Abstract
A series of novel, bioactive 5-substituted indole-2-carboxamide derivatives (10a–t and 14a–k) are synthesized by the coupling of 5-substituted indole-2-carboxylic acids with various amines in the presence of EDC HCl/HOBt in DMF/CH2Cl2 as a solvent. In vitro, antibacterial activity of titled compounds against pathogenic bacteria Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa, Salmonella typhi and antifungal activity against pathogenic fungi Candida albicans, Cryptococcus neoformans, Aspergillus fumigatus, and Candida parapsilosis are evaluated using gentamicin/ciprofloxacin and fluconazole/oxiconazole as standard drugs, respectively. The majority of the synthesized compounds exhibited good antibacterial activity, but surprisingly none showed antifungal activity. Compounds 10c, 10d, 10i, 10j, 10l–n, 14g, 14h, 14i, 14j, and 14k exhibited high inhibitory antibacterial activity with MIC values in the range of 0.12–6.25 µg/mL. Interestingly, compounds 14i, 14j, and 14k exhibited excellent antibacterial activity against K. pneumoniae and E. coli compare to synthesized compound. All the experimental results promote us to consider this series as a starting point for the development of novel and more potent antibacterial agents in the future.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For the normal growth of animals and microbes, the interactions between them are intimate and vital, yet they also have darker side, since microbes trigger a broad range of human diseases. History observes continual battle between human beings and disease-causing microbes. In the field of medicine, invention of and advancements in antibiotics are among the most astonishing achievements of the last century. However, these advances in medical care are threatened by a natural phenomenon known as antimicrobial resistance [1–4]. Antimicrobial resistance is the result of inadequate, empirical, self-medicated, improper, and overuse of antibiotics, a gift of the medical field, for the treatment of diseases. Antibiotic resistance is not new; it is ancient [5].
Infection-causing Gram-negative bacteria, about 50 % of the multidrug-resistant bacteria [6], including Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii [7], have become a serious threat in hospitals and community clinics [8]. Increasing multidrug-resistant (MDR) infections caused by Gram-negative bacteria are more difficult to treat, since they increase mortality, morbidity [9, 10], and health care cost. In spite of a large number of antibiotics and chemotherapeutics available for medical use, the emergence of multidrug bacterial resistance to current antibiotics have created an urgent need for either in the development of novel antibiotics or modification of existing antibiotics to fight against MDR infections, which threaten the progress of about 60 years in medicine [11–13]. The majority of antimicrobial agents act on a small group of well-validated targets, which suggests the most effective way to kill cell. In view of the above, the design and synthesis of newer, efficient, and broad-spectrum antimicrobials with fewer side effects will always remain an area of immense significance.
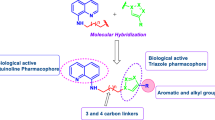
Merck scientists reported that L-161, 240 (aryl oxazoline hydroxamic acid derivative [14]) are potent inhibitors of LpxC [15–19] from E. coli [20]. We designed and synthesized novel compounds of general motif, shown in Fig. 1, by changing the Zn-binding hydroxamic acid functionality of L-161, 240 to amide functionality, the oxazoline moiety to indole moiety (Fig. 1). We focused on the role of hydrophobic substituent of the aryl group, indole ring, and aliphatic or aromatic amines of amide functionality on antimicrobial activity. Indoles are omnipresent in natural products, as well as in several synthetic bioactive compounds [21–40]. Indole moiety is the most privileged structural motif in the process of discovery of new drugs [41–46]. The inbuilt ability of indole derivatives such as mimicking the structure of proteins and binding reversibly to enzymes [47] creates huge opportunities to discover novel bioactive molecules with different mechanisms of action. U.S. retail reports that many indole-containing drugs are among the best selling drugs [48]. Moreover, 1H-indole-2-carboxamide templates are present in natural products [49], as well as in several synthetic bioactive compounds. This scaffold shows broad-spectrum biological activity [50–52]. Hence, among the pharmacophores responsible for antimicrobial activity, the indole derivatives seem to be a viable lead structure for a more efficacious and systematic antimicrobial drug. However, to the best of our knowledge, the antibacterial and antifungal activity of these bioactive pharmacophores has not been reported to date.
Inspired by these encouraging facts and as a part of our ongoing interest toward the design and synthesis of novel bioactive heterocycles harboring indole nucleus as antimicrobial agents, we have synthesized a series of novel 5-arylindole-2-carboxamide derivatives (10a–t and 14a–k) and screened them for antimicrobial activity.
Results and discussion
Chemistry
The target compounds (10a–t) and (14a–k) were synthesized following procedures outlined in the scheme (Scheme 1). In the first step, an ice-cold aqueous solution of NaNO2 was added to an ice-cold solution of 4-bromoaniline (1) in 12 M HCl, and the resulting solution was stirred at 0 °C for 15 min. To the stirred solution, an ice-cold solution of SnCl2 in 12 M HCl was added drop-wise, and the resulting solution was stirred at 0 °C for 4 h to afford 4-bromophenylhydrazine (2). Indole-2-carboxylic ester (4) was obtained by condensation of 2 with ethyl pyruvate followed by cyclization using polyphosphoric acid. Suzuki coupling of 4 with corresponding phenyl boronic acids in presence of Pd(dppf)2Cl2·CH2Cl2 at 90 °C under inert atmosphere gave ester 5a/5b. Ester 5a/5b underwent N-benzylation with 4-chlorobenzylchloride in DMF in presence of Cs2CO3 to give ester 6a/6b which was hydrolyzed with LiOH in THF, H2O and EtOH system to furnish corresponding carboxylic acid 7a/7b. Hydrolysis of 5a with LiOH in THF, H2O and EtOH system gave carboxylic acid (7c). To the stirred solution of carboxylic acid (7a/7b/7c), EDC.HCl, HOBt, and DIPEA/TEA in DMF, an appropriate amine was added and the resulting solution was stirred for 20–30 h at room temperature to afford the target carboxamide (10a–t and 14a–k) in good to excellent yields.
Synthesis of 5-substituted indole-2-carboxamide derivatives. Reagents and conditions: a (i) NaNO2, HCl, 0 °C, 15 min; (ii) SnCl2, HCl, 0 °C, 4 h; b ethyl pyruvate, EtOH, argon, reflux 5.5 h; c PPA, 120 °C, 20–30 min; d 4-Flouro-3-methylphenylboronic acid or 4-methoxyphenylboronic acid, Pd(dppf)2Cl2–CH2Cl2, KOAc, 1,4-dioxane, H2O, 80–90 °C, 3 h; e 4-chlorobenzyl chloride, Cs2CO3, DMF, 60 °C, 6 h; f LiOH, THF, H2O, EtOH, Stirr, rt, 3–4 h; h LiOH, THF, H2O, EtOH, Stirr, rt, 3–4 h; g, i amine, EDC-HCl, HOBt, DIPEA, DMF, 0 °C to rt, 20–30 h
Biological activity
In the endeavor to identify the antimicrobial activity of 5-arylindole-2-carboxamides, we synthesized a series of 31 compounds (10a–t and 14a–k) and screened them against pathogenic Gram-negative bacteria Klebsiella pneumoniae, E. coli, P. aeruginosa, and Salmonella typhi and pathogenic fungi Candida albicans, Cryptococcus neoformans, Aspergillus fumigatus, and Candida parapsilosis by using the broth microdilution technique described by the Clinical and Laboratory Standards Institute (CLSI), 2012 (Formerly NCCLS) [49] and the results are depicted in Table 1. Many of the synthesized carboxamides exhibited excellent antibacterial activity that was manyfold better than the activity of standard drugs, gentamicin and ciprofloxacin.
The compounds 10c, 10d, 10j, 10n, 14i, 14j, and 14k showed high antibacterial activity against K. pneumoniae with respective MIC value of 1.56, 1.25, 1.56, 1.56, 0.39, 0.39, and 0.12 μg/mL. However, 14i, 14j, and 14k exhibited excellent antibacterial activity against K. pneumoniae, E. coli and moderate activity against P. aeruginosa when compared to both standards. Compounds 10d, 10j, 10l–n, 14i, 14j, and 14k have exhibited promising antibacterial activity against E. coli in the range of 0.56–3.25 μg/mL. Compound 14h was found to be as potent as ciprofloxacin against S. typhi. Interestingly, 14k inhibited K. pneumoniae, E. coli, and P. aeruginosa more efficiently than gentamicin and ciprofloxacin. All the tested compounds were inactive (MIC > 50 μg/mL) (Table 1) against tested fungi when compared to standards, fluconazole and oxiconazole, except 14a, which exhibited moderate activity only against C. neoformans and A. fumigatus (MIC ~ 25–50 μg/mL).
Structure–activity relationship study
We explored diversity in the designed molecule (5-arylindole-2-carboxamide) by varying the aryl group and especially amines in the amide functionality. Replacement of para-OMe substituent of the phenyl ring by hydrophobic para-F and meta-Me groups increased antibacterial activity by 2–4 and twofold against K. pneumoniae and E. coli. respectively. and moderately with P. aeruginosa. In many cases, deprotection of indole N–H increased antibacterial activity to an acceptable extent against K. pneumoniae, E. coli. and P. aeruginosa indicating the importance of free indole N–H (Fig. 2). Substitution of aromatic amines by few specific alicyclic amines improved the antibacterial activity by 2–10-fold (Table 1). Change in either the aryl group or amines of amide functionality did not change the antifungal activity of titled compounds.
Conclusion
We have designed, synthesized, and screened a series of novel 5-arylindole-2-carboxamides (10a–t and 14a–k) for in vitro antibacterial and antifungal activity. All the synthesized compounds show moderate to excellent antibacterial activity compared to standard drugs. However, none of the titled compounds show antifungal activity except 14a, indicating potency of 5-arylindole-2-carboxamides towards bacterial strains than fungi. Compounds 14i, 14j, and 14k show excellent antibacterial activity against K. pneumoniae and E. coli than standard drugs and are promising to act as potential antibacterial agent. Compound 14k have been found to be most efficient than both standards against K. pneumoniae, E. coli, and P. aeruginosa. Our findings underscore the promising potential of the 5-arylindole-2-carboxamides as an important lead structural scaffold in the design and synthesis of new antibacterial agents.
