Abstract
A comparative study was carried out on the photocatalytic properties depending on Jodifferent morphologies of Ag3PO4 fabricated by different methods, i.e. as precipitated, oil bath heat treatment, hydrothermal, and microwave-assisted hydrothermal. The crystal structures of the prepared samples were analyzed by X-ray diffraction. The different morphologies of Ag3PO4 synthesized by different methods were observed from SEM micrographs. The micrographs show that the as-precipitated Ag3PO4 sample has smaller grain morphology whereas samples synthesized by other methods have distinct large-grain morphologies. Sharp-edged rhombic dodecahedron, cubic and polyhedral Ag3PO4 crystals were observed that were synthesized by microwave-assisted hydrothermal and hydrothermal methods. However, sharp-edged polyhedral Ag3PO4 crystals were not observed from the oil bath heat-treatment method. The optical properties of the different samples were measured by using UV–Vis diffuse reflectance spectroscopy. The samples fabricated by the microwave-assisted hydrothermal process showed better photocatalytic decoloring of methylene blue than those prepared by the hydrothermal and oil bath heat-treatment methods.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Semiconductor photocatalysts have attracted great attention due to their great potential in solving numerous environmental and energy problems ranging from degrading organic contaminants and water splitting to artificial photosynthesis, and most of them are chemically stable [1,2,3,4,5,6,7]. Among the various photocatalysts, TiO2 has been widely studied due to its appropriate band gap, stability and low cost, non-toxicity, and environment friendly advantages. However, they are photoactive only under UV light, which covers only 3–4% of the solar spectrum [8], and so are limited in the solar spectrum that consists of 45% of visible and about 51% infrared light [9]. To address this issue, numerous studies have been conducted for the exploration and fabrication of photocatalysts to improve the photocatalytic properties in the visible region of the solar spectrum during the past few decades.
Recently, Yi et al. [10] reported a Ag3PO4 (silver phosphate) semiconductor photocatalytic application, which exhibits extremely high photooxidative capabilities for the evolution of O2 from water and organic dye decomposition under visible light irradiation. It has therefore attracted great attention in the application of photocatalysts under visible light irradiation [11, 12]. Several synthesis techniques, such as the precipitation route [13], ion exchange route [14], electrochemical oxidization method [15] and hydrothermal method [16], have been used for the synthesis of Ag3PO4 compounds.
In this paper, rhombic dodecahedron Ag3PO4 crystals were prepared by the microwave-assisted hydrothermal (HW) method, and compared with conventional oil bath heat treatment (O) and hydrothermal (H) methods. The MW method enables fast heating to the required temperature and extremely fast rates of crystallization. The effect of this synthesis method and reaction condition on the surface morphologies and photocatalytic properties of rhombic dodecahedron Ag3PO4 have been explored.
Experimental
Materials
Silver nitrate (AgNO3), ammonium hydroxide (NH4OH), and disodium hydrogen orthophosphate (Na2HPO4) were received from Sigma Aldrich. All the chemicals were of analytical grade and used for the synthesis of Ag3PO4 without any further purification.
Synthesis of rhombic dodecahedron Ag3PO4
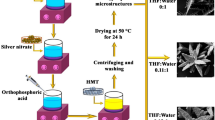
The rhombic dodecahedron Ag3PO4 crystals were prepared by the HW, H and O processes. The AgNO3 (0.3 M) and Na2HPO4 (0.2 M) were dissolved separately in 50 ml distilled water. Na2HPO4 aqueous solution was added drop by drop to the AgNO3 under magnetic stirring for 10 min. Consequently, the above solution was precipitated and classified as the precipitated sample (P). Precipitated solutions were finally transferred to a Teflon flask for the HW process. The mixture was heated at 120 °C for 1, 2 and 4 h, and the prepared samples were denoted as HW1, HW2 and HW4, respectively. For the H process, the mixture was heated at 120 °C for 12, 30 and 48 h, and samples were denoted as H12, H30 and H48, respectively. Similarly, the sample mixture was subject to the O treatment at 120 °C for 6, 8 and 10 h, and samples were denoted as O6, O8 and O10, respectively. The resulting products were washed with distilled water and ethanol several times to remove any impurities. The final products were dried at 70 °C.
