Abstract
Novel 1,4-phenylene-bis-N-acetyl- (3a–h) and bis-N-phenylpyrazoline derivatives (4a–h) were obtained by addition of hydrazine hydrate and phenylhydrazine to bis-chalcone derivatives (1a–h) in acetic acid and acetic acid/ethanol for 4 and 8 h in reflux conditions, respectively. The structures of the obtained bis-N-acetylpyrazoline and bis-N-phenylpyrazoline derivatives were characterized by nuclear magnetic resonance (NMR) and infrared (IR) spectroscopic methods and elemental analysis. Compounds 3a–h and 4a–h were investigated to evaluate their anticancer activities against C6 (rat brain tumor cells) and HeLa (human uterus carcinoma) in vitro using a dose-dependent assay from 5 to 100 μM with 5-fluorouracil (5-FU) as standard anticancer drug. Compound 3a showed higher cell-selective activity compared with 5-FU against HeLa cells. Compounds 3a–h (except 3d) were shown to have better activities than 5-FU against both cells, particularly at high concentration. Compound 4c showed higher cell-selective activity compared with 5-FU against C6 cells. Compound 3a may be particularly promising as an anticancer drug against HeLa cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer has become one of the major health problems ravaging the world today, and one of the ways of treating cancer is known as chemotherapy [1–3]. Chemotherapy causes intense side-effects, due to its cytotoxic effect on normal cells [4]. For this reason, it is important that anticancer drugs show antiproliferative and cytotoxic activity against tumor cells without affecting normal tissues. Although numerous cytotoxic agents have been developed, there is a need to develop more potent and safer chemotherapeutic agents [5].
In this area, pyrazolines are of great interest due to their synthetic feasibility and biological importance [6–12]. Derivatives of pyrazolines are found to show good anticancer and radioprotective activity [13]. Pyrazoline is a prominent structural motif found in numerous antitumor agents [14]. Several 1,3,5-triaryl-4-alkylpyrazolines have been evaluated as breast cancer treatments with the goal of both reducing toxicity and increasing the response rate as an antitumor agent [15]. In addition, among anticancer pyrazolines, 1,3-diphenyl pyrazolines have been reported to be highly potent and efficient cytotoxic agents [16–20]. Also, a series of novel 3,5-diarylpyrazolines [21] and thiazolylpyrazoline derivatives were recently reported as potent anticancer agents [22].
Prompted by these results, we decided to investigate and report the synthesis and anticancer activity of a series of novel 1,4-phenylene-bis-N-acetylpyrazoline (3a–h) and 1,4-phenylene-bis-N-phenylpyrazoline derivatives (4a–h).
Results and discussion
Chemistry
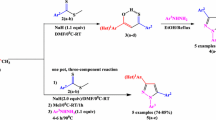
The approach for synthesis of our target compounds is shown in Scheme 1. Initially, the known key 1,4-phenylene-bis-chalcones intermediates 1a–h were resynthesized according to published procedure [23–26]. Bis-N-acetylpyrazoline derivatives 3a–h were obtained in good yields (75–93 %) by refluxing 4 equivalents of hydrazine hydrate and the corresponding chalcones 1a–h in acetic acid for 4 h (Scheme 1; Table 1). The compounds 3e–h are known [27]; the others are novel, and they were synthesized by literature procedure [28]. The reaction involved nucleophilic attack of amino group on polarized carbonyl group, followed by intramolecular cyclization. Evidence for N-acetyl-4,5-dihydropyrazole ring formation was obtained from 1H NMR spectra. The 1H NMR spectra of the compounds displayed a typical ABX-type pattern of doublet of doublets due to three pyrazoline protons. Methine proton of pyrazoline was found at around 5.50 ppm, as a doublet of doublets with coupling constants of nearly 12 Hz and 4.0–4.8 Hz. Two methylene protons displayed two signals: a doublet of doublets at around 3.80–3.65 ppm with coupling constants of nearly 17 and 12 Hz, and a doublet of doublets at around 3.30–3.10 ppm. The 13C NMR spectra of compounds 3a–h confirmed their proposed structures, since C4 and C5 of pyrazoline ring resonated at around 40–45 and 55–60 ppm, respectively. Furthermore, in the 13C NMR spectra, signals appearing at around 20–25 ppm indicated acetyl group connected to pyrazoline ring.
Similarly, heating at reflux of 4 equivalents of phenylhydrazine and the chalcones 1a–h in acetic acid/ethanol mixture for 8 h afforded the corresponding bis-N-phenylpyrazoline derivatives 4a–h in some good yields (76–93 %) (Scheme 1) [29]. Compound 4b is novel; the others are known [30–32]. The structures of compounds 4a–h were determined on the basis of spectral data. The spectral and microanalytical data of compounds 4a–h were consistent with their structures.
Biological evaluation: anticancer activity
All compounds were tested for their potential growth inhibitory activity against rat brain tumor cells (C6) and human uterus carcinoma (HeLa) cell line using a proliferation BrdU enzyme-linked immunosorbent assay (ELISA). The tests were performed at concentrations of 5, 10, 20, 30, 40, 50, 75, and 100 μM with 5-fluorouracil (5-FU) as standard. The antiproliferative activities of the compounds increased with dose.
