Abstract
The 12-tungstophosphoric acid supported on silica gel (PW/SiO2) exhibits excellent activity in the synthesis of 2,3-dihydroquinazolin-4(1H)-ones by cyclocondensation reaction of 2-aminobenzamide with carbonyl compounds in water under reflux conditions. The desired products have been obtained in short reaction times in high yields. Our method has been successfully applied for both aldehydes and ketones (aromatic and aliphatic). Easy recovery and reusable catalyst, easy work-up and avoidance of using harmful organic solvents are the major advantages of our method in comparison to existing methods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2,3-Dihydroquinazolineone-4(1H)-one is a class of heterocyclic compounds which is found in various natural products [1–3]. They exhibit a broad range of pharmacological activities such as antibacterial, anticancer, antitumor, analgesic, antihypertonic, antidepressant, antihistamine, diuretic activities and plant growth regulation ability [4–10]. They are also efficient in various cellular processes [11]. Therefore, the development of new methods for the synthesis of quinazolineas is of great importance due to their potential biological and pharmaceutical activities. These compounds can easily be oxidized to corresponding quinazoline-4(3H)-ones by using KMnO4 and are useful as growth inhibitors against leukemia cells [11]. Several classical procedures have been developed to prepare these classes of compounds. Among them, the condensation of 2-aminobenzamide with aldehydes or ketones is the simplest and most direct method for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones. Various acid catalysts such as cerium(IV)ammonium nitrate (CAN) [12], cellulose-SO3H [11], succinic acid [13], p-TSA [14], nanocomposites [15], ionic liquid [16], Sc(OTf)3 [17], boric acid-functionalized MCM-41 [18], ammonium chloride [19], ZrCl4 [20] β-cyclodextrin-SO3H [21],clay [22], succinimide-N-sulfonic acid [23], lactic acid [24], l-proline nitrate [25], and molecular sieve-supported lanthanum catalyst [26], as well as catalyst-free [27, 28] have been utilized to accomplish this transformation. Some of these methods require the use of organic solvents, expensive reagents, extended reaction times, and also tedious work-up procedures. Moreover, most of them are only applied for either aldehydes or ketones. Thus, the development of a simple, efficient, and high-yielding method for synthesizing 2,3 dihydroquinazolin-4(1H)-ones from both aldehydes and ketones is still in demand.
Heteropolyacids have numerous applications due to their super-acidic properties. Among heteropolyacids, polytungstic acids are widely used in organic synthesis. Non-toxicity, environmental compatibility, thermal stability, experimental simplicity, low reducibility and ease of handling are the major advantages of polytungstic acids that make them useful in organic synthesis [29–31]. Since heteropolyacids have low surface area (8–10 m2/g), supporting them on acidic or neutral solids such as silica improves the surface area and causes better accessibility of reactants to the active sites. Supported 12-tungstophosphoric acid on silica gel has been used as an effective catalyst for many organic reactions such as the synthesis of ß-acetamido ketones [32], Friedel–Crafts reactions [33], Fries rearrangement [34] and Diels–Alder reaction [35].
In the present study, we have reported an efficient synthesis with high-to-excellent yields and simple workup of 2-substituted 2,3-dihydroquinazolin-4(1H)-ones via cyclocondensation of o-aminobenzamide with different aldehydes and ketones in the presence of SiO2–H3PW12O40 in water (Scheme 1). Adopting water as the solvent is a better alternative. Organic reactions in water have attracted more attention because water is cheap, abundant and environmentally benign.
Experimental
General procedure for preparation of 12-tungstophosphoric acid supported on silica gel
The 12-tungstophosphoric acid supported on silica gel (PW/SiO2) was prepared by mixing silica gel (1.50 g, Merck grade 60, 230–400 mesh) with a solution of tungstophosphoric acid (0.50 g) in distilled water (10 mL). The resulting mixture was stirred for 30 min and then water was removed in a rotary evaporator and the solid powder was dried at 70 °C for 3 h [29].
