Abstract
A new magnetic nanocomposite based on layered double hydroxides (LDHs) was synthesized by coprecipitation of (Cu/Ni)–Al LDHs and nano-Fe3O4 and characterized by Fourier-transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) analysis, scanning electron microscopy (SEM), and energy-dispersive X-ray (EDX) techniques. The size of the composite was about 10 nm. Photodegradation of methylene blue as an organic pollutant by this nanocomposite via oxidation under visible-light irradiation was studied in comparison with nano-Fe3O4 and (Cu/Ni)–Al LDHs. The results showed that the degradation by the nanocomposite was more effective compared with Fe3O4 or (Cu/Ni)–Al LDHs alone. After four runs of use as photocatalyst, the composite remained powerful and effective in the degradation reaction. The influence of acidic, neutral, and basic pH and chloride anion on photodegradation of methylene blue was also investigated. The degradation occurred for a long time in acidic and basic pH but was faster in presence of Cl− anion.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are colored compounds with complex aromatic molecular structure, being applied in food, dyeing, plastics, paper, and leather industries; they may convert to carcinogenic, mutagenic, or inert, nonbiodegradable compounds when discharged into water [1]. In addition to the unacceptable color of dyes, dangerous byproducts of their oxidation, hydrolysis, or other chemical reactions can occur in wastewater [2]. Elimination of organic dyes from wastewater by traditional techniques such as coagulation, filtration, adsorption, reverse osmosis, and ozone treatment has been developed [3].
Recently, the mechanisms of advanced oxidation processes (AOPs) such as aqueous-phase oxidation methods have been found to rely on highly reactive hydroxyl radicals to degrade organic pollutants in wastewater [2, 4]. Among AOPs, heterogeneous photocatalytic reactions have shown potential as an efficient tool for efficient mineralization of pollutants into innocuous CO2, H2O, and mineral acids [3,4,5].
The catalytic activity of a heterogeneous catalyst is mainly determined by its morphology and particle size. The catalytic activity can be improved by reducing the particle size to nanometer scale [6]. However, use of such nanosystems (nanoscales) can increase operating costs and have negative environmental impacts, since their separation from water is difficult. Hence, magnetic separation techniques have been applied for removal of suspended or dispersed magnetizable particles from different media, having extensive applications such as filtration, sedimentation, flocculation, flotation, extraction, stabilized fluidized beds, biomedicine, and protein recovery [7, 8]. In this technique, separation was performed based on very small, weakly magnetic materials. In addition, the nonmagnetic fraction of minerals can be upgraded by use of many of these materials. It is expected that application of magnetic separation techniques to small, nonmagnetic particles will be further studied [8].
In recent years, LDHs have attracted considerable interest for use in environmental waste treatment due to their easy processing. Their applicability is increased by properties such as their special layered structure, exchangeable anions, high anion exchange capacity, and low cost [9].
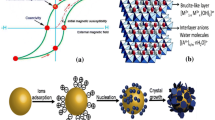
Layered double hydroxides (LDHs) are defined by the general formula
where MII and MIII are divalent and trivalent metal cations, A n− is a (n ·)-valent anion, and x usually takes values between 0.20 and 0.33, as shown in Fig. 1 [10].
LDHs act based on photogenerated electrons (e−) provided by defect energy between the valence and conduction band. These photons can be shifted to the conduction band by visible-light photons with lower energy [8, 9], then be absorbed by the absorption edge (photocatalysis) [11]. Since LDHs have anion exchange ability, especially for bulky inorganic ions, many other methods have been utilized [12]. Numerous studies have reported the utility of layered double hydroxides (LDHs) as adsorbents [13], using different interlayer anions for exchange with other ions in aqueous solution [11]. The morphology of LDHs has been substantially investigated, for instance, powders, spheres, nanosized belts, fibrous structures, and films on substrates [14]. Thermal evaluation revealed that LDHs can be successfully employed as photocatalysts for elimination of organic pollutants because of their high anion exchange capacity, expansion properties, low cost, and environmental friendliness [12, 15,16,17].
The purpose of the present work is to compare the photocatalytic activity of (Cu/Ni)–Al LDHs@Fe3O4, Fe3O4, and (Cu/Ni)–Al LDHs for degradation of methylene blue with H2O2 under visible-light irradiation. The magnetic nanocomposite was synthesized by coprecipitation of Fe3O4 and (Cu/Ni)–Al LDHs. The reasons for use of this nanocomposite are its easy preparation, low cost, large surface area, ion exchange ability, adsorbent nature, compositional flexibility, and especially the ability to remove it from the reaction mixture due to its magnetic property.