Experimental
All the solvents and reagents were purchased from commercial suppliers, Spectrochem Pvt. Ltd., Sigma Aldrich, and Rankem India Ltd., and were used without further purification. The progress of each reaction was monitored by ascending TLC using TLC aluminum sheets, pre-coated silica gel F254 (Merck, Germany), and by locating the spots using ultraviolet (UV) light as the visualizing agent or iodine vapors. Melting points were taken in an open capillary method and are uncorrected. All compounds were purified by recrystallization/slica gel (100–200 mesh) gravity column with suitable organic solvents. Mass spectra were recorded on Shimadzu GC-MS-QP-2010 model using direct inlet probe technique. 1H NMR, 13C NMR was determined in CDCl3 , and DMSO-d 6 solution on a Bruker Ac 200 MHz or Bruker Ac 400 MHz spectrometer. High-resolution mass spectra (HRMS) were recorded on an Agilent 6520 (QTOF) ESI-HRMS instrument and elemental analyses (C, H, N) performed at the PerkineElmer 2400 CHN analyzer.
Synthesis of 4-bromophenylhydrazine (2)
To an ice-cold aqueous solution of 12 M HCl (30 mL), 4-bromoaniline 1 (8.00 g, 46.51 mmol) and, dropwise, an ice-cold solution of NaNO2 (3.20 g, 46.37 mmol) in H2O (10 mL) was added. The reaction was stirred for 15 min at 0 °C. To the resulting mixture, an ice-cold solution of SnCl2 (26.44 g, 139.89 mmol) in 12 M HCl (70 mL) was added dropwise. The reaction mixture was stirred for 4 h at 0 °C. The precipitate was filtered, washed with 1 M HCl, and poured into water where the pH value was adjusted to 10 by adding 1 M NaOH. The crude product was extracted with Et2O, dried over Na2SO4, filtered, and concentrated under reduced pressure to give an orange solid 2.
Synthesis of ethyl pyruvate 4-bromophenylhydrazone (3)
A mixture of 4-bromophenylhydrazine 2 (5.0 g, 26.73 mmol), ethyl pyruvate (3.72 g, 32.07 mmol) in EtOH (30 mL) was refluxed under argon for 5 h. The cooled reaction mixture was then filtered, and the precipitate was washed with water. The crude product was triturated with cyclohexane and filtered to give a yellow solid 3.
Synthesis of ethyl 5-bromo-1H-indole-2-carboxylate (4)
A mixture of ethyl pyruvate 4-bromophenylhydrazone 3 (5.0 g, 17.54 mmol) and polyphosphoric acid (44 g) was heated to 120 °C for 0.5 h. The reaction mixture was then cooled, poured into ice-cold water, and neutralized with saturated aqueous sodium bicarbonate. The crude product was extracted with EtOAc, dried over Na2SO4, filtered, and concentrated under reduced pressure to give a pale yellow solid 4.
Synthesis of ethyl 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate (5a) and Ethyl 5-(4-methoxyphenyl)-1H-indole-2-carboxylate (5b)
Potassium acetate (0.914 g, 13.98 mmol) in water (3 mL) was added to a solution of 4 (1.5 g, 05.59 mmol) and 4-fluoro-3-methylphenylboronic acid (06.71 mmol)/4-methoxyphenylboronic acid (06.71 mmol) in 1,4-dioxane (25 mL). The reaction mixture was degassed using argon and Pd(dppf)2Cl2 (0.228 g, 0.279 mmol) was added. The reaction mixture was stirred for 16 h at 120 °C. After completion of the reaction, monitored by TLC, the mixture was cooled to room temperature and the solvent was removed under reduced pressure. Water (10 mL) was added to the residue and extracted with EtOAc (3 × 10 mL). The organic layer was washed with brine (1 × 10 mL), dried over anhydrous Na2SO4 and solvent was evaporated in vacuum. The product was purified by silica gel chromatography using Hexane: EtOAc (5:95) to afford 0.463 g (28 %) of compound 5a/0.469 g (30 %) of compound 5b as light yellow solids.
Synthesis of ethyl 1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate (6a) and ethyl 1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxylate (6b)
4-Chloro benzyl chloride (0.25 mL, 01.55 mmol) was added to a suspension of cesium carbonate (0.546 g, 01.55 mmol) and ethyl 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate 5a (1.55 mmol)/ethyl 5-(4-methoxyphenyl)-1H-indole-2-carboxylate 5b (1.55 mmol) in DMF (20 mL). The reaction mixture was stirred at 60 °C for 6 h (monitored by TLC). The reaction mixture was poured into water (20 mL) and extracted with EtOAc (3 × 10 mL). The organic layer was washed with saturated aqueous NaHCO3 (1 × 20 mL), water (1 × 10 mL), brine (1 × 10 mL), dried over anhydrous Na2SO4 , and concentrated in vacuum. The product was isolated by silica gel chromatography using Hexane: EtOAc (90:10) to afford 0.438 g (88 %) of compound 6a/0.436 g (88 %) of compound 6b as white solids.
Synthesis of 1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylic acid (7a), 1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxylic acid (7b) and 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylic acid (7c)
Ethyl 1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate 6a (1.24 mmol)/ethyl 1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxylate 6b (1.24 mmol)/ethyl 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate 5a (1.24 mmol) was dissolved in THF (15 mL) and to that lithium hydroxide (0.044 g, 1.86 mmol) dissolved in 4 mL of water, was added dropwise. The reaction mixture was stirred at room temperature for 16 h. The pH of the reaction mixture was lowered to 2–3 with 1 M HCl. The mixture was extracted with EtOAc (3 × 30 mL). The combined organic layer was dried over anhydrous Na2SO4 and then concentrated in vacuum. The product was isolated by silica gel chromatography using hexane:EtOAc (50:50) to obtain 0.343 g (94 %) of compound 7a, 0.340 g (94 %) of compound 7b, and 0.255 g (94 %) of compound 7c as white solids.
General procedure for 1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamide, 1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxamide (10 a–t ) and 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamide ( 14a–k )
Compound 7a (0.253 mmol)/compound 7b (0.253 mmol)/compound 7c (0.371 mmol) was dissolved in DMF/CH2Cl2 (15 mL) and cooled to 0 °C to rt. To that EDC.HCl (0.42 mmol), HOBt (0.42 mmol) and DIPEA/TEA (0.50 mmol) were added. The reaction mixture was stirred for 0.5 h and then appropriate Amine (0.352 mmol) was added and it was stirred for 20–30 h at room temperature. After completion of the reaction (monitored by TLC), the reaction mixture was diluted with dichloromethane (20 mL) and washed with saturated aq. NH4Cl solution (1 × 20 mL), water (1 × 20 mL) and brine (1 × 20 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated in vacuum. The product was isolated by flash chromatography on silica gel using hexane:EtOAc (95:5) to afford desired targets (10a–t) and (14a–k) (70–94 %) as white to off-white solids. In the case of DMF, after completion of the reaction, the reaction mixture was poured into water and the obtained solids were filtered and washed thoroughly with water to afford the desired products.
Spectral data
4-Bromophenylhydrazine ( 2 )
Yield 82 %, Mp. 224 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 7.24 (d, 2H, J = 8.8 Hz, Ar), 6.92 (s, 1H, NH), 6.73 (d, 2H, J = 8.8 Hz, Ar–H) and 4.42 (s, 2H, NH2); 13C NMR (100 MHz, DMSO-d 6 ) δ C 113.4, 117.2, 133.6, and 134.3; HRMS (M+H) Calcd = 185.9793, Found 187.0104.
Ethyl pyruvate 4-bromophenylhydrazone ( 3 )
Yield 76 %, Mp. 90–92 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 9.96 (s, 1H, NH), 7.46 (d, 2H, J = 8.8 Hz, Ar–H), 7.22 (d, 2H, J = 8.8 Hz, Ar–H), 4.21 (q, 2H, J = 7.2 Hz, OCH2CH3), 2.1 (s, 3H, –CH3), and 1.29 (t, 3H, J = 7.2 Hz, –CH2–CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 12.4, 14.7, 60.8, 112.6, 116.1, 132.2, 133.3, 144.2, and 165.3; HRMS (M+H) Calcd = 284.0160, Found 285.1304.
Ethyl 5-bromo-1H-indole-2-carboxylate ( 4 )
Yield 82 %, Mp. 165 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 12.08 (s, 1H, NH), 7.84 (d, 1H, J = 0.8 Hz, Ar–H), 7.40 (dd, 1H, J = 8.8 and 0.8 Hz, Ar–H), 7.39 (dd, 1H, J = 8.8 and 1.2 Hz, Ar–H), 7.10 (d, 1H, J = 1.2 Hz, Ar–H), 4.32 (q, 2H, J = 7.2 Hz, –OCH2CH3), and 1.32 (t, 3H, J = 7.2 Hz, –CH2–CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 13.8, 61.2, 109.1, 113.8, 114.1, 121.8, 124.9, 126.1, 133.4, 135.6, and 162.3; HRMS (M+H) Calcd = 266.9895, Found 268.0017.
Ethyl 5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate ( 5a )
Yield 28 %, Mp. 262 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 11.98 (s, 1H, NH), 7.95 (s, 1H, Ar–H), 7.60 (d, 1H, J = 8.0 Hz, Ar–H), 7.53 (d, 1H, J = 8.0 Hz, Ar–H), 7.43 (t, 2H, Ar–H), 7.33 (t, 1H, Ar–H), 7.20 (s, 1H, Ar–H), 4.36 (q, 2H, J = 8 Hz, –OCH2CH3), 2.27 (s, 3H, –CH3), and 1.36 (t, 3H, J = 8 Hz, –CH2CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 13.7, 14.4, 60.8, 108.1, 113.2, 113.3, 120.1, 122.6, 124.1, 127.9, 129.8, 132.3, 137.3, 141.3, 141.4, 143.2, 160.3, 162.7, and 163.2 (J = 248 Hz); HRMS (M+H) Calcd = 297.1960, Found 298.1994.