General procedure for the preparation of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by SiO2–H3PW12O40
A stirred mixture of carbonyl compound (aromatic aldehyde and ketone (1 mmol), aliphatic aldehyde and ketone (2 mmol)), 2-aminobenzamide (1 mmol) and PW/SiO2 (50 mg) in water (5 mL) was refluxed for an appropriate time. After completion of the reaction, as indicated by TLC (ethyl acetate:n-hexane, 3:2), the reaction mixture was cooled to room temperature and water was removed by simple filtration. Then, ethyl acetate (10 mL) was added to the mixture and the catalyst was separated by filtration. Then ethyl acetate was removed under reduced pressure, and crude solid product was purified by recrystallization from ethanol. For the other carbonyl compounds, the reactions were performed in the presence of aqueous CTAB solution (0.02 g/mL). A stirred mixture of carbonyl compound (1 mmol) 2-aminobenzamide (1 mmol) and PW/SiO2 (50 mg) in the presence of aqueous CTAB solution (0.02 g/mL) was refluxed for an appropriate time. After completion of the reaction, as indicated by TLC (ethyl acetate:n-hexane, 3:2), the reaction mixture was cooled to room temperature, then ethyl acetate (5–10 mL) was added to the mixture and the catalyst was filtered. To remove CTAB from the reaction mixture, the organic and aqueous layers were separated with a separator funnel and CTAB was removed from the aqueous layer. The organic product was isolated by extraction with ethyl acetate. The ethyl acetate was dried (sodium sulfate) and then evaporated under reduced pressure, and the products were purified further by crystallization from ethanol, to afford the pure 2,3-dihydroquinazolin-4(1H)-ones which were characterized by 1H NMR, 13CNMR, IR, CHN analysis, and melting point, and compared with literature data.
Results and discussion
Initially, to optimize the reaction conditions, the effects of the solvent were investigated using 2-aminobenzamide (1 mmol) and 4-cyanobenzaldehyde (1 mmol) in the presence of SiO2–H3PW12O40 under reflux of the solvent as a model (Table 1, entries 1–7). This reaction was observed to perform well in water and methanol in comparison with the commonly used organic solvents such as CH3OH, CH3CN, THF and dioxin (Table 1, entries 2 and 7). We have chosen water as a solvent because it is in accordance with green chemistry. Then, we examined varying the temperatures to improve the reaction conditions (Table 1, entries 7–11). The results indicate that the reflux temperature is the best condition for this reaction. In the next step, we studied the effect of the amount of catalyst on the conversion rate of the reaction by varying the amount of the catalyst. The results are shown in Table 1 (entries 12–15).
As shown from Table 1, the best results were obtained using 0.05 g of SiO2–H3PW12O40 as the catalyst. We studied the effect of 12-tungstophosphoric acid (H3PW12O40) and SiO2 (mesh 230–400) on the 2, 3-dihydroquinazolin-4(1H)-ones formation (Table 1, entries 16–18). However, their catalytic activities were much lower than SiO2–H3PW12O40. To understand the role of the catalyst, the reaction was performed in its absence, when the desired product was isolated in only 30 % yield after 7 h (Table 1, entry 19).
To explore the scope and limitation of this reaction, we extended the cyclocondensation of 2-aminobenzamide with various carbonyl compounds (Scheme 1) in the presence of SiO2–H3PW12O40 in water under reflux conditions and the results are summarized in Table 2.
As indicated in Table 2, aromatic aldehydes with electron-withdrawing substituents performed well with 2-aminobenzamide in the presence of SiO2–H3PW12O40 in water under reflux conditions in affording the desired products with excellent yields (Table 2, entries 1–8). Aromatic aldehyde containing electron-donating substituents (Table 2, entries 10–15) afforded 2,3-dihydroquinazolin-4(1H)-ones in the presence of cetyltrimethylammonium bromide (CTAB). In the case of benzene-1,4-dicarbaldehyde, two different products were obtained which are related to its molar ratio to o-aminobenzamide (Table 2, entries 16, 17). Remarkably, acid-sensitive cinnamaldehyde (Table 2, entries 18, 19), aromatic heterocyclic aldehydes (Table 2, entries 20, 21) and aromatic ketones (Table 2, entries 22, 23) also gave the desired product in high to excellent yield in the presence of cetyltrimethylammonium bromide (CTAB). For aliphatic aldehydes and ketones, the reactions were performed in the presence of 2 mmol carbonyl compounds and the products were obtained in excellent yield under optimal reaction conditions (Table 2, entries 24–28).
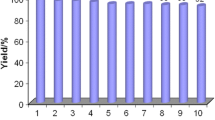
At the last step, the recyclability of the catalyst was investigated. After the reaction was completed, followed by the filtration, the filtered catalyst was dried for 3 h at 70 °C and then saved for the next reaction. The recyclability of the catalyst was tested at least three times and the desired product was isolated in high yields in each run (Table 3).
