L.D. Stashina, M.M. Konyushkin, S.I. Raihstat, A.A. Firgang, M.P. Philchenkov, L.M. Zavelevich, V.F. Kuiava, D.Y. Chekhun, A.S. Blokhin, M.N. Kiselyov, V.V. Semenova, Bioorg. Med. Chem. 22, 738–755 (2014)
R. Gujjar, A. Marwaha, J. White, L. White, S. Creason, D.M. Shackleford, J. Baldwin, W.N. Charman, F.S. Buckner, S. Charman, P.K. Rathod, M.A. Phillips, J. Med. Chem. 52, 1864–1872 (2009)
B.A. Johns, J.G. Weatherhead, S.H. Allen, J.B. Thompson, E.P. Garvey, S.A. Foster, Bioorg. Med. Chem. Lett. 19, 1802–1806 (2009)
S.C. Beasley, N. Cooper, L. Gowers, J.P. Gregory, A.A.F. Haughan, P.G. Hellewell, D. Macar, J. Miotla, J.G. Montana, T. Morgan, R. Taylor, K.A. Runcie, B. Tuladhar, J.B.H. Warneck, Bioorg. Med. Chem. Lett. 8, 2629–2634 (1998)
C.E. De, Clin. Microbiol. Rev. 10, 674–693 (1997)
T. Weide, S.A. Saldanha, D. Minond, T.P. Spicer, J.R. Fotsing, M. Spaargaren, J. Frere, C. Bebrone, K.B. Sharpless, P.S. Hodder, V.V. Fokin, A.C.S. Med, Chem. Lett. 1, 150–154 (2010)
G. Heubach, B. Sachse, H. Buerstell, Ger. Offen 2, 760–826 (1979)
B. Chaia, X. Qianb, S. Caoa, H. Liua, G. Song, ARKIVOC ii, 141-145, (2003)
A.R. Bhat, G.V. Bhat, G.G. Shenoy, J. Pharm. Pharmacol. 53, 267–272 (2001)
N.B. Patel, I.H. Khan, S.D. Rajani, Eur. J. Med. Chem. 45, 4293–4299 (2010)
G.R. Jadhav, M.U. Shaikh, R.P. Kale, M.R. Shiradkar, C.H. Gill, Eur. J. Med. Chem. 44, 2930–2935 (2009)
H. Foks, M. Janowiec, Z. Zwolska, E. Augustynowicz-Kopeć, Phosphorus Sulfur Silicon Relat. Elem. 180, 537–543 (2005)
D. Castagnolo, M. Radi, F. Dessi, F. Manetti, M. Saddi, R. Meleddu, A.D. Logu, M. Botta, Bioorg. Med. Chem. Lett. 19, 2203–2205 (2009)
M. Shiradkar, S. Kumar, V. Dasari, S. Tatikonda, K.C. Akula, R. Shah, Eur. J. Med. Chem. 42, 807–816 (2007)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, A.T. Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911–3922 (2012)
M.H. Shaikh, D.D. Subhedar, L. Nawale, D. Sarkar, F.A. Kalam Khan, J.N. Sangshetti, B.B. Shingate, Med. Chem. Commun 6, 1104–1116 (2015)
P. Shanmugavelan, S. Nagarajan, M. Sathishkumar, A. Ponnuswamy, P. Yogeeswari, D. Sriram, Bioorg. Med. Chem. Lett. 21, 7273–7276 (2011)
N. Boechat, V.F. Ferreira, S.B. Ferreira, M.L.G. Ferreira, F.C. da Silva, M.M. Bastos, M.S. Costa, M.C.S. Lourenço, A.C. Pinto, A.U. Krettli, A.C. Aguiar, B.M. Teixeira, N.V. da Silva, P.R.C. Martins, F.A.F.M. Bezerra, A.L.S. Camilo, G.P. da Silva, C.C.P. Costa, J. Med. Chem. 54, 5988–5999 (2011)