Ethyl 5-(4-methoxyphenyl)-1H-indole-2-carboxylate ( 5b )
Yield 30 %, Mp. 230 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 11.84 (s, 1H, NH), 7.86 (d, 1H, J = 7.9 Hz, Ar–H), 7.78 (s, 1H, Ar–H), 7.62 (d, 2H, J = 8.2 Hz, Ar–H), 7.46 (d, 1H, J = 7.9 Hz, Ar–H), 7.32 (s, 1H, Ar–H), 7.20 (d, 2H, J = 8.2 Hz, Ar–H), 4.14 (q, 2H, J = 7.1 Hz, –OCH2CH3), 3.68 (s, 3H, –OCH3), and 1.18 (t, 3H, J = 7.1 Hz, –CH2CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 14.2, 56.2, 60.2, 107.9, 133.7, 113.6, 115.2, 118.0, 124.6, 129.3, 130.1, 132.9, 133.8, 142.6, 158.8, and 161.2; HRMS (M+H) Calcd = 295.1208, Found 296.1326.
Ethyl 1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylate ( 6a )
Yield 88 %, Mp. 281 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 8.02 (s, 1H, Ar–H), 7.54 (s, 2H, Ar–H), 7.39 (m, 3H, Ar–H), 7.32 (d, 3H, Ar–H), 7.09 (d, 2H, Ar–H), 5.84 (s, 2H, –CH2), 4.19 (q, 2H, J = 7.2 Hz, –OCH2CH3), 2.27 (s, 3H, –CH3), and 1.25 (t, 3H, J = 7.2 Hz, –CH2CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 14.1, 14.7, 49.6, 60.7, 111.6, 112.4, 113.6, 120.9, 123.1, 124.9, 126.8, 128.4, 129.0, 129.3, 132.0, 132.3, 132.6, 138.0, 139.1, 160.3, 162.7, and 163.2 (J = 250 Hz); HRMS (M+H) Calcd = 421.1245, Found 422.1364.
Ethyl 1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxylate ( 6b )
Yield 88 %, Mp. 258 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 8.01 (s, 1H, Ar–H), 7.79 (d, 1H, J = 8.1 Hz, Ar–H), 7.72 (d, 1H, J = 8.1 Hz, Ar–H), 7.64 (d, 2H, J = 8.0 Hz, Ar–H), 7.40 (d, 2H, J = 7.9 Hz, Ar–H), 7.23 (s, 1H, Ar–H), 7.16 (d, 2H, J = 7.9 Hz, Ar–H), 7.06 (d, 2H, J = 8.0 Hz, Ar–H), 5.79 (s, 2H, –CH2), 4.28 (q, 2H, J = 7.1 Hz, –OCH2CH3), 3.72 (s, 3H, –OCH3), and 1.27 (t, 3H, J = 7.1 Hz, –CH2CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 14.3, 49.9, 56.2, 60.8, 110.5, 111.3, 115.1, 116.6, 119.2, 128.3, 128.7, 129.8, 130.9, 131.4, 132.8, 134.7, 137.4, 142.3, 159.5, and 160.3; HRMS (M+H) Calcd = 419.1288, Found 420.1405.
1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylic acid ( 7a )
Yield 94 %, Mp. 294 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 12.01 (s, 1H, –COOH), 8.0 (s, 1H, Ar–H), 7.60 (s, 2H, Ar–H), 7.41 (m, 3H, Ar–H), 7.31 (d, 3H, Ar–H), 7.01 (d, 2H, Ar–H), 5.87 (s, 2H, –CH2), and 2.24 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 13.9, 46.6, 111.2, 111.8, 112.9, 113.1, 120.3, 122.4, 122.6, 124.4, 126.2, 128.2, 128.6, 128.9, 131.7, 132.0, 132.1, 137.7, 138.6, 140.7, 160.0, 162.4, and 162.9 (J = 248 Hz); HRMS (M+H) Calcd = 393.0932, Found 394.1472.
1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxylic acid ( 7b )
Yield 94 %, Mp. 248 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 8.0 (s, 1H, Ar–H), 7.6 (m, 3H, Ar–H), 7.4 (m, 3H, Ar–H), 7.3 (d, 1H, Ar–H), 7.2 (d, 1H, Ar–H), 7.1 (m, 2H, Ar–H), 6.9 (d, 1H, Ar–H), 5.9 (s, 2H, –CH2), and 3.6 (s, 3H, –OCH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 46.4, 55.0, 110.4, 111.4, 112.2, 112.8, 113.8, 119.1, 120.7, 126.2, 128.2, 129.9, 131.5, 132.9, 137.6, 139.0, 141.0, 142.3, and 159.6; HRMS (M+H) Calcd = 391.0975, Found 392.1047.
5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxylic acid ( 7c )
Yield 94 %, Mp. 220 °C, 1H NMR (400 MHz, DMSO-d 6 ) δ H 13.04 (s, 1H, –COOH), 11.87 (s, 1H, NH), 7.95 (s, 1H, Ar–H), 7.58 (d, 1H, J = 8.0 Hz, Ar–H), 7.51 (d, 1H, J = 8.0 Hz, Ar–H), 7.44 (t, 2H, Ar–H), 7.34 (t, 1H, Ar–H), 7.16 (s, 1H, Ar–H), and 2.27 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d 6 ) δ C 14.3, 108.2, 113.2, 113.4, 120.2, 122.7, 124.1, 127.9, 129.8, 132.4, 137.4, 141.5, 141.6, 160.4, 162.8, and 163.2 (J = 250 Hz); HRMS (M+H) Calcd = 269.0852, Found 270.1074.
1-(4-chlorobenzyl)-N-(4-methoxybenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxamide ( 10a )
Yield: 72 %; mp 282 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.7 (t, J = 6.0 Hz, 1H, NH), 7.6 (m, 2H, ArH), 7.4 (m, 4H, ArH), 7.1 (m, 4H, ArH), 6.9 (m, 3H, ArH), 6.7 (d, 2H, ArH), 6.5 (s, 1H, ArH), 5.2 (s, 2H, CH2Ar), 4.2 (d, J = 6.0, Hz, 2H, CH2), 3.6 (s, 6H, OCH3); 13C NMR (100 MHz, DMSO-d 6 ): δ 42.9, 48.7, 54.8, 110.4, 113.7, 114.2, 114.9, 115.4, 118.5, 127.6, 129.2, 130.7, 131.3, 132.7, 134.4, 141.2, 142.5, 157.3, 158.9, 162.0; MS (ESI) m/e = 510 (M+), 512 (M+2)+; Anal. Calcd. for C31H27ClN2O3: C, 72.86 %, H, 5.33 %, N, 5.48 %, Found: C, 72.82 %, H, 5.28 %, N, 5.40 %.
1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-N-(4-methoxybenzyl)-1H-indole-2-carboxamide ( 10b )
Yield: 76 %; mp 297 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.6 (t, J = 6.0 Hz, 1H, NH), 7.9 (s, 1H, ArH), 7.7 (d, 1H, ArH), 7.6 (d, 1H, ArH), 7.5 (m, 2H, ArH), 7.4 (m, 3H, ArH), 7.2 (d, 2H, ArH), 7.1 (d, 2H, ArH), 6.8 (d, 2H, ArH), 6.7 (s, 1H, ArH), 5.5 (s, 2H, CH2Ar), 4.2 (d, J = 6.0 Hz, 2H, CH2), 3.7 (s, 3H, OCH3), 2.3 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ 14.0, 43.2, 46.6, 55.2, 141.8, 113.9, 114.8, 115.4, 116.3, 119.6, 124.1, 128.1, 128.6, 130.1, 130.3, 131.3, 132.2, 132.4, 135.2, 137.1, 141.9, 143.4, 157.6, 160.4, 162.2; MS (ESI) m/e = 512 (M+), 514 (M+2)+; Anal. Calcd. for C31H26ClFN2O2: C, 72.58 %, H, 5.11 %, N, 5.46 %; Found: C, 72.49 %, H, 5.14 %, N, 5.39 %.
1-(4-chlorobenzyl)-N-(4-iodobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxamide ( 10c )
Yield: 78 %; mp > 300 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.7 (t, J = 6.0 Hz, 1H, NH), 7.8 (m, 2H, ArH), 7.6 (m, 4H, ArH), 7.4 (m, 4H, ArH), 7.2 (m, 3H, ArH), 7.0 (d, 2H, ArH), 6.7 (s, 1H, ArH), 5.7 (s, 2H, CH2Ar), 4.2 (d, J = 6.0 Hz, 2H, CH2), 3.8 (s, 3H, OCH3); 13C NMR (100 MHz, DMSO-d 6 ): δ 43.4, 49.0, 55.2, 91.8, 110.7, 113.4, 114.8, 115.9, 119.1, 127.9, 128.2, 129.7, 130.8, 131.9, 132.8, 135.0, 136.2, 136.7, 141.2, 141.9, 143.1, 159.3, 161.2; MS (ESI) m/e = 606 (M+), 608 (M+2)+; Anal. Calcd. for C30H24ClIN2O2: C, 59.37 %, H, 3.99 %, N, 4.62 %; Found: C, 59.31 %, H, 3.90 %, N, 4.56 %.