The activities of the N-acetylpyrazoline derivatives 3a–h against C6 are shown in Fig. 1a and Table 1. All tested compounds 3a–h showed anticancer activity with 50 % inhibitory concentration (IC50) values ranging from <5 to 29.43 μM. In most cases, compounds 3a–h were more active than 5-FU; in particular, compounds 3a, b, f–h with IC50 values <5 µM exhibited significant activity at all concentrations.
The activities of the N-acetylpyrazoline derivatives 3a–h against HeLa are shown in Fig. 1b and Table 1. As seen in Fig. 1b, the anticancer activities of all compounds 3a–h (except 3d, e) were better than 5-FU at high concentrations (75 and 100 μM). Moreover, compound 3a exhibited very high activity with 95 % inhibition value at 100 to 20 μM concentrations. According to the IC50 values, the most active compounds were 3a, g with IC50 value <5 μM, followed by 3b (9.79 μM), 3c (24.72 μM), and 3f (28.93 μM).
The results for the anticancer activities of the N-phenylpyrazoline derivatives 4a–h against C6 cells are shown in Fig. 2a and Table 1. According to Fig. 2a, the anticancer activity of 4c was better than 5-FU at high concentrations (75 and 100 μM), and it showed significant activity at all concentrations. Compound 4d showed almost the same activity as 5-FU. Compounds 4b, e–h showed moderate anticancer effects. However, 4a did not show significant activity compared with 5-FU. In addition, the compounds had very low IC50 values, except 4h; while the IC50 values of 4a, b, d, f, g were <5 μM, the others were 23.88 μM for 4a, 13.44 μM for 4e, and 53.56 μM for 4h (Table 1).
The activities of 4a–h against HeLa are shown in Fig. 2b and Table 1. According to these results, whereas compounds 4b–d, f showed moderate activity at 100 µM concentration, the others were almost inactive compared with 5-FU.
According to these results, it was observed that compounds 3a–h showed higher anticancer activities than compounds 4a–h against both cell lines. Moreover, while compounds 3a–h exhibited significant activities against both cell lines, compounds 4a–h demonstrated significant activities against the C6 cell line, particularly at high concentrations.
Structure–activity relationship (SAR)
Comparing compounds 3a–h with 4a–h, it is obvious that the N-acetylpyrazolines 3a–h showed higher growth inhibitory effect than the N-phenylpyrazolines 4a–h against both cell lines. The higher cytotoxicity of 3a–h is due to the acetyl (CH3CO–) group attached to the pyrazoline ring, while in the case of compounds 4a–h, the Ph group lowered the cytotoxicity.
Considering the N-acetylpyrazolines 3a–h, all compounds showed high growth inhibitory effects against the C6 cell line at almost all concentrations. Compounds 3a and d with 1-naphthyl and furyl substituents, respectively, showed lower activities, whereas the others exhibited activities higher than 5-FU against the C6 cell line at 100–50 μM concentrations.
The activities of all the compounds (except 3d containing furyl ring) were better than 5-FU against HeLa cell lines at high concentrations. In addition, the activity of 3e was low compared with 5-FU. However, compounds 3a and g with 2-naphthyl and 4-methoxyphenyl substitution, respectively, exhibited activities higher than 5-FU against HeLa cell lines at 100 to 20 μM concentrations. This high cytotoxicity of both compounds 3a and g is attributed to the presence of naphthyl moieties and methoxide group, respectively.
Considering the N-phenylpyrazolines 4a–h, all compounds 4a–h showed selective growth inhibitory effects against the C6 cell line. All compounds (except 4a containing naphthyl ring) exhibited remarkable activities, particularly at high concentrations. Among these compounds, the most active was 4c (containing 2-thienyl ring), which showed significant activity at all concentrations. The remarkable activity of 4c is due to the presence of the thiophene moiety.
All compounds showed very low activities except at 100 μM concentration against the HeLa cell line. The most active compound was 4d, which is attributed to the presence of furan ring.
Conclusions
A series of 1,4-phenylene-bis-N-acetylpyrazoline (3a–h) and 1,4-phenylene-bis-N-phenylpyrazoline derivatives (4a–h) were synthesized in good yields by addition of hydrazine hydrate and phenyl hydrazine to 1,4-phenylene-bis-chalcones and evaluated for their anticancer activities against C6 (rat brain tumor) and HeLa (human uterus carcinoma) cell lines. Compounds 3a–h showed potent anticancer activities against both cell lines. Most of them were higher in activity than 5-FU against the C6 and HeLa cell lines at high concentration. Compound 3a exhibited very high activity against HeLa cell line cell selectively at 100 to 20 μM concentrations. Also, compounds 4a–h (except 4a) demonstrated significant activities against the C6 cell line, when compared with 5-FU at high concentrations. Moreover, only one compound (4d) showed remarkable activity against HeLa cell lines at 100 and 75 μM concentrations.