Material characterization
The crystalline structure of the catalysts was characterized by powder X-Ray diffraction (XRD) employing a scanning rate of 4° per minute in a range from 20 to 80° by a Rigaku X-Ray diffractometer using monochromatized CuKα (λ = 1.54 Å) radiation. The morphologies and sizes of the samples were observed by scanning electron microscopy (SEM; SEC). UV–Vis diffuse reflectance spectra (DRS) were recorded by UV–Vis spectrophotometer (NIR JASCO 570; Micrometrics Instrumen; Edinburgh Instrument F900). Photocatalytic experiments were performed under a portable solar simulator and analyzed by UV–Vis spectrophotometer (Optizen View; Mexasys).
Photocatalytic experiment
Methylene blue (MB) organic dye was used as a model molecule in order to test the photocatalytic activity under simulated solar light conditions. In the photocatalytic experiment, 0.1 g of the photocatalyst powder was placed in a Pyrex glass cell (50 mm × 70 mm) and then 50 ml of 20 ppm methylene blue solution was slowly added. The above solution was kept in the dark for 30 min to achieve adsorption–desorption equilibrium. Then, the simulated solar light was turned on to induce MB to be oxidized. After withdrawing 1 ml from the photoreactor, its UV–Vis absorption spectrum was used to analyze its concentration. The photoreaction was performed for 120 min of irradiation by withdrawing a sample each 30 min.
Results and discussion
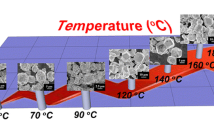
The crystal morphologies of as-precipitated (P) and microwave-assisted hydrothermal (HW)-treated Ag3PO4 are shown in Fig. 1. The sample ‘P’ without calcination is shown in Fig. 1a as having irregular morphology with size ranging from 0.5 to 1.0 µm. The size of sample P was very small as compared to the MW-treated samples, as shown in Fig. 1b–d. The small crystal morphology of sample P was due to the lack of heat treatment which obviously hindered the growth mechanism, whereas the HW treatment favored the growth of particles with definite crystal morphology. In the HW process, the 1-h-treated sample (HW1) shows less distinct polyhedral crystal morphology, of size ranging from 10 to 20 µm. However, by increasing the HW treatment to 2 h, the polyhedron crystals showed more regular and larger structures in the range of 20–40 µm, as shown in Fig. 1c. A further increase in the treatment time to 4 h gave rise to a higher definition of the crystals showing sharp edges, along with the appearance of several sub-micron particles adhered to the parent ones, as shown in Fig. 1d.
The HW method has been considered for the fabrication of different types of powder morphologies within a short span of time; however, homogenous polyhedral morphology was not obtained in our investigation and thus other methods were also applied, such as the H and O treatment methods. The powder morphologies obtained with these methods are shown in Fig. 2. The H method was deployed for the fabrication of regular homogenous polyhedral crystal structures. Different holding times were taken into account to optimize the regular crystal structures, i.e. 12, 30 and 48 h at 120 °C. In the 12-h holding time, large polyhedron structures with many sub-micron particles were observed, as shown in Fig. 2a. The crystal structures that were obtained were polyhedron and some were rhombic dodecahedron, with sizes ranging from 10 to 50 µm. The homogenous crystal structures were obtained with 30-h holding time, as shown in Fig. 2b. Some of the crystal structures are rhombic dodecahedrons with sizes ranging from 10 to 20 µm. The sub-micron particles were reduced in the 30-h holding time but homogenous structures were not obtained. However, the 48-h holding time shows sharp-edge polyhedron crystal structures with sub-micron particles, as shown in Fig. 2c. The smooth surface without any regular polyhedron crystal structures was obtained with samples prepared by the O treatment at 120 °C with various holding times, i.e. 6, 8, and 10 h, as shown in Fig. 2d–f. In addition, no sub-micron particles were detected in the O-treated samples. As for the morphologies of the powders prepared by different methods, the HW method at higher holding times (2 and 4 h) shows sharp-edge polyhedral crystalline particles fabricated in a short span of time.