1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-N-(4-iodobenzyl)-1H-indole-2-carboxamide ( 10d )
Yield: 74 %; mp 291 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.8 (t, J = 6.0 Hz, 1H, NH), 8.1 (s, 1H, ArH), 7.8 (d, 1H, ArH), 7.7 (d, 1H, ArH), 7.6 (m, 2H, ArH), 7.5 (m, 3H, ArH), 7.4 (d, 2H, ArH), 7.2 (d, 2H, ArH), 7.0 (d, 2H, ArH), 6.8 (s, 1H, ArH), 5.7 (s, 2H, CH2Ar), 4.1 (d, J = 6.0 Hz, 2H, CH2), 2.2 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ 14.4, 43.2, 49.1, 92.5, 110.9, 114.7, 115.2, 116.2, 118.8, 123.7, 127.8, 128.3, 128.8, 130.6, 131.7, 132.0, 134.8, 135.9, 136.6, 137.1, 141.2, 141.9, 143.2, 160.3, 159.9; MS (ESI) m/e = 608 (M+), 610 (M+2)+; Anal. Calcd. for C30H23ClFIN2O: C, 59.18 %, H, 3.81 %, N, 4.60 % Found: C, 59.14 %, H, 3.78 %, N, 4.54 %.
1′-(1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 10e )
Yield: 70 %; mp 295 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 7.7 (m, 2H, ArH), 7.5 (m, 5H, ArH), 7.2 (m, 5H, ArH), 7.0 (m, 3H, ArH), 6.6 (s, 1H, ArH), 5.4 (s, 2H, CH2Ar), 4.2 (b, 2H, CH2), 3.6 (s, 3H, OCH3), 2.6 (s, 2H, CH2), 1.6 (b, 4H, CH2), 1.1 (b, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ 34.1, 38.4, 44.2, 49.1, 55.1, 68.2, 112.5, 114.6, 115.0, 116.4, 119.0, 120.1, 121.2, 127.3, 128.4, 129.7, 131.0, 131.8, 133.0, 133.4, 135.1, 141.3, 142.0, 143.2, 159.3, 160.3, 165.1, 190.6; HRMS, m/z, Calcd. = 590.1972, Found 591.2050 (M+H)+; Anal. Calcd. for C36H31ClN2O4: C, 73.15 %, H, 5.29 %, N, 4.74 % Found: C, 73.09 %, H, 5.23 %, N, 4.70 %.
1′-(1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 10f )
Yield: 73 %; mp 297 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.2 (dd, 2H, CH2), 8.1 (d, 1H, CH2), 7.7 (d, 1H, CH2), 7.6 (dd, 2H, CH2), 7.4 (s, 1H, CH2), 7.3 (d, J = 7.8 Hz, 2H, CH2), 7.2 (d, J = 7.8 Hz, 2H, CH2), 7.1 (d, 2H, CH2), 6.9 (dd, 2H, CH2), 5.7 (s, 2H, CH2Ar), 3.4 (m, 2H, CH2), 3.3 (m, 2H, CH2), 2.7 (s, 2H, CH2), 2.2 (s, 3H, CH3), 1.9 (m, 2H, CH2), 1.7 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ 14.6, 34.1, 38.6, 44.2, 49.2, 68.8, 111.4, 114.0, 115.1, 115.7, 116.5, 119.5, 120.4, 121.4, 124.2, 128.0, 128.9, 132.2, 132.6, 133.3, 135.2, 137.3, 141.5, 142.2, 143.4, 161.2, 165.1, 190.4; HRMS, m/z, Calcd. = 592.1929, Found 593.2013 (M+H)+; Anal. Calcd. for C36H30ClFN2O3: C, 72.90 %, H, 5.10 %, N, 4.72 % Found: C, 72.86 %, H, 5.04 %, N, 4.68 %.
7-bromo-1′-(1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 10g )
Yield: 71 %; mp > 300 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.2 (dd, 2H, ArH), 8.1 (d, 1H, ArH), 7.7 (d, 1H, ArH), 7.6 (d, J = 8.2 Hz, 2H, ArH), 7.4 (s, 2H, ArH), 7.3 (d, J = 7.8 Hz, 2H, ArH), 7.2 (d, J = 7.8 Hz, 2H, ArH), 7.1 (d, 1H, ArH), 6.9 (d, J = 8.2 Hz, 2H, ArH), 5.8 (s, 2H, CH2Ar), 3.8 (s, 3H, OCH3), 3.3 (m, 2H, CH2), 3.2 (m, 2H, CH2), 2.7 (s, 2H, CH2), 1.8 (m, 2H, CH2), 1.6 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ 34.2, 38.7, 44.6, 49.4, 55.9, 68.6, 111.4, 114.6, 115.3, 116.5, 117.4, 119.5, 120.3, 123.3, 126.7, 128.5, 130.3, 131.1, 132.4, 133.5, 135.1, 141.6, 142.4, 143.3, 159.2, 159.6, 165.1, 190.4; MS (ESI) m/e = 668 (M+), 670 (M+2)+, 672 (M+4); Anal. Calcd. for C36H30BrClN2O4: C, 64.54 %, H, 4.51 %, N, 4.18 % Found: C, 64.49 %, H, 4.47 %, N, 4.14 %.
7-bromo-1′-(1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 10 h )
Yield: 75 %; mp > 300 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.1 (dd, 2H, ArH), 8.0 (d, 1H, ArH), 7.8 (d, 2H, ArH), 7.7 (d, J = 8.2 Hz, 2H, ArH), 7.3 (s, 2H, ArH), 7.2 (d, 3H, ArH), 6.9 (d, J = 8.2 Hz, 2H, ArH), 5.7 (s, 2H, CH2Ar), 3.4 (m, 2H, CH2), 3.3 (m, 2H, CH2), 2.6 (s, 2H, CH2), 2.2 (s, 3H, CH3), 1.9 (m, 2H, CH2), 1.7 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ 14.6, 34.1, 38.7, 44.3, 49.7, 68.6, 111.6, 115.6, 116.4, 117.3, 119.5, 120.6, 123.3, 124.7, 127.1, 128.1, 128.8, 131.5, 132.1, 135.5, 137.3, 141.7, 142.3, 143.5, 159.1, 160.3, 165.1, 190.5; MS (ESI) m/e = 670 (M+), 672 (M+2)+, 674 (M+4)+; Anal. Calcd. for C36H29BrClFN2O3: C, 64.34 %, H, 4.35 %, N, 4.17 % Found: C, 64.28 %, H, 4.31 %, N, 4.13 %.
1-(4-chlorobenzyl)-N-cyclohexyl-5-(4-methoxyphenyl)-1H-indole-2-carboxamide ( 10i )
Yield: 94 %, MP. 279 °C, 1H NMR (200 MHz, DMSO-d 6 ): δ H 7.90 (s, 1H, ArH), 7.82 (d, 1H, J = 7.9 Hz, ArH), 7.76 (d, J = 8.0 Hz, 1H, NH), 7.60 (d, 1H, J = 7.9 Hz, ArH), 7.52 (d, 2H, J = 8.2 Hz, ArH), 7.43 (d, 2H, J = 8.4 Hz, ArH), 7.34 (s, 1H, ArH), 7.26 (d, 2H, J = 8.4 Hz, ArH), 7.14 (d, 2H, J = 8.2 Hz, ArH), 5.82 (s, 2H, CH2), 3.92 (m, 1H, CHN–), 3.78 (s, 3H, OCH3), 2.1 (d, 2H, CH2), 1.79 (t, 2H, CH2–), 1.68 (m, 2H, CH2–), 1.36 (m, 2H, CH2–), 1.24 (m, 2H, CH2); 13C NMR (50 MHz, DMSO-d 6 ): δ C 24.8, 25.7, 32.3, 49.4, 50.8, 55.4, 111.2, 114.2, 115.0, 116.2, 118.9, 128.6, 129.8, 130.7, 131.8, 132.6, 134.7, 141.2, 142.0, 143.3, 159.2, and 160.2; HRMS, m/z, Calcd = 472.1918, Found 473.2109 (M+H)+; Anal. Calcd for C29H29ClN2O2: C, 73.64 %, H, 6.18 %, N, 5.92 %; Found: C, 73.59 %, H, 6.14 %, N, 5.89 %.
1-(4-chlorobenzyl)-N-cyclohexyl-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamide ( 10j )
Yield: 92 %; mp 276 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 7.82 (s, 1H, ArH), 7.50 (d, J = 8.0 Hz, 1H, NH), 7.38 (d, 1H, ArH), 7.28 (m, 6H, ArH), 7.09 (d, 2H, ArH), 6.93 (s, 1H, ArH), 5.80 (s, 2H, –CH2Ar), 3.94 (m, 1H, –CHN), 2.33 (s, 3H, CH3), 2.0 (d, 2H, CH2), 1.78 (t, 2H, CH2), 1.61 (m, 2H, CH2), 1.29 (m, 2H, CH2), 1.24 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.2, 24.9, 25.5, 33.2, 47.5, 48.5, 104.5, 110.9, 113.7, 119.9, 122.5, 123.9, 126.8, 128.0, 128.7, 131.7, 132.9, 136.8, 138.1, 161.4; HRMS (M+H) Calcd. = 474.1874, Found 475.1947; Anal. Calcd. for C29H28ClFN2O: C, 73.33 %, H, 5.94 %, N, 5.90 % Found: C, 73.28 %, H, 5.89 %, N, 5.84 %.
(1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indol-2-yl)(piperidin-1-yl)methanone ( 10k )
Yield: 87 %; mp 283 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.1 (s, 1H, ArH), 7.6 (m, 3H, ArH), 7.4 (m, 3H, ArH), 7.3 (m, 2H, ArH), 7.2 (m, 2H, ArH), 7.0 (d, 1H, ArH), 5.9 (s, 2H, CH2Ar), 3.8 (s, 3H, OCH3), 3.4 (b, 4H, CH2), 1.4 (b, 6H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 24.4, 25.6, 47.3, 49.2, 55.8, 116.4, 114.5, 115.3, 116.7, 119.2, 128.5, 130.4, 131.2, 132.3, 133.2, 135.1, 141.7, 142.6, 159.4, 165.5; MS (ESI) m/e = 458 (M+), 460 (M+2)+; Anal. Calcd. for C28H27ClN2O2: C, 73.27 %, H, 5.93 %, N, 6.10 % Found: C, 73.21 %, H, 5.88 %, N, 6.04 %.