Experimental
The major chemicals were purchased from Sigma-Aldrich and Fluka. IR spectra (KCl disc) were recorded on a Jasco FT/IR-430 spectrometer. 1H and 13C NMR spectra were recorded on a Bruker Avance DPX-400 instrument. Tetramethylsilane (TMS) and CDCl3 served as internal standard for 1H and 13C NMR spectroscopy with δ values of 0.00 and 77.0 ppm, respectively. J values are given in Hz. Signal multiplicities in the 1H NMR spectra are abbreviated as s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), br (broad), and combinations thereof. Melting points were measured on Electrothermal 9100 apparatus. Elemental analyses were obtained using a LECO CHNS 932 elemental analyzer.
General method for synthesis of 1,4-phenylene-bis-N-acetylpyrazoline (3a–h) and 1,4-phenylene-bis-N-phenylpyrazoline derivatives (4a–h)
Bis-chalcone (1a–h) (1 mmol) and hydrazine hydrate (4 mmol) in acetic acid and/or phenylhydrazine (4 mmol) in acetic acid/ethanol mixture were refluxed for 4 and 8 h, respectively. The reaction mixture was poured into ice bath at the end of the reaction, and was checked with pH paper to ensure neutralization of the medium by addition of ammonia. It was observed that the collapse occurred when the mixture was allowed to stand overnight, and the resulting precipitate was filtered off and allowed to dry. The resulting material was obtained as pure.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(naphthalen-1-yl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3a )
Yield: 86 %. M.p. 250–252 °C. IR (KCl) v/cm−1: 3046, 1648, 1508, 1419, 1394, 1268, 1143, 1014, 958, 804, 773, 557. 1H NMR (400 MHz, DMSO, ppm): δ 9.24 (d, J = 8.4 Hz, 2H), 8.03–7.98 (m, 4H), 7.75 (d, J = 6.8 Hz, 2H), 7.68 (t, J = 7.2 Hz, 2H), 7.60 (t, J = 7.2 Hz, 2H), 7.55–7.52 (m, 2H), 7.24 (brs, 4H), 5.55–5.51 (dd, J = 11.4, 3.2 Hz, 2H), 4.10–4.03 (dd, J = 17.6, 12.4 Hz, 2H), 3.33–3.28 (m, 2H), 2.41 (s, 6H). 13C NMR (100 MHz, DMSO, ppm): δ 168.4 (2C), 155.5 (2C), 141.6 (2C), 134.1 (2C), 131.5 (2C), 130.1 (2C), 129.5 (2C), 129.2 (4C), 128.3 (2C), 127.4 (2C), 126.8 (2C), 126.6 (2C), 126.3 (2C), 125.7 (2C), 58.5 (2C), 45.0 (2C), 22.2 (2C). Anal. Calcd. for C36H30N4O2: C, 78.52; H, 5.49; N, 10.17. Found: C, 78.48; H, 5.32; N, 10.11.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(thiophen-3-yl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3b )
Yield: 77 %. M.p. 223–225 °C. IR (KCl) v/cm−1: 3023, 2924, 1644, 1438, 1409, 1338, 1102, 1037, 1018, 982, 813, 701, 635, 548. 1H NMR (400 MHz, DMSO, ppm): δ 7.55–7.54 (m, 2H), 7.47–7.46 (m, 2H), 7.42–7.38 (m, 2H), 7.18–7.16 (m, 4H), 5.57–5.51 (dd, J = 11.6, 4.8 Hz, 2H), 3.73–3.65 (dd, J = 17.6, 11.6 Hz, 2H), 3.14–3.07 (dt, J = 17.6, 4.8 Hz, 2H), 2.43 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 168.8 (2C), 150.2 (2C), 141.0 (2C), 133.9 (2C), 126.9 (2C), 126.4 (4C), 126.1 (2C), 125.5 (2C), 59.5 (2C), 42.8 (2C), 21.9 (2C). Anal. Calcd. for C24H22N4O2S2: C, 62.31; H, 4.79; N, 12.11. Found: C, 62.28; H, 4.67; N, 12.06.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3c )
Yield: 75 %. M.p. 306–308 °C. IR (KCl) v/cm−1: 3033, 2929, 1644, 1440, 1413, 1326, 1141, 1037, 1018, 952, 831, 761, 630, 555. 1H NMR (400 MHz, DMSO, ppm): δ 7.45–7.41 (dd, J = 5.2, 0.8 Hz, 2H), 7.21–7.20 (m, 6H), 7.09–7.07 (dd, J = 5.0, 3.6 Hz, 2H), 5.58–5.55 (dd, J = 11.6, 4.4 Hz, 2H), 3.77–3.69 (dd, J = 17.4, 11.6 Hz, 2H), 3.19–3.13 (dd, J = 17.2, 4.4 Hz, 2H), 2.40 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 168.7 (2C), 149.6 (2C), 140.9 (2C), 134.8 (2C), 128.8 (2C), 128.7 (2C), 127.6 (2C), 126.1 (4C), 59.7 (2C), 42.8 (2C), 21.9 (2C). Anal. Calcd. for C24H22N4O2S2: C, 62.31; H, 4.79; N, 12.11. Found: C, 62.25; H, 4.72; N, 12.09.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(furan-2-yl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3d )
Yield: 93 %. M.p. 251–253 °C. IR (KCl) v/cm−1: 3124, 1643, 1421, 1388, 1010, 983, 827, 769, 563. 1H NMR (400 MHz, DMSO, ppm): δ 7.57 (s, 2H), 7.18 (s, 4H), 6.73 (d, J = 2.8 Hz, 2H), 6.54–6.52 (m, 2H), 5.58–5.54 (dd, J = 12.0, 4.8 Hz, 2H), 3.71–3.63 (dd, J = 17.6, 12.0 Hz, 2H), 3.12–3.06 (dt, J = 17.6, 3.6 Hz, 2H), 2.42 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 168.8 (2C), 146.7 (2C), 145.7 (2C), 144.8 (2C), 140.8 (2C), 126.1 (4C), 112.8 (2C), 111.9 (2C), 59.1 (2C), 41.9 (2C), 21.9 (2C). Anal. Calcd. for C24H22N4O4: C, 66.97; H, 5.15; N, 13.02. Found: C, 66.89; H, 5.09; N, 12.98.