Experimental
General
All chemical reagents were purchased from Merck and Aldrich chemical companies and used without any further purification.
Synthesis of Fe3O4 nanoparticles
Fe3O4 nanoparticles were prepared by coprecipitation of ferrous chloride tetrahydrate and ferric chloride hexahydrate in ammonia solution with treatment under hydrothermal conditions according to a previously reported method [18]. In a 250-mL flask, 3.0 g ferrous chloride tetrahydrate (FeCl2·4H2O) and 8.1 g ferric chloride hexahydrate (FeCl3·6H2O) (molar ratio 1:2) were taken and dissolved by addition of 150 mL water under stirring. Chemical precipitation was then achieved by adding 38 mL ammonium hydroxide (NH4OH) solution (29.6%) dropwise for a period of 30 min at room temperature. Black precipitate appeared immediately after addition of ammonium hydroxide solution. During the reaction process, the pH was maintained at about 10. The precipitate was then heated at 80 °C for 60 min. The thus-obtained Fe3O4 magnetic nanoparticles were washed several times with water and ethanol, until the pH became completely neutral. The Fe3O4 particles were then dried under reduced pressure in an air circulating oven at 80 °C for a period of 3 h.
Synthesis of magnetic nanocomposite
Magnetic (Cu/Ni)–Al LDHs@Fe3O4 composite was prepared by coprecipitation without further aging. Firstly, 0.3 g Fe3O4 was ultrasonically dispersed into 150 mL double-distilled water for 20 min to obtain uniform suspension, which was then transferred into a 500-mL flask with vigorous stirring. Alkaline solution (100 mL, 0.32 g Na2CO3, 0.48 g NaOH) was added dropwise into the suspension until pH ca. 10.0 and kept for 5 min, then 100 mL salt solution [0.29 g Cu(NO3)2·3H2O, 0.99 g Ni(NO3)2·6H2O, 0.56 g Al(NO3)3·9H2O] and the above alkaline solution were simultaneously added maintaining pH of 9.5–10. The resultant was stirred for 5 min followed by separation using a magnet, followed by thorough washing with deionized water and alcohol and drying at 60 °C overnight to give product (Cu/Ni)–Al LDHs@Fe3O4 [19].
Photodegradation
Photocatalytic experiments were carried out using a homemade photoreactor and 400-W metal halide lamps for visible-light irradiation [5].
In a typical experiment, 10 mL aqueous MB with initial concentration of 1 × 10−5 M was placed in a 25-mL round-bottomed flask. Photocatalyst (0.01 g) was added with 1 mL H2O2 (30%), and the suspension was stirred for 30 min in the dark at room temperature to ensure establishment of adsorption–desorption equilibrium. The lamp was then turned on while the suspension was magnetically stirred. The photocatalyst was then filtered out of the mixed reaction using an external magnet. Sample (4 mL) was transferred into a spectrophotometer cell for measurement of absorbance of MB at 900 to 500 nm.
Characterization
Powder XRD data were obtained on a Shimadzu XRD-6000 diffractometer (Cu Kα radiation) from Kansaran Binalood Company. FT-IR spectra were recorded on a Bruker Vector-22 FT-IR spectrophotometer. Morphology was analyzed using a Zeiss Supra 55 field-emission scanning electron microscope (Razi Metallurgical Research Center). EDX data were recorded by field-emission SEM (Mira 3-XMU instrument, Razi Metallurgical Research Center).
Results
Synthesis of magnetic nanocomposite
As shown in Scheme 1, nanomagnetic Fe3O4 as a core or bed was prepared by coprecipitation of Fe2+ and Fe3+ in aqueous solution. The nanocomposite was then synthesized by coprecipitation of Cu2+, Ni2+, and Al3+ metal ions on the Fe3O4 bed or core nanoparticles in alkaline medium.
Characterization of magnetic nanocomposite
All compounds were characterized by FT-IR, XRD, SEM, and EDX techniques. The degradation process was followed by ultraviolet–visible (UV–Vis) spectroscopy.