(1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indol-2-yl)(piperidin-1-yl)methanone ( 10l )
Yield: 84 %; mp 279 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 7.9 (s, 1H, ArH), 7.6 (d, 1H, ArH), 7.5 (d, 1H, ArH), 7.4 (t, 2H, ArH), 7.3 (m, 3H, ArH), 7.1 (d, 2H, ArH), 6.7 (s, 1H, ArH), 5.6 (s, 2H, CH2Ar), 3.5 (m, 4H, CH2), 2.3 (s, 3H, CH3), 1.5–1.2 (m, 6H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.3, 24.4, 46.7, 104.0, 111.5, 113.4, 119.7, 122.7, 127.2, 128.9, 129.3, 131.9, 132.4, 133.3, 137.0, 137.7, 141.5, 160.4, 162.0, 162.8; HRMS, m/z, Calcd. = 460.1718, Found 461.1788 (M+H)+; Anal. Calcd. For C28H26ClFN2O: C, 72.95 %, H, 5.69 %, N, 6.08 % Found: C, 72.91 %, H, 5.64 %, N, 6.02 %.
(1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indol-2-yl)(pyrrolidin-1-yl)methanone ( 10 m )
Yield: 90 %; mp 274 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.1 (d, 2H, ArH), 7.9 (d, 1H, ArH), 7.6 (d, 2H, J = 8.1 Hz, ArH), 7.3 (d, 2H, J = 7.8 Hz, ArH), 7.2 (s, 1H, ArH), 7.1 (d, 2H, J = 7.8 Hz, ArH), 7.0 (d, 2H, J = 8.1 Hz, ArH), 5.7 (s, 2H, CH2Ar), 3.7 (s, 3H, OCH3), 3.5 (t, 4H, CH2), 2.0 (t, 4H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 25.3, 47.3, 49.2, 55.7, 111.4, 114.6, 115.1, 116.6, 119.3, 128.5, 130.5, 131.4, 132.3, 133.4, 135.2, 141.6, 142.3, 143.5, 159.1, 165.3; MS (ESI) m/e = 444 (M+), 446 (M+2)+; Anal. Calcd. for C27H25ClN2O2: C, 72.88 %, H, 5.66 %, N, 6.30 % Found: C, 72.83 %, H, 5.63 %, N, 6.26 %.
(1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indol-2-yl)(pyrrolidin-1-yl)methanone ( 10n )
Yield: 89 %; mp 268 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.2 (d, 2H, ArH), 8.0 (s, 1H, ArH), 7.7 (d, 1H, ArH), 7.4 (d, 2H, J = 7.6 Hz, ArH), 7.3 (s, 1H, ArH), 7.20 (d, 2H, ArH), 7.1 (d, 2H, J = 7.6 Hz, ArH), 5.8 (s, 2H, CH2Ar), 3.5 (t, 4H, CH2), 2.3 (s, 3H, CH3), 2.0 (t, 4H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.7, 25.2, 47.3, 49.3, 116.5, 115.2, 116.6, 119.3, 124.6, 128.4, 128.9, 131.3, 132.3, 132.5, 135.4, 137.3, 141.7, 142.3, 143.5, 160.2, 165.3; MS (ESI) m/e = 446 (M+), 448 (M+2)+; Anal. Calcd. for C27H24ClFN2O: C, 72.56 %, H, 5.41 %, N, 6.27 % Found: C, 72.52 %, H, 5.38 %, N, 6.23 %.
Tert-butyl-4-(1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-1H-indole-2-carboxamido)piperidine-1-carboxylate ( 10o )
Yield: 80 %; mp 293 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.3 (d, J = 8.0 Hz, 1H, NH), 8.1 (d, 2H, ArH), 8.0 (d, 1H, ArH), 7.6 (d, J = 8.2 Hz, 2H, ArH), 7.4 (d, J = 7.8 Hz, 2H, ArH), 7.3 (s, 1H, ArH), 7.1 (d, J = 7.8 Hz, 2H, ArH), 7.0 (d, J = 8.2 Hz, 2H, ArH), 5.8 (s, 2H, CH2Ar), 3.7 (s, 3H, OCH3), 3.6 (m, 1H), 3.4 (m, 2H), 3.3 (m, 2H), 1.8 (m, 2H), 1.6 (m, 2H), 1.4 (s, 9H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 28.3, 26.7, 43.4, 47.8, 49.3, 55.7, 79.6, 111.4, 114.9, 115.3, 116.7, 119.3, 128.6, 130.2, 131.2, 132.0, 133.1, 135.2, 141.7, 142.0, 143.6, 159.4, 159.6, 160.4; MS (ESI) m/e = 573 (M+), 575 (M+2)+; Anal. Calcd. for C33H36ClN3O4: C, 69.04 %, H, 6.32 %, N, 7.32 % Found: C, 69.0 %, H, 6.27 %, N, 7.28 %.
Tert-butyl-4-(1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamido)piperidine-1-carboxylate ( 10p )
Yield: 76 %; mp 287 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.5 (d, J = 8.0 Hz, 1H, NH), 7.9 (s, 1H, ArH), 7.5 (dd, 2H, ArH), 7.4 (dd, 2H, ArH), 7.3 (d, 3H, ArH), 7.2 (s, 1H, ArH), 7.1 (d, 2H, ArH), 5.8 (s, 2H, CH2Ar), 3.9 (b, 3H), 2.3 (s, 3H, CH3), 1.8 (s, 2H), 1.4 (s, 13H); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.3, 28.6, 331.7, 46.7, 46.9, 79.1, 106.3, 111.8, 113.3, 113.5, 120.0, 120.8, 126.9, 128.8, 129.0, 132.0, 133.0, 138.0, 138.3, 141.3, 141.4, 154.4, 160.4, 161.4, 162.7; MS (ESI) m/e = 575 (M+), 577 (M+2)+; Anal. Calcd. for C33H35ClFN3O3: C, 68.80 %, H, 6.12 %, N, 7.29 % Found: C, 68.74 %, H, 6.08 %, N, 7.26 %.
1-(4-chlorobenzyl)-N′-isonicotinoyl-5-(4-methoxyphenyl)-1H-indole-2-carbohydrazide ( 10q )
Yield: 83 %; mp 263 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 9.1 (s, 2H, NH), 8.7 (d, J = 8.2 Hz, 2H, ArH), 8.2 (d, 2H, ArH), 8.0 (d, 1H, ArH), 7.8 (d, J = 8.2 Hz, 2H, ArH), 7.6 (d, J = 8.0 Hz, 2H, ArH), 7.4 (d, J = 7.8 Hz, 2H, ArH), 7.3 (s, 1H, ArH), 7.1 (d, J = 7.8 Hz, 2H, ArH), 7.0 (d, J = 8.0 Hz, 2H, ArH), 5.8 (s, 2H, CH2Ar), 3.6 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 50.2, 55.7, 111.6, 114.6, 115.1, 116.6, 119.2, 121.4, 128.5, 130.0, 131.1, 132.0, 133.1, 135.3, 140.6, 141.6, 142.0, 143.5, 149.6, 159.4, 161.2, 164.6; MS (ESI) m/e = 510 (M+), 512 (M+2)+; Anal. Calcd. for C29H23ClN4O3: C, 68.17 %, H, 4.54 %, N, 10.96 % Found: C, 68.12 %, H, 4.49 %, N, 10.90 %.
1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-N′-isonicotinoyl-1H-indole-2-carbohydrazide ( 10r )
Yield: 71 %; mp 289 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 9.0 (s, 2H), 8.8 (d, J = 8.2 Hz, 2H, ArH), 8.2 (d, 2H, ArH), 8.0 (d, 1H), 7.7 (d, J = 8.2 Hz, 2H, ArH), 7.6 (d, 1H, ArH), 7.4 (s, 1H, ArH), 7.3 (d, J = 8.0 Hz, 2H, ArH), 7.2 (d, 2H, ArH), 7.0 (d, J = 8.0 Hz, 2H, ArH), 5.8 (s, 2H, CH2Ar), 2.3 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.6, 50.2, 111.6, 115.1, 115.8, 116.6, 119.2, 121.5, 124.2, 128.0, 128.5, 131.1, 132.0, 132.2, 135.3, 137.1, 140.6, 141.6, 142.0, 143.5, 149.5, 160.2, 161.4, 164.6; MS (ESI) m/e = 512 (M+), 514 (M+2)+; Anal. Calcd. for C29H22ClFN4O2: C, 67.90 %, H, 4.32 %, N, 10.92 % Found: C, 67.84 %, H, 4.26 %, N, 10.88 %.
1-(4-chlorobenzyl)-5-(4-methoxyphenyl)-N-(1-(2-methoxyphenyl)propan-2-yl)-N-methyl-1H-indole-2-carboxamide ( 10s )
Yield: 84 %; mp 277 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.2 (d, 2H, ArH), 8.0 (d, 1H, ArH), 7.6 (d, 2H, J = 8.1 Hz, ArH), 7.4 (d, 2H, J = 7.8 Hz, ArH), 7.1 (d, 2H, J = 7.8 Hz, ArH), 7.0 (d, 2H, J = 8.1 Hz, ArH), 7.3 (s, 1H, ArH), 6.9–7.1 (m, 4H, ArH), 5.8 (s, 2H, CH2Ar), 4.1 (m, 1H, CH), 3.8 (s, 6H, OCH3), 3.4 (s, 3H, NCH3), 2.9 (dd, 1H, CH), 2.6 (dd, 1H, CH), 1.2 (d, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 18.2, 33.2, 49.2, 55.3, 56.1, 60.1, 111.6, 112.3, 114.5, 115.1, 116.7, 119.4, 120.8, 127.2, 127.8, 128.5, 130.2, 130.2, 130.4, 131.4, 131.1, 132.0, 133.0, 135.2, 141.6, 142.2, 143.5, 158.6, 159.3, 161.5; MS (ESI) m/e = 552 (M+), 554 (M+2)+; Anal. Calcd. for C34H33ClN2O3: C, 73.83 %, H, 6.01 %, N, 5.06 % Found: C, 73.78 %, H, 5.97 %, N, 5.01 %.