XRD patterns of different samples are shown in Fig. 3a that were synthesized by different methods. The peaks of the fabricated samples were matched with JCPDS No. O6-0505, and thus confirmed the formation of Ag3PO4 crystals. The XRD patterns are of samples P, HW4, H48 and O10. The (110) plane for sample HW4 had a higher intensity than that of the (200) plane and it suggests the higher number of rhombic dodecahedron structure in HW4 as shown in Fig. 3b. However, the samples fabricated by other methods show that the intensity of the (200) plane is higher than the (110) plane and concludes that more cubic, spherical and polyhedral structures rather than rhombic dodecahedrons have been formed [17, 18].
a XRD patterns of as-precipitated (P) Ag3PO4 and Ag3PO4 samples prepared at 120 °C by microwave for 4 h (HW4), hydrothermal for 48 h (H48) and oil bath-treated for 10 h (O10). b XRD patterns showing the intensities of the (110) and (200) planes for as-precipitated (P) Ag3PO4 and Ag3PO4 samples prepared at 120 °C by microwave for 4 h (HW4), hydrothermal for 48 h (H48) and oil bath-treated for 10 h (O10)
Figure 4a displays the UV–Vis DRS of samples P, HW1, HW2 and HW4. It can be observed that the absorption edge of the sample P as in the range of 530 nm while, for the HW samples, the absorption edge is nearly extended to 550 nm and the absorption intensity is slightly increased. From the DRS analysis, the microwave-treated Ag3PO4 samples have absorbed more visible light than the as-precipitated sample, as shown in Fig. 4a. The absorption edge for the H-treated samples has been extended to 550 nm, as shown in Fig. 4b. Similarly, Fig. 4c shows the similar trend to the HW and H samples except for sample O6 having an absorption edge similar to the P sample around 530 nm, as shown in Fig. 4a, except for the O-treated sample for lower holding time (6 h) having similar absorbance to the as-precipitated sample. The absorption coefficient α is calculated according to the Kubelka–Munk function based on the DRS [19,20,21]; the estimated band gap energies for all the samples are shown in Table 1. The samples P and O6 have higher energy band gap values of 2.42 and 2.41 eV, respectively, whereas other samples have lower values of 2.37 eV. All the Ag3PO4 samples show a good absorbance in the visible region.
Comparison of UV–Vis diffuse reflectance spectra of the as-precipitated sample and those fabricated by different methods. a Microwave-assisted hydrothermal-treated samples at different holding times: 1 h (HW1), 2 h (HW2) and 4 h (HW4); b hydrothermal-treated samples at different holding times: 12 h (H12), 30 h (H30) and 48 h (H48); c oil bath heat-treated samples at different holding times: 6 h (O6), 8 h (O8) and 10 h (O10). Insets show Kubelka–Munk functions to calculate the energy band gap
The kinetic plot of the photocatalytic decoloring of methylene blue organic dye solution under simulated solar light is shown in Fig. 5. The photocatalytic activities of Ag3PO4 samples prepared by different methods were compared with the precipitated sample under identical conditions. The methylene blue suspension was magnetically stirred in the dark for 30 min to reach an adsorption and desorption equilibrium prior to the photocatalytic reaction. Among the four types of Ag3PO4 samples, HW4 exhibited the best decoloring performance on methylene blue. The rate of photo-decoloring was found to be in the order 4 > 2 > 1 h > precipitated samples as shown in Fig. 5a. The sample HW4 includes hetero-crystals, a sharp polyhedral edge with a rough surface and were polyhedral which demonstrates that rhombic dodecahedral crystals having 12 facets with high surface energies can enhance the photocatalytic reaction [22]. Thus, HW4 shows better photo-decoloring behavior. The half-life (t 1/2) reaction for the samples H48 (Fig. 5b), O10 (Fig. 5c) and P are 30.33, 76.84 and 267 min, respectively. Moreover, HW4 showed the highest photo-activity with a half-life time (t 1/2) of 20.33 min. The rate of photocatalysis was found to be in the order HW4 > H48 > O10 > P (Fig. 5d). The rate constants (k) of all the samples were calculated and are listed in Table 1. The rate constants for HW4, H48 and O10 were 0.034, 0.022 and 0.009 min−1, respectively. The most pronounced photocatalytic performance was observed for the sample HW4. The overall results show that the microstructures with sharp edges, and defects on the surface due to the submicron particles adhering to the microcrystals of the Ag3PO4, had higher photocatalytic activity.