1,1′-(5,5′-(1,4-Phenylene)bis(3-phenyl-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3e )
Yield: 78 %. M.p. 296–298 °C. IR (KCl) v/cm−1: 3060, 1644, 1454, 1409, 1118, 958, 829, 771, 555. 1H NMR (400 MHz, DMSO, ppm): δ 7.78 (d, J = 4.0 Hz, 4H), 7.46 (s, 6H), 7.14 (s, 4H), 5.54–5.51 (dd, J = 11.8, 4.0 Hz, 2H), 3.87–3.80 (dd, J = 18.0, 12.0 Hz, 2H), 3.15–3.10 (dd, J = 18.0, 4.0 Hz, 2H), 2.43 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 167.8 (2C), 154.6 (2C), 141.8 (2C), 131.5 (2C), 130.7 (2C), 129.2 (4C), 127.4 (2C), 127.0 (4C), 126.2 (2C), 59.5 (2C), 42.4 (2C), 22.1 (2C). Anal. Calcd. for C28H26N4O2: C, 74.65; H, 5.82; N, 12.44. Found: C, 74.59; H, 5.79; N, 12.38.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(p-tolyl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3f )
Yield: 78 %. M.p. 268–270 °C. IR (KCl) v/cm−1: 3027, 2921, 1662, 1417, 1326, 1143, 1027, 1010, 960, 867, 819, 543. 1H NMR (400 MHz, CDCl3, ppm): δ 7.63 (d, J = 7.2 Hz, 4H), 7.27 (d, J = 7.6 Hz, 4H), 7.18 (s, 4H), 5.59–5.53 (dd, J = 12.0, 4.8 Hz, 2H), 3.73–3.66 (dd, J = 17.4, 12.0 Hz, 2H), 3.19–3.12 (dt, J = 17.4, 4.4 Hz, 2H), 2.45 (s, 6H), 2.42 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 168.8 (2C), 154.2 (2C), 141.1 (2C), 140.7 (2C), 129.4 (4C), 128.4 (2C), 126.5 (4C), 126.1 (4C), 59.5 (2C), 42.2 (2C), 21.9 (2C), 21.5 (2C). Anal. Calcd. for C30H30N4O2: C, 75.29; H, 6.32; N, 11.71. Found: C, 75.21; H, 6.27; N, 11.63.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3g )
Yield: 79 %. M.p. 248–250 °C. IR (KCl) v/cm−1: 3002, 2933, 2836, 1656, 1606, 1517, 1423, 1255, 1174, 1035, 960, 831, 547. 1H NMR (400 MHz, CDCl3, ppm): δ 7.71–7.66 (dd, J = 8.8, 2.8 Hz, 4H), 7.18 (s, 4H), 6.97–6.93 (dd, J = 8.8, 2.4 Hz, 4H), 5.58–5.54 (dd, J = 12.0, 4.8 Hz, 2H), 3.87 (s, 6H), 3.72–3.64 (dd, J = 17.4, 12.0 Hz, 2H), 3.17–3.12 (dt, J = 17.4, 4.4 Hz, 2H), 2.42 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 168.7 (2C), 161.3 (2C), 153.9 (2C), 141.1 (2C), 128.2 (4C), 126.2 (2C), 126.1 (2C), 123.9 (2C), 114.5 (4C), 59.5 (2C), 55.4 (2C), 42.2 (2C), 21.9 (2C). Anal. Calcd. for C30H30N4O4: C, 70.57; H, 5.92; N, 10.97. Found: C, 70.52; H, 5.83; N, 10.84.