1-(4-chlorobenzyl)-5-(4-fluoro-3-methylphenyl)-N-(1-(2-methoxyphenyl)propan-2-yl)-N-methyl-1H-indole-2-carboxamide ( 10t )
Yield: 79 %; mp 281 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 8.2 (d, 2H, ArH), 7.9 (d, 1H, ArH), 7.7 (d, 1H, ArH), 7.5 (d, 2H, J = 7.6 Hz, ArH), 7.4 (s, 1H, ArH), 7.3 (d, 2H, ArH), 7.2 (d, 2H, J = 7.6 Hz, ArH), 6.9–7.1 (m, 4H, ArH), 5.8 (s, 2H, CH2Ar), 4.1 (m, 1H, CH), 3.7 (s, 3H, OCH3), 3.5 (s, 3H, NCH3), 2.9 (dd, 1H, CH), 2.6 (dd, 1H, CH), 2.2 (s, 3H, CH3), 1.1 (d, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.7, 18.2, 33.4, 49.3, 56.2, 60.2, 111.6, 112.2, 115.1, 115.7, 116.6, 119.2, 121.0, 124.2, 127.2, 127.5, 128.1, 128.6, 130.4, 131.1, 132.2, 132.5, 135.2, 137.1, 141.7, 142.2, 143.5, 158.5, 160.4, 161.5; MS (ESI) m/e = 554 (M+), 556 (M+2)+; Anal. Calcd. for C34H32ClFN2O2: C, 73.57 %, H, 5.81 %, N, 5.05 % Found: C, 73.51 %, H, 5.77 %, N, 5.01 %.
(5-(4-fluoro-3-methylphenyl)-1H-indol-2-yl)(3-phenoxyazetidin-1-yl)methanone ( 14a )
Yield: 74 %; mp 279 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.8 (s, 1H, NH), 7.9 (s, 1H, ArH), 7.6 (d, 1H, Ar–H), 7.5 (d, 1H, Ar–H), 7.4 (t, 2H, Ar–H), 7.3 (d, 3H, Ar–H), 7.1 (s, 1H, Ar–H), 7.0 (d, 3H, ArH), 4.4 (m, 1H, CH), 4.3 (d, 2H, CH2), 4.0 (d, 2H, CH2), 2.3 (s, 3H, –CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.6, 52.5, 70.7, 111.4, 114.3, 114.8, 115.7, 116.6, 119.2, 120.1, 124.2, 128.1, 129.1, 131.6, 132.3, 137.1, 138.6, 143.5, 157.2, 160.2,172.4; MS (ESI) m/e = 400 (M+); Anal. Calcd. for C25H21FN2O2: C, 74.98 %, H, 5.29 %, N, 7.0 % Found: C, 74.92 %, H, 5.23 %, N, 6.96 %.
5-(4-fluoro-3-methylphenyl)-N-(4-methoxybenzyl)-1H-indole-2-carboxamide ( 14b )
Yield: 89 %; mp 295 °C; 1H NMR (200 MHz, DMSO-d 6 ): δ H 11.7 (t, J = 6.0 Hz, 1H, NH), 9.0 (t, 1H, NH), 7.9 (s, 1H, Ar–H), 7.4 (m, 4H, Ar–H), 7.3 (m, 4H, Ar–H), 6.9 (d, 2H, Ar–H), 4.5 (d, J = 6.0 Hz, 2H, CH2N), 3.4 (s, 3H, OCH3), 2.2 (s, 3H, CH3); 13C NMR (50 MHz, DMSO-d 6 ): δ C 13.8, 41.7, 55.0, 103.0, 112.6, 112.8, 113.0, 113.7, 119.4, 122.2, 122.6, 127.7, 128.6, 130.7, 130.8, 131.5, 131.8, 131.9, 132.5, 136.2, 141.1, 141.3, 158.3, 158.7, 163.5; MS (ESI) m/e = 388 (M+); Anal. Calcd. for C24H21FN2O2: C, 74.21 %, H, 5.45 %, N, 7.21 % Found: C, 74.15 %, H, 5.40 %, N, 7.18 %.
5-(4-fluoro-3-methylphenyl)-N-(4-iodobenzyl)-1H-indole-2-carboxamide ( 14c )
Yield: 87 %; mp 286 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.8 (t, J = 6.0 Hz, 1H, NH), 9.1 (t, 1H, NH), 7.9 (s, 1H, Ar–H), 7.6 (m, 4H, Ar–H), 7.3 (m, 4H, Ar–H), 6.8 (d, 2H, Ar–H), 4.4 (d, J = 6.0 Hz, 2H, CH2N), 2.2 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.7, 43.5, 92.1, 111.6, 114.7, 115.8, 116.6, 119.2, 124.2, 128.1, 128.6, 131.7, 132.1, 136.7, 137.1, 137.5, 138.4, 138.7, 143.5, 160.1, 161.2; MS (ESI) m/e = 484 (M+); Anal. Calcd. for C23H18FIN2O: C, 57.04 %, H, 3.75 %, N, 5.78 % Found: C, 56.98 %, H, 3.71 %, N, 5.74 %.
5-(4-fluoro-3-methylphenyl)-N′-isonicotinoyl-1H-indole-2-carbohydrazide ( 14d )
Yield: 71 %; mp 270 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.9 (s, 1H, NH), 8.8 (d, 2H, J = 8.2 Hz, Ar–H), 8.2 (s, 2H, NH), 7.8 (d, 1H, ArH), 7.7 (d, 2H, J = 8.2 Hz, Ar–H), 7.6 (d, 1H, Ar–H), 7.5 (d, 2H, Ar–H), 7.4 (s, 1H, ArH), 7.2 (d, 2H, Ar–H), 2.2 (s, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.6, 111.6, 114.7, 115.8, 116.6, 119.2, 121.2, 124.2, 128.1, 131.6, 132.2, 137.1, 138.6, 140.6, 143.5, 149.5, 160.2, 161.1, 164.6; MS (ESI) m/e = 388 (M+); Anal. Calcd. for C22H17FN4O2: C, 68.03 %, H, 4.41 %, N, 14.43 % Found: C, 67.97 %, H, 4.37 %, N, 14.38 %.
1′-(5-(4-fluoro-3-methylphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 14e )
Yield: 73 %; mp 293 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.7 (s, 1H, NH), 7.9 (s, 1H, Ar–H), 7.7 (d, 1H, ArH), 7.6 (d, 1H, ArH), 7.4 (m, 5H, Ar–H), 7.2 (m, 2H, Ar–H), 6.9 (s, 1H, Ar–H), 3.4 (s, 4H, CH2), 2.8 (s, 2H, CH2), 2.2 (s, 3H, CH3), 2.1 (m, 2H, CH2), 1.8 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.8, 34.1, 38.7, 44.3, 68.6, 111.6, 114.1, 114.7, 115.7, 116.6, 119.2, 120.1, 121.5, 124.2, 127.6, 131.6, 132.1,133.5, 137.1, 138.6, 143.1, 160.1, 160.4, 172.1, 190.6; MS (ESI) m/e = 468 (M+); Anal. Calcd. for C29H25FN2O3: C, 74.34 %, H, 5.38 %, N, 5.98 % Found: C, 74.0 %, H, 5.31 %, N, 5.93 %.
7-bromo-1′-(5-(4-fluoro-3-methylphenyl)-1H-indole-2-carbonyl)spiro[chroman-2,4′-piperidin]-4-one ( 14f )
Yield: 70 %; mp > 300 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.9 (s, 1H, NH), 7.8 (d, 3H, Ar–H), 7.7 (d, 2H, ArH), 7.4 (s, 1H, Ar–H), 7.3 (d, 1H, Ar–H), 7.2 (d, 3H, Ar–H), 3.4 (m, 2H, CH2), 3.3 (m, 2H, CH2), 2.7 (s, 2H, CH2), 2.2 (s, 3H, CH3), 1.9 (m, 2H, CH2), 1.7 (m, 2H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.7, 34.2, 38.8, 44.5, 68.8, 111.6, 114.7, 115.8, 116.6, 117.4, 119.2, 120.5, 124.3, 126.7, 128.1, 130.4, 131.6, 132.2, 137.1, 138.6, 143.2, 159.2, 160.2, 172.1, 190.8; HRMS (M+Na+2) Calcd. = 546.0954, Found 571.0825; Anal. Calcd. for C29H24BrFN2O3: C, 63.63 %, H, 4.42 %, N, 5.12 % Found: C, 62.97 %, H, 4.37 %, N, 5.09 %.
Tert-butyl 4-(5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamido)piperidine-1-carboxylate ( 14g )
Yield: 78 %; mp 290 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.8 (d, J = 8.0 Hz, 1H, NH), 8.2 (d, 1H, NH), 7.8 (d, 2H, ArH), 7.7 (d, 2H, Ar–H), 7.4 (s, 1H, Ar–H), 7.2 (d, 2H, Ar–H), 3.6 (m, 1H, CH), 3.4 (m, 2H, CH2), 3.3 (m, 2H, CH2), 2.2 (s, 3H, CH3), 1.8 (m, 2H, CH2), 1.6 (m, 2H, CH2), 1.4 (s, 9H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.8, 28.3, 26.5, 43.4, 47.5, 79.9, 111.6, 114.7, 115.8, 116.6, 119.2, 124.1, 128.1, 131.6, 132.2, 137.1, 138.6, 143.5, 159.4, 160.2, 161.1; HRMS (M+Na) Calcd. = 451.2271, Found 474.2155; Anal. Calcd. for C26H30FN3O3: C, 69.16 %, H, 6.70 %, N, 9.31 % Found: C, 69.10 %, H, 6.65 %, N, 9.27 %.