Photocatalytic decoloring curves of Ag3PO4 samples fabricated by different methods and compared with as precipitated sample (P). a Microwave-assisted hydrothermal-treated samples at different holding times: 1 h (HW1), 2 h (HW2) and 4 h (HW4); b hydrothermal-treated samples at different holding times: 12 h (H12), 30 h (H30) and 48 h (H48); c oil bath heat-treated samples at different holding times: 6 h (O6), 8 h (O8) and 10 h (O10); d comparison between the as-precipitated, microwave-assisted hydrothermal (4 h), hydrothermal (48 h) and oil bath (10 h) samples
Conclusions
In conclusion, the Ag3PO4 samples were successfully synthesized by microwave-assisted hydrothermal, hydrothermal and conventional oil bath heat treatment of as-precipitated Ag3PO4 powders. The rhombic dodecahedron, cubic and polyhedral microcrystals of Ag3PO4 were obtained by the microwave-assisted hydrothermal and hydrothermal methods. On the other hand, oil bath heat-treated samples show more or less spherical microcrystals. Among the three methods, the microwave-assisted hydrothermal method has been found to be the most efficient one because of its short synthesis time, less energy consumption, and better photocatalytic properties. Moreover, the 4-h microwave-assisted hydrothermally-treated sample exhibited excellent photocatalytic activity for methylene blue decoloring under simulated solar light irradiation.
References
K. Maeda, K. Teramura, D. Lu, T. Takata, N. Saito, Y. Inoue, K. Domen, Nature 440, 295 (2006)
T. Hayashi, M. Deura, K. Ohkawa, Jpn. J. Appl. Phys. 51, 112 (2012)
K. Hashimoto, H. Irie, A. Fujishima, Jpn. J. Appl. Phys. 44, 8269 (2005)
C. Pan, Y. Zhu, Environ. Sci. Technol. 44, 5570 (2010)
M.R. Hoffmann, J.A. Moss, M.M. Baum, Dalton Trans. 40, 5151 (2011)
M.J. Height, S.E. Pratsinis, O. Mekasuwandumrong, P. Praserthdam, Appl. Catal. B Environ. 63, 305 (2006)
S. Šegota, L. Ćurković, D. Ljubas, V. Svetličić, I.F. Houra, N. Tomašić, Ceram. Int. 37, 1153 (2011)
A. Fujishima, T.N. Rao, D.A. Tryk, J. Photochem. Photobiol. C 1, 1 (2000)
J. Zhoua, Y. Cheng, J. Yu, J. Photochem. Photobiol. A 223, 82 (2011)
Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo, Z. Li, Y. Liu, R.L. Withers, Nat. Mater. 9, 559 (2010)
G.F. Huang, Z.L. Ma, W.Q. Huang, Y. Tian, C. Jiao, Z.M. Yang et al., J. Nanomater. 1, 2013 (2013)
A. Khan, M. Qamar, M. Muneer, Chem. Phys. Lett. 54, 519 (2012)
H. Wang, L. He, L. Wang, P. Hu, L. Guo, X. Han et al., Cryst. Eng. Comm. 14, 8342 (2012)
M. Ge, N. Zhu, Y. Zhao, J. Li, L. Liu, Ind. Eng. Chem. Res. 51, 5167 (2012)
Z. Lou, B. Huang, Z. Wang, R. Zhang, Y. Yang, X. Qin et al., Cryst. Eng. Comm. 15, 5070 (2013)
J. Wang, F. Teng, M. Chen, J. Xu, Y. Song, X. Zhou, Cryst. Eng. Comm. 15, 39 (2013)
H.F. Cheng, B.B. Huang, Y.Y. Liu, Z.Y. Wang, X.Y. Qin, X.Y. Zhang, Y. Dai, Chem. Comm. 48, 9729 (2012)
H.J. Fan, U. Gcsele, M. Zacharias, Small 3, 1660 (2007)
N. Serpone, D. Lawless, R. Khairutdinov, J. Phys. Chem. 991, 6646 (1995)
E.M. Patterson, C.E. Shelden, B.H. Stockton, Appl. Opt. 16, 729 (1977)
J.G. Yu, X.X. Yu, Environ. Sci. Technol. 42, 4902 (2008)
Y. Bi, S. Ouyang, J. Cao, J. Ye, Phys. Chem. Phys. 13, 10071 (2011)
Acknowledgements
This research was supported by Global Research Laboratory Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (MEST) of Korea (Grant No.: 2010-00339) and also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A1A01052893).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Special Issue of the 1st International Symposium on Photocatalysis at Fuzhou University.
Rights and permissions
About this article
Cite this article
Jo, YH., Joshi, B., Sekino, T. et al. Comparative study on the photocatalytic properties of Ag3PO4 fabricated by different methods. Res Chem Intermed 43, 5261–5269 (2017). https://doi.org/10.1007/s11164-017-3055-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3055-8