FT-IR
The FT-IR spectra of Fe3O4, (Cu/Ni)–Al LDHs, and (Cu/Ni)–Al LDHs@Fe3O4 are shown in Fig. 2. The spectrum of the LDHs showed absorption bands characteristic of LDHs synthesized by coprecipitation at 3432, 1637, 1380, and 800 to 400 cm−1. The appearance of strong and broad absorption bands at around 3432 cm−1 is due to O–H stretching of hydroxyl group in the surface of LDHs, both in the brucite-like layers and from interlayer water molecules [20]. This broad band is usually followed by adsorption bands at around 1637 cm−1 indicating presence of stretching vibrations of interlayer water molecules. A sharp and intense band was observed at 1380 cm−1, corresponding to stretching vibration of intercalated NO3 ions in the LDHs [21]. The weak absorption bands in the 400–900 cm−1 region are associated with M–O stretching and M–OH bending vibrations in all compounds.
The infrared spectrum of the composite showed bands at 3399, 1635, 1380, and 590 cm−1, attributed to LDHs, and also bands at 892 and 791 cm−1, attributed to Fe3O4. The spectrum was similar to those of the LDHs and Fe3O4, confirming preparation of the composite.
XRD
The XRD patterns of (Cu/Ni)–Al LDHs and (Cu/Ni)–Al LDHs@Fe3O4 are shown in Fig. 3. The XRD patterns of the two compounds are similar. The peaks labeled with star symbols in the XRD pattern of the (Cu/Ni)–Al LDHs were also observed in that of the (Cu/Ni)–Al LDHs@Fe3O4 composite, while the peaks labeled with heart symbols in the XRD pattern of the (Cu/Ni)–Al LDHs@Fe3O4 composite originated from Fe3O4. The XRD data therefore also confirm synthesis of the magnetic nanocomposite.
SEM
SEM images of Fe3O4, (Cu/Ni)–Al LDHs, and (Cu/Ni)–Al LDHs@Fe3O4 are shown in Fig. 4. The morphology of all the compounds was sub-micro-spheres with diameter of about 4–10 nm, but the surface of the (Cu/Ni)–Al LDHs@Fe3O4 nanocomposite showed rougher sheet-like morphology than the surface of Fe3O4.
EDX
The composition of the (Cu/Ni)–Al LDHs@Fe3O4 composite was investigated by EDX in comparison with (Cu/Ni)–Al LDHs and Fe3O4 (Fig. 5); the related data are presented in Table 1. Clearly, Ni, Cu, Al, and O elements were all present in the (Cu/Ni)–Al LDHs and (Cu/Ni)–Al LDHs@Fe3O4 composite. However, the (Cu/Ni)–Al LDHs@Fe3O4 composite contained all of these elements as well as Fe, providing the best evidence for the formation of the magnetic nanocomposite.
The results of these analyses therefore confirm that the magnetic nanocomposite of LDHs and Fe3O4 was synthesized and stable in aqueous solution via electronic interaction forces. The electronic interaction between the negatively charged magnetite nanoparticles and positively charged LDHs was sufficient to induce stable self-assembly of the two components. This interaction can form a stable colloidal suspension of the composite in aqueous solution [22].
Photodegradation
Adsorption–desorption equilibrium
A portion of the MB will be adsorbed onto the surface of the solid phase of the photocatalyst, slightly decreasing the absorption peak of MB. Thus, initially, the mixture for the photodegradation reaction must be allowed to reach adsorption–desorption equilibrium in the dark. The electronic absorption spectrum of the organic dye is useful to detect this equilibrium. Therefore, the variation in the MB absorption at λ max (around 670 nm) was recorded at 5–60 min in dark condition. The absorption peak was found to decrease from 0 to 30 min, but remained almost the same thereafter (Table 2), indicating constant MB concentration.
Figure 6 shows the evolution of the MB absorption spectrum with illumination time for a representative sample. In general, it appears that, the longer the illumination time of the methylene blue solution, the greater the reduction in the intensity of the peak at 660 nm, indicating photodegradation of MB. Here, absorption time of 0 min starts after 30 min of adsorption–desorption equilibrium under dark condition. MB solution was decolorized after 5 min of irradiation by visible light in presence of the nanocomposite, while decolorization occurred after 420 min.
Therefore, the magnetic nanocomposite is a powerful photocatalyst for degradation of MB.