(5-(4-fluoro-3-methylphenyl)-1H-indol-2-yl)(piperidin-1-yl)methanone ( 14h )
Yield: 92 %; mp 260 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.0 (s, 1H, NH), 7.9 (s, 1H, NH), 7.7 (d, 1H, ArH), 7.4 (d, 1H, Ar–H), 7.3 (m, 3H, Ar–H), 6.8 (s, 1H, Ar–H), 3.7 (b, 4H, CH2), 2.2 (s, 3H, CH3), 1.7 (b, 6H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.6, 24.1, 25.2, 47.1, 111.6, 114.7, 115.7, 116.6, 119.2, 124.3, 128.1, 131.7, 132.3, 137.1, 138.7, 143.6, 160.3, 172.1; MS (ESI) m/e = 336 (M+); Anal. Calcd. for C21H21FN2O: C, 74.98 %, H, 6.29 %, N, 8.33 % Found: C, 74.92 %, H, 6.24 %, N, 8.28 %.
(5-(4-fluoro-3-methylphenyl)-1H-indol-2-yl)(pyrrolidin-1-yl)methanone ( 14i )
Yield: 90 %; mp 254 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.8 (s, 1H, NH), 7.8 (d, 2H, ArH), 7.7 (d, 2H, ArH), 7.4 (s, 1H, Ar–H), 7.2 (d, 2H, Ar–H), 3.5 (t, 4H, CH2), 2.2 (s, 3H, CH3), 1.9 (t, 4H, CH2); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.8, 25.2, 47.3, 111.6, 114.8, 115.6, 116.5, 119.3, 124.1, 128.1, 131.5, 132.1, 137.3, 138.6, 143.7, 160.5, 172.2; HRMS (M+H) Calcd. = 322.1481, Found 323.1548; Anal. Calcd. for C20H19FN2O: C, 74.51 %, H, 5.94 %, N, 8.69 % Found: C, 74.47 %, H, 5.89 %, N, 8.64 %.
N-cyclohexyl-5-(4-fluoro-3-methylphenyl)-1H-indole-2-carboxamide ( 14j )
Yield: 94 %, MP. 268 °C, 1H NMR (400 MHz, DMSO-d 6 ): δ H 10.64 (d, J = 8.0 Hz, 1H, NH), 7.32 (d, 1H, NH), 6.93 (s, 1H, Ar–H), 6.52 (d, 2H, Ar–H), 6.46 (d, 2H, Ar–H), 6.36 (t, 1H, Ar–H), 6.25 (s, 1H, Ar–H), 1.54 (s, 1H, –CH–), 1.29 (s, 3H, –CH3), 0.84 (d, 4H, –CH2–), 0.65 (d, 1H, –CH–), 0.36–0.39 (m, 4H, –CH2–) and 0.17–0.19 (m, 1H, –CH–); 13C NMR (100 MHz, DMSO-d 6 ): δ C 13.9, 25.2, 32.6, 48.1, 103.1, 112.8, 113.0, 119.4, 121.9, 122.1, 122.3, 122.5, 127.7, 130.8, 132.0, 132.9, 136.1, 141.3, 160.0, 160.1, 162.4; HRMS (M+H) Calcd. = 350.1794, Found 351.1836; Anal. Calcd for C22H23FN2O: C, 75.40 %, H, 6.62 %, N, 7.99 %; Found: C, 75.41 %, H, 6.61 %, N, 7.98 %.
5-(4-fluoro-3-methylphenyl)-N-(1-(2-methoxyphenyl)propan-2-yl)-N-methyl-1H-indole-2-carboxamide ( 14k )
Yield: 74 %; mp 281 °C; 1H NMR (400 MHz, DMSO-d 6 ): δ H 11.8 (s, 1H, NH), 7.8 (d, 2H, ArH), 7.7 (d, 2H, ArH), 7.4 (s, 1H, Ar–H), 7.2 (d, 3H, Ar–H), 6.8–6.9 (m, 3H, ArH), 4.1 (m, 1H, CHN), 3.7 (s, 3H, OCH3), 3.4 (s, 3H, CH3N), 2.9 (d, 1H, CH), 2.6 (d, 1H, CH), 2.2 (s, 3H, CH3), 1.1 (d, 3H, CH3); 13C NMR (100 MHz, DMSO-d 6 ): δ C 14.7, 18.2, 33.4, 35.6, 56.0, 60.3, 111.6, 112.2, 114.7, 115.8, 116.6, 119.2, 121.0, 124.3, 127.1, 127.5, 128.1, 130.4, 131.6, 132.2, 137.1, 138.6, 143.5, 158.5, 160.2, 161.6; MS (ESI) m/e = 430 (M+); Anal. Calcd. for C27H27FN2O2: C, 75.33 %, H, 6.32 %, N, 6.51 % Found: C, 75.28 %, H, 6.28 %, N, 6.48 %.
Experimental protocol for biological activity
Antibacterial activity assay [53]
The in vitro antimicrobial susceptibility of prepared compounds (10a–t and 14a–k) (in terms of minimum inhibitory concentration; MIC) against strains of pathogenic Gram-negative bacteria K. pneumoniae (ATCC 27736), E. coli (ATCC 9637), P. aeruginosa (ATCC BAA427), and S. typhi were evaluated by the broth microdilution technique described by the Clinical and Laboratory Standards Institute (CLSI), 2012 (formerly NCCLS) [53]. The minimum inhibitory concentration (MIC, μg/mL) was defined as the lowest concentration of an antimicrobial agent that will inhibit the visible growth of microbe. Gentamicin and ciprofloxacin were used as standard drugs for comparison of antibacterial activity. Dimethyl sulfoxide (DMSO) was used as a solvent or negative control. To clarify any effect of DMSO on the antifungal activity, separate studies were carried out with solutions alone of DMSO, and these studies showed no activity against any microbial strains. The MIC of tested compounds was determined using the twofold serial dilution technique by assaying at 51.2, 25.6, 12.8, 6.4, 3.2, 1.6, 0.8, 0.4, 0.2, 0.1, and 0.05 μg/mL concentrations along with standards at the same concentrations.
Antifungal activity assay [54, 55]
The in vitro antifungal susceptibility (AFST) of prepared compounds (10a–t and 14a–k) (in terms of minimum inhibitory concentration; MIC) against strains of pathogenic fungi, for example, C. albicans, C. neoformans, A. fumigatus (all strains are patients' isolates), and C. parapsilosis (ATCC 22019) were evaluated by the broth microdilution technique described by the Clinical and Laboratory Standards Institute (CLSI), 2012 (formerly NCCLS) [54, 55]. Fluconazole and oxiconazole were used as standard drugs for comparison of antifungal activity. DMSO was used as a solvent or negative control. To clarify any effect of DMSO on the antifungal activity, separate studies were carried out with solutions alone of DMSO and these studies showed no activity against any microbial strains. MIC (the lowest concentration of an antimicrobial agent that will inhibit the visible growth of microbe) of tested compounds was determined using the twofold serial dilution technique by assaying at 64, 32, 16, 8, 4, 2, 1, and 0.5 μg/mL concentrations along with standards at the same concentrations.