1,1′-(5,5′-(1,4-Phenylene)bis(3-(4-chlorophenyl)-4,5-dihydro-1H-pyrazole-5,1-diyl))diethanone ( 3h )
Yield: 81 %. M.p. 253–255 °C. IR (KCl) v/cm−1: 1666, 1592, 1417, 1398, 1322, 1145, 1091, 1010, 956, 827, 565, 538. 1H NMR (400 MHz, CDCl3 ppm): δ 7.70–7.65 (dd, J = 8.8, 2.8 Hz, 4H), 7.42–7.39 (dd, J = 8.6, 2.4 Hz, 4H), 7.19 (s, 4H), 5.61–5.55 (m, 2H), 3.73–3.62 (m, 2H), 3.17–3.10 (dt, J = 17.6, 4.4 Hz, 2H), 2.42 (s, 6H). 13C NMR (100 MHz, CDCl3, ppm): δ 169.0 (2C), 153.0 (2C), 140.9 (2C), 136.3 (2C), 129.7 (2C), 129.0 (4C), 127.8 (4C), 126.1 (4C), 59.7 (2C), 42.0 (2C), 21.9 (2C). Anal. Calcd. for C28H24Cl2N4O2: C, 64.74; H, 4.66; N, 10.79. Found: C, 64.66; H, 4.59; N, 10.72.
1,4-Bis(3-(naphthalen-1-yl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4a )
Yield: 79 %. M.p. 271–273 °C. IR (KCl) v/cm−1: 3446, 3322, 1598, 1540, 1498, 1247, 1133, 997, 802, 779, 690. 1H NMR (400 MHz, DMSO, ppm): δ 9.58 (d, J = 8.4 Hz, 2H), 8.09–7.99 (m, 4H), 7.72 (t, J = 7.2 Hz, 2H), 7.68 (t, J = 7.2 Hz, 2H), 7.46–7.41 (m, 2H), 7.25–7.21 (m, 4H), 6.99–6.97 (m, 4H), 6.90–6.87 (m, 4H), 6.06–6.02 (m, 4H), 5.33–5.23 (m, 2H), 4.08–4.01 (dd, J = 16.8, 12.4 Hz, 2H), 3.39–3.33 (dd, J = 16.8, 7.2 Hz, 2H). 13C NMR (100 MHz, DMSO, ppm): δ 152.1 (2C), 143.9 (2C), 143.3 (2C), 142.8 (2C), 137.0 (2C), 130.2 (4C), 129.6 (2C), 129.3 (4C), 128.2 (2C), 127.2 (4C), 127.1 (4C), 124.5 (4C), 120.0 (2C), 118.9 (2C), 113.2 (2C), 62.9 (2C), 40.5 (2C). Anal. Calcd. for C44H34N4: C, 85.41; H, 5.54; N, 9.05. Found: C, 85.39; H, 5.35; N, 9.02.
1,4-Bis(1-phenyl-3-(thiophen-3-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4b )
Yield: 85 %. M.p. 279–281 °C. IR (KCl) v/cm−1: 3315, 3097, 1596, 1498, 1361, 1103, 997, 746, 692. 1H NMR (400 MHz, CDCl3, ppm): δ 7.59–7.56 (dd, J = 4.8, 3.6 Hz, 2H), 7.30 (s, 4H), 7.24–7.22 (dd, J = 3.6, 1.2 Hz, 2H), 7.14 (t, J = 8.0 Hz, 6H), 6.90 (t, J = 7.2 Hz, 4H), 6.73 (t, J = 7.2 Hz, 2H), 5.39–5.35 (dd, J = 12.4, 6.4 Hz, 2H), 3.88–3.3.83 (dd, J = 17.4, 12.0 Hz, 2H), 3.10–3.02 (dd, J = 17.2, 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3, ppm): δ 153.0 (2C), 145.1 (2C), 144.8 (2C), 142.2 (2C), 129.3 (4C), 128.3 (2C), 128.1 (2C), 127.7 (4C), 126.8 (2C), 117.9 (2C), 110.8 (4C), 62.9 (2C), 40.5 (2C). Anal. Calcd. for C32H26N4S2: C, 72.42; H, 4.94; N, 10.56. Found: C, 72.39; H, 4.91; N, 10.48.
1,4-Bis(1-phenyl-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4c )
Yield: 82 %. M.p. 231–233 °C. IR (KCl) v/cm−1: 3284, 3021, 1594, 1500, 1367, 1132, 998, 740, 690. 1H NMR (400 MHz, CDCl3, ppm): δ 7.58–7.56 (dd, J = 4.8, 3.6 Hz, 2H), 7.29 (s, 4H), 7.21 (t, J = 3.6 Hz, 2H), 7.13 (t, J = 8.0 Hz, 4H), 7.09–7.07 (m, 2H), 6.90 (d, J = 7.2 Hz, 4H), 6.71 (t, J = 7.2 Hz, 2H), 5.45–5.40 (dd, J = 12.0, 6.4 Hz, 2H), 3.90–3.87 (dd, J = 17.2, 12.0 Hz, 2H), 3.10–3.08 (dd, J = 17.2, 6.4 Hz, 2H). 13C NMR (100 MHz, CDCl3, ppm): δ 163.3 (2C), 144.5 (2C), 144.1 (2C), 142.0 (2C), 129.3 (4C), 128.2 (2C), 128.0 (2C), 127.9 (4C), 127.0 (2C), 119.1 (2C), 113.2 (4C), 63.3 (2C), 44.1 (2C). Anal. Calcd. for C32H26N4S2: C, 72.42; H, 4.94; N, 10.56. Found: C, 72.35; H, 4.89; N, 10.51.