Comparison of photocatalytic activity
The photocatalytic activity of the synthesized magnetic nanocomposite was compared with that of nano-Fe3O4 and the LDHs for degradation of 10−5 M MB under visible light. The results in Table 3 show that nano-Fe3O4 and the LDHs caused decoloration of aqueous MB solution in 60 and 30 min, respectively, compared with 5 min in presence of the nanocomposite. Therefore, the nanocomposite is a faster and remarkable photocatalyst. This result confirms that the new compound combining nano-Fe3O4 and LDHs had more effective photocatalytic properties than each of the constituents alone. Therefore, the nanocomposite of LDHs and Fe3O4 (NP) is a more efficient photocatalyst than the neat LDHs or Fe3O4 (NP). Two reasons for this can be proposed. The first is the adsorption capacity of the LDHs. Study of the adsorption of organic dye (MB) on Fe3O4, LDHs, and the composite in the dark showed that the LDHs and composite exhibited about 5% adsorption while Fe3O4 showed about 0.2% (nearly 0%). This can be attributed to the high adsorption capacity of the LDHs. Numerous literature studies have reported the same result [23, 24]. Thus, the degradation of the MB dye may be increased by greater adsorption on the catalyst. The second possibility is a narrower bandgap of the composite. As Ulises et al. showed, the bandgap of a LDH–MFe2O4 composite is lower than either constituent [25]. We also reported greater photocatalytic activity on decreasing the bandgap due to easier production of electron–hole pairs [5].
Influence of MB concentration
The results in Table 4 show that, on increasing the MB concentration tenfold (from 10−5 to 10−4 M) for the same reaction conditions, the reaction time increased (by about 20 times). For 10−3 M MB, the degradation time was 420 min (about 60 times).
Influence of Cl− ion
When one drop of NaCl (1 M) solution was added to the reaction mixture, the degradation efficiency (DE) was 100% and the time for degradation was shorter than in absence of Cl− ion (less than 5 min). The proposed mechanism in the presence of chloride ion is illustrated in Scheme 2, where “X” indicates the radical fragments of MB or ·OH radical.
The chloride ion is converted to an active radical form by contact with hυ and/or other radical fragments in the reaction mixture. This radical is as effective as OH radical, so the time for degradation of MB is shorter.
Influence of pH
The pH of the dye solution is a main parameter in the degradation of organic dyes [26].
The influence of pH is observed in two forms: (1) the surface charge on the catalyst and (2) the structure or ionization state of the organic dye [27].
Comparative experiments were performed at different pH values: one acidic (3), one basic (9), and one neutral (7), achieved by adding one drop of HCl (1 M) or NaOH (1 M) to the reaction mixture, or without acid or base solution, respectively. The results are presented in Table 5. The time for degradation changed when varying the pH of the reaction mixture. In acidic and basic conditions, the degradation time was longer than in neutral condition. The order of reaction time was:
Structural changes due to the influence of pH can cause degradation of organic dyes, since MB has various forms at different pH values (Fig. 7). In acidic condition, MB+ is converted to the leuco-MB2+ form, thus the charge increases. Leuco-MB2+ is repelled more than MB+ from the positive surface of the LDHs by electrical repulsion. However, leuco-MB is less adsorbed on the surface of the LDHs, thus the degradation rate is decreased [28].
At various pH values, the surface of the LDHs shows different charges resulting from adsorption of ions such as H+ or OH− from solution [29].
In acidic solution:
For pH values below 7, the hydrated surface of the LDHs is protonated and becomes positively charged, causing greater repulsion of the dye from the surface of the LDHs. As a result, the degradation becomes less.
In basic solution:
The surface of the LDHs is deprotonated at pH values above 7 and becomes negatively charged.
The OH radical is nonselective and a strong oxidizing agent with high oxidation potential compared with common oxidizing agents such as H2O2, O3, O2, etc.
In basic condition, (1) a portion of the oxidant is destroyed by decomposition of H2O2 [30], (2) OH− is exchanged by NO −3 anion in the interlayer of the LDHs, and (3) according to Eq. (2), some hydroxide is used to convert the surface from positive to negative charge. Thus, the amount of OH radical in the reaction mixture is decreased, as is the degradation rate.