G.R. Labadie, A. de la Iglesia, H.R. Morbidoni, Mol. Divers. 15, 1017–1024 (2011)
C. Gill, G. Jadhav, M. Shaikh, R. Kale, A. Ghawalkar, D. Nagargoje, M. Shiradkar, Bioorg. Med. Chem. Lett. 18, 6244–6247 (2008)
S.R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, T. Anjana Devi, S.V. Kalivendi, S. Kantevari, J. Med. Chem. 55, 3911–3922 (2012)
L. Chen, D.J. Wilson, Y. Xu, C.C. Aldrich, K. Felczak, Y.Y. Sham, K.W. Pankiewicz, J. Med. Chem. 53, 4768–4778 (2010)
R.S. Upadhayaya, G.M. Kulkarni, N.R. Vasireddy, J.K. Vandavasi, S.S. Dixit, V. Sharma, J. Chattopadhyaya, Bioorg. Med. Chem. 17, 4681–4692 (2009)
C. Menendez, S. Gau, C. Lherbet, F. Rodriguez, C. Inard, M.R. Pasca, M. Baltas, Eur. J. Med. Chem. 46, 5524–5531 (2011)
S.P. Pardeshi, S.S. Patil, V.D. Bobade, Bioorg. Med. Chem. Lett. 21, 6559–6562 (2011)
T. Tomasic, S. Katsamakas, Z. Hodnik, J. Ilas, M. Brvar, T. Solmajer, S. Montalvao, P. Tammela, M. Banjanac, G. Ergovic, M. Anderluh, L.P. Masic, D. Kikelj, J. Med. Chem. 58, 5501–5521 (2015)
Z.Y. Liu, Y.M. Wang, Z.R. Li, J.D. Jiang, D.W. Boykin, Bioorg. Med. Chem. Lett. 19, 5661–5664 (2009)
S.A.F. Rostom, I.M. El-Ashmawy, H.A. Abd El Razik, M.H. Badr, H.M.A. Ashour, Bioorg. Med. Chem. Lett. 17, 882–895 (2009)
D. Sampson, X.Y. Zhu, S.V.K. Eyunni, J.R. Etukala, E. Ofori, B. Bricker, N.S. Lamango, V. Setola, B. Roth, S.Y. Ablordeppey, Bioorg. Med. Chem. Lett. 22, 3105–3114 (2014)
G.S. Inamdar, A.N. Pandya, H.M. Thakar, V. Sudarsanam, S. Kachler, S. Moro, D. Sabbadin, K.N. Klotz, K.K. Vasu, Eur. J. Med. Chem. 63, 924–934 (2013)
Y.S. Lee, H. Kim, Y.H. Kim, E.J. Roh, H. Han, K.J. Shin, Bioorg. Med. Chem. Lett. 22, 7555–7561 (2012)
Y.K. Abhale, K.K. Deshmukh, A.V. Sasane, A.P. Chavan, P.C. Mhaske, J. Heterocycl. Chem. 53, 229–233 (2016)
P.C. Mhaske, S.H. Shelke, M. Bhoye, V.D. Bobade, J. Heterocycl. Chem. 54, 1590–1597 (2017)
S. Malik, R.S. Bahare, S.A. Khan, Eur. J. Med. Chem. 67, 1–13 (2013)
M.F. Arshad, N. Siddiqui, A. Elkerdasy, H. Abdulmohsen, A.L. Rohaimi, S.A.A. Khan, J. Pharmacol. Toxicol. 9, 132–138 (2014)
J.L. Falco, A. Palomer, A. Guglietta, US 2008/0200473 A12008
F. Hayat, E. Yoo, H. Rhim, H.Y.P. Choo, Bull. Korean Chem. Soc. 34, 495–499 (2013)
R.B. Clark, D. Lamppu, L. Libertine, A. McDonough, A. Kumar, G.L. Rosa, R. Rush, D. Elbaum, J. Med. Chem. 57, 3966–3983 (2014)
J.A. Shiran, A. Yahyazadeh, M. Mamaghani, M. Rassa, J. Mol. Struct. 1039, 113–118 (2013)