1,4-Bis(3-(furan-2-yl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4d )
Yield: 80 %. M.p. 246–248 °C. IR (KCl) v/cm−1: 3122, 2915, 1594, 1504, 1394, 1257, 1130, 1027, 829, 748, 690. 1H NMR (400 MHz, CDCl3, ppm): δ 7.52–7.51 (dd, J = 3.2, 1.6 Hz, 2H), 7.29 (s, 4H), 7.20–7.17 (m, 4H), 7.05–7.03 (m, 4H), 6.81 (t, J = 6.8 Hz, 2H), 6.57 (t, J = 3.2 Hz, 2H), 6.50–6.48 (m, 2H), 5.26–5.23 (dd, J = 12.4, 4.8 Hz, 2H), 3.82–3.74 (dd, J = 16.8, 2.4 Hz, 2H), 3.11–3.05 (dd, J = 17.0, 6.8 Hz, 2H). 13C NMR (100 MHz, CDCl3, ppm): δ 149.8 (2C), 139.6 (2C), 129.3 (4C), 129.1 (2C), 127.0 (2C), 119.1 (2C), 118.8 (2C), 113.3 (4C), 112.5 (4C), 112.4 (2C), 111.3 (2C), 62.6 (2C), 43.3 (2C). Anal. Calcd. for C32H26N4O2: C, 77.09; H, 5.26; N, 11.24. Found: C, 77.01; H, 5.19; N, 11.18.
1,4-Bis(1,3-diphenyl-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4e )
Yield: 93 %. M.p. 224–226 °C. IR (KCl) v/cm−1: 3100, 1594, 1498, 1380, 1317, 1228, 1122, 1106, 827, 748, 709, 690. 1H NMR (400 MHz, DMSO, ppm): δ 7.74–7.71 (dd, J = 7.6, 2.4 Hz, 4H), 7.43–7.39 (m, 6H), 7.29 (s, 4H), 7.14 (t, J = 7.6 Hz, 4H), 6.98 (d, J = 7.6 Hz, 4H), 6.73–6.69 (dd, J = 12.2, 6.8 Hz, 2H), 5.45–5.40 (dd, J = 12.4, 6.8 Hz, 2H), 3.93–3.85 (dd, J = 17.4 12.4 Hz, 2H), 3.10–3.04 (dd, J = 17.4, 6.8 Hz, 2H). 13C NMR (100 MHz, CDCl3, ppm): δ 147.6 (2C), 144.5 (2C), 142.2 (2C), 132.6 (2C), 129.3 (4C), 129.1 (4C), 129.0 (4C), 127.0 (2C), 126.1 (4C), 119.1 (2C), 113.3 (4C), 63.3 (2C), 43.4 (2C). Anal. Calcd. for C36H30N4: C, 83.37; H, 5.83; N, 10.80. Found: C, 83.29; H, 5.79; N, 10.78.
1,4-Bis(1-phenyl-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4f )
Yield: 76 %. M.p. 278–280 °C. IR (KCl) v/cm−1: 3018, 2919, 1596, 1498, 1386, 1321, 1122, 817, 746, 690. 1H NMR (400 MHz, CDCl3, ppm): δ 7.64–7.63 (dd, J = 7.4, 4.4 Hz, 4H), 7.29 (s, 4H), 7.22–7.20 (m, 8H), 7.08 (d, J = 7.6 Hz, 4 H), 6.83–6.75 (m, 2H), 5.26–5.21 (dd, J = 12.2, 7.2 Hz, 2H), 3.85–3.77 (dd, J = 15.0, 2.0 Hz, 2H), 3.15–3.09 (dd, J = 16.8, 7.2 Hz, 2H), 2.40 (s, 6H). 13C NMR (100 MHz, DMSO, ppm): δ 147.6 (2C), 143.5 (2C), 142.2 (2C), 137.1 (2C), 132.6 (2C), 129.9 (2C), 129.3 (4C), 129.0 (4C), 127.0 (2C), 126.1 (4C), 123.5 (2C), 113.3 (4C), 63.3 (2C), 43.4 (2C), 20.1 (2C). Anal. Calcd. for C38H34N4: C, 83.48; H, 6.27; N, 10.25. Found: C, 83.37; H, 6.19; N, 10.15.