Recyclability study
After the complete run 1, the photocatalyst was filtered by a magnetofiltration process using an external magnet (Fig. 8) and washed with distilled water, ethanol, and diethyl ether (2 × 10 mL) in each run, then heated at 80 °C for 4 h, until the catalyst was activated. This filtrated compound was then used in subsequent photoreactions (runs 2–4).
The photodegradation time increased slightly, because a portion of the catalyst was lost during the filtration process and the active sites on the photocatalyst probably decreased in each run. These results show that the magnetic nanocomposite is a more effective and reusable catalyst for photodegradation of MB or other organic dyes.
As shown in Fig. 9, the ability of the nanocomposite was studied for four photocatalytic reactions. After four runs, the catalyst remained powerful and effective in the degradation reaction.
Conclusions
A novel nanomagnetic composite photocatalyst is introduced for degradation of methylene blue under visible light. The nanocomposite was synthesized by coprecipitation of Cu2+, Ni2+, and Al3+ cations from alkaline solution onto Fe3O4 nanoparticles. The size of the nanocomposite was about 10 nm. The nanocomposite was found to be powerful, more efficient, inexpensive, and easily prepared, and more active as a photocatalyst than neat Fe3O4 or the LDHs alone. Degradation of methylene blue was faster in presence of Cl− anion. The decolorization of the organic dye was affected by the solution pH, with photocatalytic activity in the order: neutral > acidic > basic medium.
References
V. Venkateswaran, P. Kalaamani, N. Karpagam, Adsorptive removal of methylene blue using activated Prosopis spicigera: a low cost adsorbent. Chem. Sci. Trans. 4(2), 347–354 (2015)
Y. Chiang, C. Lin, Photocatalytic decolorization of methylene blue in aqueous solutions using coupled ZnO/SnO2 photocatalysts. Powder Technol. 246, 137–143 (2013)
M.H. Farzana, S. Meenakshi, Visible light-driven photoactivity of zinc oxide impregnated chitosan beads for the detoxification of textile dyes. Appl. Catal. A Gen. 503, 124–134 (2015)
M. Klavarioti, D. Mantzavinos, D. Kassinos, Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Interact. 35, 402–417 (2009)
H.R. Mardani, M. Forouzani, M. Ziari, P. Biparva, Visible light photo-degradation of methylene blue over Fe or Cu promoted ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 141, 27–33 (2015)
S. Xing, Z. Zhou, Z. Ma, Y. Wu, Characterization and reactivity of Fe3O4/FeMnO x core/shell nanoparticles for methylene blue discoloration with H2O2. Appl. Catal. B Environ. 107, 386–392 (2011)
Y. Lin, Y. Wei, Y. Sun, J. Wang, Synthesis and magnetic characterization of magnetite obtained by monowavelength visible light irradiation. Mater. Res. Bull. 47, 614–618 (2012)
D. Ljubas, M. Franzreb, H. Christian, B. Hansen, P.G. Weidler, Magnetizing of nano-materials on example of Degussa’s P-25 TiO2 photocatalyst: synthesis of magnetic aggregates, characterization and possible use. Sep. Purif. Technol. 136, 274–285 (2014)
S.J. Xia, F.X. Liu, Z.M. Ni, W. Shi, J.L. Xue, P.P. Qian, Ti-based layered double hydroxides: efficient photocatalysts for azo dyes degradation under visible light. Appl. Catal. B Environ. 144, 570–579 (2014)
F. Kovanda, E. Jindová, K. Lang, P. Kubát, Z. Sedláková, Preparation of layered double hydroxides intercalated with organic anions and their application in LDH/poly(butyl methacrylate) nanocomposites. Appl. Clay Sci. 48, 260–270 (2010)
Z.P. Xu, J. Zhang, M.O. Adebajo, H. Zhang, C. Zhou, Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 53, 139–150 (2011)
M. Sipiczki, Functional Materials Syntheses, Characterisation and Catalytic Applications. PhD Thesis, Supervisors: Dr. P. Sipos, Dr. I. Palinko, University of Szeged (2013)