References
D. Abbanat, M. Macielag, K. Bush, Expert Opin. Investig. Drugs 12, 379 (2003)
J. Pootoolal, J. Neu, G.D. Wright, Annu. Rev. Pharmacol. Toxicol. 42, 381 (2002)
I.I. Raad, H.A. Hanna, R.Y. Hachem, T. Dvorak, R.B. Arbuckle, G. Chaiban, R.B. Rice, Antimicrob. Agents Chemother. 48, 3583 (2004)
F.W. Goldstein, Clin. Microbiol. Infect. 13, 2 (2007)
V.M. Dcosta, E.K. Christine, L. Kalan, M. Morar, W.L. Wilson, Nature 477, 457 (2011)
S.B. Levy, B. Marshall, Nat. Med. 10, 122 (2004)
M.O. Wright, Am. J. Infect. Control 33, 419 (2005)
D.J. Diekema, B.J. BootsMiller, T.E. Vaughan, R.F. Woolson, J.W. Yankey, E.J. Ernst, S.D. Flach, M.M. Ward, C.L. Franciscus, M.A. Pfaller, B.N. Doebbeling, Clin. Infect. Dis. 38, 78 (2004)
H.W. Boucher, G.H. Talbot, J.S. Bradley, J.E. Edwards, D. Gilbert, L.B. Rice, M. Scheld, B. Spellberg, J. Bartlett, Clin. Infect. Dis. 48, 1 (2009)
L.B. Rice, Clevel. Clin. Q. 74, 12 (2007)
D.F. Fidler, Emerg. Infect. Dis. 4, 169 (1998)
A. Macchiarulo, G. Constantino, D. Fringuelli, A. Vecchiarelli, F. Schiaffella, R. Fringuelli, Bioorg. Med. Chem. 10, 3415 (2002)
M. Leeb, Nature 431, 892 (2004)
T. Kline, N.H. Andersen, E.A. Harwood, J. Bowman, A. Malanda, S. Endsley, A.L. Erwin, M. Doyle, S. Fong, A.L. Harris, B. Mendelsohn, K. Mdluli, C.R.H. Raetz, C.K. Stover, P.R. Witte, A. Yabannavar, S. Zhu, Potent. J. Med. Chem. 45, 3112 (2002)
K. Young, D.B. Silver, P. Cameron, S.S. Eveland, C.R.H. Raetz, S.A. Hyland, M.S. Anderson, J. Biol. Chem. 270, 30384 (1995)
J.E. Jackman, C.R.H. Raetz, C.A. Fierke, Biochemistry 40, 514 (2001)
D. Leung, G. Abbenante, D.P. Fairlie, J. Med. Chem. 43, 305 (2000)
A. Scozzafava, C.T. Supuran, J. Med. Chem. 43, 3677 (2000)
J.E. Jackman, C.A. Fierke, L.N. Tumey, M. Pirrung, U. Uchiyama, H.S. Tahir, O. Hindsgual, C.R.H. Raetz, J. Biol. Chem. 275, 11002 (2000)
M.H. Chen, M.G. Steiner, S.E. de Laszlo, A.A. Patchett, M.S. Anderson, S.A. Hyland, H.R. Onishi, L.L. Silver, C.R.H. Ratez, Bioorg. Med. Chem. Lett. 9, 313 (1999)
N.K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C.H. Kim, A.K. Verma, E.H. Choi, Molecules 18, 6620 (2013)
S. Pandey, S.S. Chauhan, R. Shivahare, A. Sharma, S. Jaiswal, S. Gupta, J. Lal, P.M.S. Chauhan, Eur. J. Med. Chem. 110, 237 (2016)
F. Ban, E. Leblanc, H. Li, R.S.N. Munuganti, K. Frewin, P.S. Rennie, A. Cherkasov, J. Med. Chem. 57, 6867 (2014)
M.M. Mahmoud, H.I. Ali, K.H. Ahn, A. Damaraju, S. Samala, V.K. Pulipati, S. Kolluru, D.A. Kendall, D. Lu, J. Med. Chem. (2013). doi:10.1021/jm4009828
C.J. Qiao, H.I. Ali, K.H. Ahn, S. Kolluru, D.A. Kendall, D. Lu, Eur. J. Med. Chem. 121, 517 (2016)
E.E. Cawston, M. Connor, V.D. Marzo, R. Silvestri, M. Glass, J. Med. Chem. (2015). doi:10.1021/acs.jmedchem.5b00579
K.X. Chen, B. Vibulbhan, W. Yang, M. Sannigrahi, F. Velazquez, T. Chan, S. Venkatraman, G.N. Anilkumar, Q. Zeng, F. Bennet, Y. Jiang, C.A. Lesburg, J. Duca, P. Pinto, S. Gavalas, Y. Huang, W. Wu, O. Selyutin, S. Agrawal, B. Feld, H. Huang, C. Li, K. Cheng, N. Shih, J.A. Kozlowski, S.B. Rosenblum, F.G. Njoroge, J. Med. Chem. 55, 754 (2012)
K. Onda, R. Shiraki, T. Ogiyama, K. Yokoyama, K. Momose, N. Katayama, M. Orita, T. Yamaguchi, M. Furutani, N. Hamada, M. Takeuchi, M. Okada, M. Ohta, S. Tsukamoto, Bioorg. Med. Chem. 16, 10001 (2012)
Z. Liu, L. Tang, H. Zhu, T. Xu, C. Qiu, S. Zheng, Y. Gu, J. Feng, Y. Zhang, G. Liang, J. Med. Chem. (2016). doi:10.1021/acs.jmedchem.5b02006
M. Brands, J.K. Ergüden, K. Hashimoto, D. Heimbach, C. Schröder, S. Siegel, J.P. Stasch, S. Weigand, Bioorg. Med. Chem. Lett. 15, 4201 (2005)
K.X. Chen, S. Venkatraman, G.N. Anilkumar, Q. Zeng, C.A. Lesburg, B. Vibulbhan, F. Velazquez, T.Y. Chan, F. Bennet, Y. Jiang, P. Pinto, Y. Huang, O. Selyutin, S. Agrawal, H.C. Huang, C. Li, K.C. Cheng, N.Y. Shih, J.A. Kozlowski, S.B. Rosenblum, F.G. Njoroge, ACS Med. Chem. Lett. (2013). doi:10.1021/ml400192w
P.M. Cowley, J. Baker, K.I. Buchanan, I. Carlyle, J.K. Clark, T.R. Clarkson, M. Deehan, D. Edwards, Y. Kiyoi, I. Martin, D. Osbourn, G. Walker, N. Ward, G. Wishart, Bioorg. Med. Chem. Lett. 21, 2034 (2011)
J. Stec, O.K. Onajole, S. Lun, H. Guo, B. Merenbloom, G. Vistoli, W.R. Bishai, A.P. Kozikowski, J. Med. Chem. (2016). doi:10.1021/acs.jmedchem.6b00415
R.R. Kondreddi, J. Jiricek, S.P.S. Rao, S.B. Lakshminarayana, L.R. Camacho, R. Rao, M. Herve, P. Bifani, N.L. Ma, K. Kuhen, A. Goh, A.K. Chatterjee, T. Dick, T.T. Diagana, U.H. Manjunatha, P.W. Smith, J. Med. Chem. (2013). doi:10.1021/jm4012774
G.L. Regina, A. Coluccia, A. Brancale, F. Piscitelli, V. Gatti, G. Maga, A. Samuele, C. Pannecouque, D. Schols, J. Balzarini, E. Novellino, R. Silvestri, J. Med. Chem. 54, 1587 (2011)
V. Famiglini, G.L. Regina, A. Coluccia, S. Pelliccia, A. Brancale, G. Maga, E. Crespan, R. Badia, R.M. Eva, J.A. Esté, R. Ferretti, R. Cirilli, C. Zamperini, M. Botta, D. Schols, V. Limongelli, B. Agostino, E. Novellino, R. Silvestri, J. Med. Chem. (2014). doi:10.1021/jm5011622
M.Z. Zhang, Q. Chen, G.F. Yang, Eur. J. Med. Chem. 89, 421 (2015)
L.A.T. Cleghorn, S. Albrecht, L. Stojanovski, F.R.J. Simeons, S. Norval, R. Kime, I.T. Collie, M.D. Rycker, L. Campbell, I. Hallyburton, J.A. Frearson, P.G. Wyatt, K.D. Read, I.H. Gilbert, J. Med. Chem. (2015). doi:10.1021/acs.jmedchem.5b00596
K. Sweidan, D.A. Sabbah, S. Bardaweel, K.A. Dush, G.A. Sheikha, M.S. Mubarak, J. Bioorg. Med. Chem. Lett. 26, 2685 (2016)
S. Ghassan, A.Q. Tariq, A.S. Ghassan, A.H. Yusuf, S. Kamal, A.Q. Rania, H. Suhair, H. Lama, A.K. Sameer, J. Enzyme Inhib. Med. Chem. 28, 863 (2013)
F.R. Desa-Alves, E.J. Barreiro, C.A. Fraga, Mini Rev. Med. Chem. 9, 782 (2009)
M.T. El-Sayed, S. Suzen, N. Altanlar, K. Ohlsen, A. Hilgeroth, Bioorg. Med. Chem. Lett. 26, 218 (2016)
Y. Song, F. Wu, C. Zhang, G. Liang, G. Zhou, J. Yu, Bioorg. Med. Chem. Lett. 25, 259 (2015)
Pooja, P. Prasher, P. Singh, K. Pawar, K.S. Vikramdeo, N. Mondal, S.S. Komath, Eur. J. Med. Chem. 80, 325 (2014)
G.D. Morse, R.C. Reichman, M.A. Fischl, M. Para, J. Leedom, W. Powderly, L.M. Demeter, L. Resnick, Y. Bassiakos, J. Timpone, S. Cox, D. Batts, Antiviral Res. 45, 47 (2010)
T. Nguyen, N. German, A.M. Decker, J. Li, J.L. Wiley, B.F. Thomas, T.P. Kenakin, Y. Zhang, Bioorg. Med. Chem. 23, 2195 (2015)
M.E. Welsch, S.A. Snyder, B.R. Stockwell, Curr. Opin. Chem. Biol. 14, 347 (2010)
R.E. Dolle, J. Comb. Chem. 3, 477 (2001)
E.P. Stout, B.I. Morinaka, Y.G. Wang, D. Romo, T.F. Molinski, J. Nat. Prod. 75, 527 (2012)
D.J. Hoover, S. Lefkowitz-Snow, J.L. Burgess-Henry, W.H. Martin, S.J. Armento, I.A. Stock, R.K. McPherson, P.E. Genereux, E.M. Gibbs, J.L. Treadway, J. Med. Chem. 41, 2934 (1998)
C. Kuehm-Caubere, P. Caubere, B. Jamart-Gregoire, A. Negre-Salvayre, D. Bonnefont-Rousselot, J.G. Bizot-Espiard, B. Pfeiffer, D.H. Caignard, B. Guardiola-Lemaitre, P. Renard, J. Med. Chem. 40, 1201 (1997)
F. Piscitelli, A. Ligresti, G. La Regina, A. Coluccia, L. Morera, M. Allar, E. Novellino, V. Di Marzo, R. Silvestri, J. Med. Chem. 55, 5627 (2012)
CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that grow Aerobically, Approved Standard, 9th ed., CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, (2012)
CLSI, Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard—third edition, Clinical and Laboratory Standards Institute document M27-A3 28 (2008)
CLSI, Reference Method for Broth Dilution Antifungal Susceptibility Testing Filamentous Fungi, Approved Standard, 2nd ed., CLSI document M38-A2, 950 West Valley Roadn Suite 2500, Wayne, Pennsylvania 19087, USA, (2008)
Acknowledgments
The author, Y. D. Mane, is grateful to the Department of Postgraduate Studies and Research in Chemistry, Dnyanopasak College, Parbhani, and Shri Chhatrapati Shivaji College, Omerga, for providing laboratory facilities, Indian Institute of Chemical Technology, Hyderabad, and National Chemical Laboratory for spectral data, and finally to Smita S. Patil for proofreading.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mane, Y.D., Sarnikar, Y.P., Surwase, S.M. et al. Design, synthesis, and antimicrobial activity of novel 5-substituted indole-2-carboxamide derivatives. Res Chem Intermed 43, 1253–1275 (2017). https://doi.org/10.1007/s11164-016-2696-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2696-3