1,4-Bis(3-(4-methoxyphenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4g )
Yield: 79 %. M.p. 265–267 °C. IR (KCl) v/cm−1: 3007, 1733, 1716, 1698, 1683, 1558, 1540, 414. 1H NMR (400 MHz, CDCl3, ppm): δ 7.67–7.66 (m, 4H), 7.31 (s, 4H), 7.23 (t, J = 7.6 Hz, 4H), 7.04 (d, J = 7.2 Hz, 4H), 6.94–6.91 (m, 4H), 6.79 (t, J = 7.2 Hz, 2H), 5.23–5.18 (dd, J = 12.2, 7.2 Hz, 2H), 3.85–3.80 (m, 8H), 3.14–3.10 (dd, J = 17.0, 7.2 Hz, 2H). 13C NMR (100 MHz, DMSO, ppm): δ 163.2 (2C), 147.7 (2C), 145.0 (2C), 142.2 (2C), 129.3 (4C), 127.7 (4C), 127.0 (2C), 125.2 (4C), 118.7 (2C), 114.5 (4C), 113.1 (4C), 63.2 (2C), 56.0 (2C), 43.6 (2C). Anal. Calcd. for C38H34N4O2: C, 78.87; H, 5.92; N, 9.68. Found: C, 78.81; H, 5.89; N, 9.59.
1,4-Bis(3-(4-chlorophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-5-yl)benzene ( 4h )
Yield: 84 %. M.p. 284–286 °C. IR (KCl) v/cm−1: 1594, 1502, 1488, 1386, 1321, 1132, 1087, 1010, 821, 742, 690. 1H NMR (400 MHz, DMSO, ppm): δ 7.70 (d, J = 8.0 Hz, 4H), 7.41 (d, J = 8.0 Hz, 4H), 7.26 (s, 4H), 7.11 (t, J = 7.2 Hz, 4H), 6.96 (d, J = 6.8 Hz, 4H), 6.69 (t, J = 6.8 Hz, 2H), 5.44–5.40 (dd, J = 12.0, 6.4 Hz, 2H), 3.90–3.82 (dd, J = 17.4, 12.0 Hz, 2H), 3.08–3.02 (dd, J = 17.4, 6.4 Hz, 2H). 13C NMR (100 MHz, DMSO, ppm): δ 150.7 (2C), 144.4 (2C), 140.0 (2C), 133.5 (2C), 133.0 (2C), 130.1 (2C), 129.9 (4C), 129.1 (4C), 128.6 (4C), 127.7 (4C), 113.2 (2C), 106.1 (2C), 63.4 (2C), 43.2 (2C). Anal. Calcd. for C36H28Cl2N4: C, 73.59; H, 4.80; N, 9.54. Found: C, 73.52; H, 4.79; N, 9.48.
Anticancer activity
Cell culture
Antiproliferative activities of the synthesized compounds were investigated on C6 (rat brain tumor cells) and HeLa (human uterus carcinoma) cells using a proliferation BrdU ELISA assay [33, 34]. Cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, Sigma), supplemented with 10 % (v/v) fetal bovine serum (Sigma, Germany) and PenStrep solution (Sigma, Germany) at 37 °C in 5 % CO2 humidified atmosphere.
Cell proliferation assays
Cells were plated in 96-well culture plates (COSTAR, Corning, USA) at density of 3 × 104 cells per well. The activities of samples were investigated at eight concentrations. 5-FU was used as standard compound. The cells were then incubated overnight before applying the BrdU Cell Proliferation ELISA assay reagent (Roche, Germany) according to the manufacturer’s procedure. The amount of cell proliferation was determined as A450 nm by using a microplate reader (Awareness Chromate, USA). Results are reported as percentage inhibition of cell proliferation, where the optical density measured from vehicle-treated cells was considered to be 100 % proliferation. Percentage of inhibition of cell proliferation was calculated as follows: [1 − (A treatment/A vehicle control)] × 100.
Stock solution of tested compounds was prepared in dimethyl sulfoxide (DMSO), and diluted with DMEM. The final DMSO concentration was below 0.1 % in all tests. IC50 and IC75 values were determined using ED50 plus v1.0. The results of the in vitro investigation are presented as mean ± standard deviation (SD) of six measurements. Differences between groups were tested with analysis of variance (ANOVA) with p values <0.01 considered significant, analyzed by SPSS (version 11.5 for Windows 2000, SPSS Inc.).