I.M. Ahmed, M.S. Gasser, Adsorption study of anionic reactive dye from aqueous solution to Mg–Fe–CO3 layered double hydroxide (LDH). Appl. Surf. Sci. 259, 650–656 (2012)
Y. Kuang, L. Zhao, S. Zhang, F. Zhang, M. Dong, S. Xu, Morphologies, preparations and applications of layered double hydroxide micro-/nanostructures. Materials 3, 5220–5235 (2010)
J. Fahel, S. Kim, P. Durand, E. André, C. Carteret, Enhanced catalytic oxidation ability of ternary layered double hydroxides for organic pollutants degradation. Dalton Trans. 45, 8224–8235 (2016)
W. He, R. Wang, L. Zhang, J. Zhu, X. Xiang, F. Li, Enhanced photoelectrochemical water oxidation on a BiVO4 photoanode modified with multi-functional layered double hydroxide nanowalls. J. Mater. Chem. A 3, 17977–17982 (2015)
Y. Li, L. Zhang, X. Xiang, D. Yan, F. Li, Engineering of ZnCo-layered double hydroxide nanowalls toward high-efficiency electrochemical water oxidation. J. Mater. Chem. A 2, 13250–13258 (2014)
S.Y. Kim, B. Ramaraj, K.R. Yoon, Preparation and characterization of polyvinyl alcohol-grafted Fe3O4 magnetic nanoparticles through glutaraldehyde. Surf. Interface Anal. 44, 1238–1242 (2012)
X. Chen, F. Mi, H. Zhang, H. Zhang, Facile synthesis of a novel magnetic core-shell hierarchical composite submicrospheres Fe3O4@CuNiAl-LDH under ambient conditions. Mater. Lett. 69, 48–51 (2012)
R. Shan, L. Yan, K. Yang, S. Yu, Y. Hao, H. Yu, B. Du, Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chem. Eng. J. 252, 38–46 (2014)
H. Abdolmohammad-Zadeh, Z. Talleb, Speciation of As(III)/As(V) in water samples by a magnetic solid phase extraction based on Fe3O4/Mg–Al layered double hydroxide nano-hybrid followed by chemiluminescence detection. Talanta 128, 147–155 (2014)
C. Chen, P. Gunawan, R. Xu, Self-assembled Fe3O4-layered double hydroxide colloidal nanohybrids with excellent performance for treatment of organic dyes in water. J. Mater. Chem. 21, 1218–1225 (2011)
J. Sun, H. Fan, B. Nan, S. Ai, Fe3O4@LDH@Ag/Ag3PO4 submicrosphere as a magnetically separable visible-light photocatalyst. Sep. Purif. Technol. 130, 84–90 (2014)
R. Shan, L. Yan, K. Yang, S. Yu, Y. Hao, H. Yu, B. Du, Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution. Chem. Eng. J. 252, 38–46 (2014)
A.A. Ulises, M.I. Oliva, S.G. Marchetti, A.C. Heredia, S.G. Casuscelli, M.E. Crivello, Synthesis and characterization of a mixture of CoFe2O4 and MgFe2O4 from layered double hydroxides: band gap energy and magnetic responses. J. Magn. Magn. Mater. 359, 249–259 (2014)
A.M. Tayeb, D.S. Hussein, Synthesis of TiO2 nanoparticles and their photocatalytic activity for methylene blue. Am. J. Nanomater. 3, 57–63 (2015)
N.P. Mohabansi, A comparative study on photo degradation of methylene blue dye effluent by advanced oxidation process by using TiO2/ZnO photo catalyst. J. Chem. 4, 814–819 (2011)
A.A. Hoffmann, S.L.P. Dias, J.R. Rodrigues, F.A. Pavan, E.V. Benvenutti, E.C. Lima, Methylene blue immobilized on cellulose acetate with titanium dioxide: an application as sensor for ascorbic acid. J. Braz. Chem. Soc. 19, 943–949 (2008)
Y. Li, B. Gao, T. Wu, B. Wang, X. Li, Adsorption properties of aluminum magnesium mixed hydroxide for the model anionic dye Reactive Brilliant Red K-2BP. J. Hazard. Mater. 164, 1098–1104 (2009)
L.M. Nunes, Effect of pH on the adsorption of Sunset Yellow FCF food dye into a layered double hydroxide (CaAl-LDH-NO3). Chem. Eng. J. 215–216, 122–127 (2013)
Acknowledgement
We are grateful for the financial support of Payame Noor University (PNU) of Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mardani, H.R. (Cu/Ni)–Al layered double hydroxides@Fe3O4 as efficient magnetic nanocomposite photocatalyst for visible-light degradation of methylene blue. Res Chem Intermed 43, 5795–5810 (2017). https://doi.org/10.1007/s11164-017-2963-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2963-y