M. Brvar, A. Perdith, G. Anderluh, D. Turk, T. Solmajer, J. Med. Chem. 55, 6413–6426 (2012)
C. Araniciu, A.E. Parvu, B. Tiperciuc, M. Palage, S. Oniga, P. Verite, O. Oniga, J Nanomater. Biostruct. 8, 699–709 (2013)
O. Oniga, J.T. Ndongo, C. Moldovan, B. Tiperciuc, S. Oniga, A. Pirnau, L. Vlase, P. Verite, Farmacia 6, 785–797 (2012)
C.B. Mark, J.W. Dale, E.A. Merritt, X. Xin, Chem. Rev. 105, 685–714 (2005)
E. Riego, D. Hernandez, F. Albericio, M. Alvarez, Synthesis 2005, 1907–1922 (2005)
J. Parvate, V.S. Bhagwat, M.M. Doshi, H.L. Mondkar, Indian Drugs 26, 222–226 (1989)
S.H. Shelke, P.C. Mhaske, P. Hande, V.D. Bobade, Phosphorus Sulfur Silicon Relat. Elem. 188, 1262–1270 (2013)
M.R. Shiradkar, K.K. Murahari, G.H. Reddy, S. Tatikonda, A.K. Chakravarthy, P. Dolly, R. Kaur, P. Burange, J. Ghogare, V. Mokalec, M. Rautc, Bioorg. Med. Chem. Lett. 15, 3997–4008 (2007)
Y.K. Abhale, A.V. Sasane, A.P. Chavan, K.K. Deshmukh, S.S. Kotapalli, R. Ummanni, S.F. Sayyad, P.C. Mhaske, Eur. J. Med. Chem. 94, 340–347 (2015)
Y.K. Abhale, A.V. Sasane, A.P. Chavan, S.H. Shekh, K.K. Deshmukh, S. Bhansali, L. Nawale, D. Sarkar, P.C. Mhaske, Eur. J. Med. Chem. 132, 333–340 (2017)
A. Khan, D. Sarkar, J. Microbiol. Methods 73, 62–68 (2008)
R. Singh, L. Nawale, M. Arkile, U. Shedbalkar, S. Wadhwani, D. Sarkar, B. Chopade, Int. J. Antimicrob. Agents 46, 183–188 (2015)
S. Sarkar, D. Sarkar, J. Biomol. Screen. 17, 966–973 (2012)
U. Singh, S. Akhtar, A. Mishra, D. Sarkar, J. Microbiol. Methods 84, 202–207 (2011)
A. Khan, S. Sarkar, D. Sarkar, Int. J. Antimicrob. Agents 32, 40–45 (2008)
T. Mosmann, J. Immunol. Methods 65, 55–63 (1983)
G. Ciapetti, E. Cenni, L. Pratelli, A. Pizzoferrato, Biomaterials 4, 359–364 (1993)
R.C. Hartkoorn, B. Chandler, A. Owen, S.A. Ward, S.B. Squire, D.J. Back, S.H. Khoo, Tuberculosis 87, 248 (2007)
Acknowledgements
The authors are grateful to CSIR-NCL, Pune for supporting the biological activity investigation. Central Analysis Facility, Savitribai Phule Pune University, Pune is also acknowledged for spectral analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shinde, V., Mahulikar, P., Mhaske, P.C. et al. Synthesis and biological evaluation of new 2-aryl-4-((4-aryl-1H-1,2,3-triazol-1-yl)methyl)thiazole derivatives. Res Chem Intermed 44, 1247–1260 (2018). https://doi.org/10.1007/s11164-017-3164-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3164-4