References
R. Rahman, S. Smith, C. Rahman, R. Grundy, J. Oncol. 2010. Art. ID: 251231, 16 p. (2010)
M. Gallorini, A. Cataldi, V. di Giacomo, Biodrugs 26, 377 (2012)
G. Kibria, H. Hatakeyama, H. Harashima, Arch. Pharm. Res. 37, 4 (2014)
A. Joseph, C.S. Shah, S.S. Kumar, A.T. Alex, N. Maliyakkal, S. Moorkoth, J.E. Mathew, Acta Pharm. 63, 397 (2013)
J. Polivka, V. Rohan, O. Topolcan, J. Ferda, Anticancer Res. 32, 2935 (2012)
S. Manfredini, R. Bazzanini, P.G. Baraldi, M. Bonora, M. Marangoni, D. Simoni, A. Pani, F. Scintu, E. Pinna, Anti Cancer Drug Des. 11, 193 (1996)
B. Caliskan, A. Yilmaz, I. Evren, S. Menevse, O. Uludag, E. Banoglu, Med. Chem. Res. 22, 782 (2013)
S. Manfredini, R. Bazzanini, P.G. Baraldi, M. Guarneri, D. Simoni, M.E. Marongiu, A. Pani, E. Tramontano, P. La Colla, J. Med. Chem. 35, 917 (1992)
H.-A. Park, K. Lee, S.-J. Park, B. Ahn, J.-C. Lee, H.Y. Cho, K.-I. Lee, Bioorg. Med. Chem. Lett. 15, 3307 (2005)
D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, C.W. Day, D.F. Smee, P. Grellier, R. Lesyk, Eur. J. Med. Chem. 66, 228 (2013)
S.L. Zhu, Y. Wu, C.J. Liu, C.Y. Wei, J.C. Tao, H.M. Liu, Eur. J. Med. Chem. 65, 70 (2013)
B. Insuasty, A. Montoya, D. Becerra, J. Quiroga, R. Abonia, S. Robledo, I. Darío Velez, Y. Upegui, M. Nogueras, J. Cobo, Eur. J. Med. Chem. 67, 252 (2013)
M.M. Ghorab, F.A. Ragab, S.I. Alqasoumi, A.M. Alafeefy, S.A. Aboulmagd, Eur. J. Med. Chem. 45, 171 (2010)
S. Emami, S. Dadashpour, Eur. J. Med. Chem. 102, 611 (2015)
V.C. Jorden, J. Med. Chem. 46, 1081 (2003)
Y. Yao, C. Liao, Z. Li, Z. Wang, Q. Sun, C. Liu, Y. Yang, Z. Tu, S. Jiang, Eur. J. Med. Chem. 86, 639 (2014)
X.-F. Huang, X. Lu, Y. Zhang, G.-Q. Song, Q.-L. He, Q.-S. Li, X.-H. Yang, Y. Wei, H.-L. Zhu, Bioorg. Med. Chem. 20, 4895 (2012)
Y. Xia, C.D. Fan, B.X. Zhao, J. Zhao, D.S. Shin, J.Y. Miao, Eur. J. Med. Chem. 43, 2347 (2008)
M. Fedele, C. Franco, B. Adriana, S. Daniela, B. Bruna, B. Olivia, T. Paola, M. Bruno, A. Stefano, T. Andrea, Bioorg. Med. Chem. Lett. 12, 3629 (2002)
P.-C. Lv, H.-Q. Li, J. Sun, Y. Zhou, H.-L. Zhu, Bioorg. Med. Chem. 18, 4606 (2010)
J.-J. Liu, H. Zhang, J. Sun, Z.-C. Wang, Y.S. Yang, D.-D. Li, F. Zhang, H.-B. Gong, H.-L. Zhu, Bioorg. Med. Chem. 20, 6089 (2012)
P.-C. Lu, D.-D. Li, Q.-S. Li, X. Lu, Z.-P. Xiao, H.-L. Zhu, Bioorg. Med. Chem. Lett. 21, 5374 (2011)
M.B. Gürdere, O. Özbek, M. Ceylan, Synth. Commun. 46, 322 (2016)
Y. Budak, Chin. J. Chem. 30, 341 (2012)
M. Ceylan, M.B. Gürdere, H. Gezegen, Y. Budak, Synth. Commun. 40, 2598 (2010)
I. Karaman, H. Gezegen, M.B. Gürdere, A. Dingil, M. Ceylan, Chem. Biodivers. 7, 400 (2010)
V. Kanagarajan, M.R. Ezhilarasi, M. Gopalakrishnan, Org. Med. Chem. Lett. 1, 1 (2011)
A. Voskiene, V. Mickevicius, G. Mikulskiene, Arkivoc. xv, 303 (2007)
P. Wang, N. Onozawa-Komatsuzaki, Y. Himeda, H. Sugihara, H. Arakawa, K. Kasuga, Tetrahedron Lett. 42, 9199 (2001)
N. El-Rayyes, A.A. Ai-Johary, J. Chem. Eng. Data 30, 500 (1985)
X.H. Zhang, W.Y. Lai, Z.Q. Gao, T.C. Wong, C.S. Lee, H.L. Kwong, S.T. Lee, S.K. Wu, Phys. Lett. Chem. 320, 77 (2000)
S.R. Sandier, K.C. Tsou, J. Chem. Phys. 39, 1062 (1963)
I. Demirtas, A. Sahin Yaglioglu, J. Chem. 2013, 1 (2013)
A. Sahin Yaglioglu, I. Demirtas, N. Goren, Phytochem. Lett. 8, 213 (2014)
Acknowledgments
The authors are indebted to the Gaziosmanpasa University Scientific Research Projects Commission (Project No. BAP2011/95) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gürdere, M.B., Gümüş, O., Yaglioglu, A.S. et al. Synthesis and anticancer activities of 1,4-phenylene-bis-N-acetyl- and N-phenylpyrazoline derivatives. Res Chem Intermed 43, 1277–1289 (2017). https://doi.org/10.1007/s11164-016-2697-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2697-2