We compared the catalytic activity of SiO2–H3PW12O40 with some other reported catalysts for the synthesis of 2-(4-chloro-phenyl)-2,3-dihydro-1H-quinazolin-4-one (Table 4). As indicated in Table 4, in most cases our method is better with respect to reaction time, solvent and yield.
The proposed mechanism for this reaction is shown in Scheme 2. First, the condensation of 2-aminobenzamide with carbonyl compound was promoted in the presence of SiO2–H3PW12O40 to produce intermediate 1. Then, the part of imine in this intermediate could be activated by SiO2–H3PW12O40 and subsequently converted to intermediate 2 by intramolecular nucleophilic attack of the nitrogen on the activated imine carbon. Then, intermediate 2 could be converted to 2,3-dihydroquinazolin-4(1H)-one by a 1,5-proton transfer.
Conclusion
We have demonstrated a very simple and highly efficient procedure for the preparation of 2,3-dihydroquinazolin-4 (1H)-ones using 12-tungstophosphoric acid supported on silica gel (PW/SiO2) as a recyclable solid acid catalyst. Recycling of the catalyst is possible for three successive times without significant loss of activity.This methodology is applicable for both aldehydes and ketones (aromatic and aliphatic). Our method offers several advantages including short reaction time, high yield with high purity, easy workup, easy recovery and reusable catalyst, avoidance of using harmful organic solvents, and cost-effective use of cheap and commercially available starting materials.
References
R.P. Maskey, M. Shaaban, I. Grun-Wollny, H. Laatsch, J. Nat. Prod. 67, 1131 (2004)
T. Sugimori, T. Okawa, S. Eguchi, A. Kakehi, E. Yashima, Y. Okamato, Tetrahedron 54, 7997 (1998)
W. Bowman, M.R.J. Elsegood, T. Stein, G.W. Weaver, Org. Biomol. Chem. 5, 103 (2007)
E. Cohen, B. Klarberg, J.R. Vaughan, J. Am. Chem. Soc. 81, 5508 (1959)
H.L. Yale, M. Kalkstein, J. Med. Chem. 10, 334 (1967)
K. Okumura, T. Oine, Y. Yamada, G. Hayashi, M. Nakama, J. Med. Chem. 11, 348 (1968)
G. Bonola, P. Dare, E. Massarani, M.J. Magistretti, I. Setnikar, J. Med. Chem. 11, 1136 (1968)
R.J. Alaimo, H.E. Russel, J. Med. Chem. 15, 335 (1972)
J.I. Levin, P.S. Chan, T. Bailey, A.S. Katocs, A.M. Venkatesan, Bioorg. Med. Chem. Let. 4, 1141 (1994)
G.M. Chinigo, M. Paige, S. Grindrod, E. Hamel, S. Dakshanamurthy, M. Chruszcz, W. Minor, M.L. Brown, J. Med. Chem. 51, 4620 (2008)
B.V. Reddy, A. Venkatesteswarlu, C. Madan, A. Vinu, Tetrahedron Lett. 52, 1891 (2011)
M. Wang, J. Gao, Z. Song, L. Wang, Chem. Heterocycl. Comp. 47, 851 (2011)
Y. Zong, Y. Zhao, W. Luo, X.H. Yu, J.K. Wang, Y. Pan, Chin. Chem. Lett. 21(7), 778 (2010)
M.J. Hour, L.J. Huang, S.C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel, K.H. Lee, J. Med. Chem. 43, 4479 (2000)
J. Safari, S. Gandomi-Ravandi, J. Mol. Catal. A Chem. 390, 1 (2014)
J. Chen, W. Su, H. Wu, M. Liub, C. Jin, Green Chem. 9, 972 (2007)
J. Chen, H. Wu, W. Su, Chin. Chem. Let. 18, 536 (2007)
P. Sivaguru, K. Parameswaran, M. Kiruthiga, P. Vadivel, A. Lalitha, JICS 12, 95 (2015)
A. Shaabani, A. Maleki, H. Mofkham, Synth. Commun. 38, 3751 (2008)
M. Abdollahi-Alibeik, E. Shabani, Chin. Chem. Lett. 22(10), 1163 (2011)
J. Wu, X. Du, J. Ma, Y. Zhang, Q. Shi, L. Luo, B. Song, S. Yang, D. Hu, Green Chem. 16, 3210 (2014)
B.A. Dar, A.K. Sahu, P. Patidar, P. Sharma, M. Sharma, B. Singh, Am. J. Chem. 2(5), 248 (2012)
M. Ghashang, S.S. Mansoor, K. Aswin, Res. Chem. Intermed. 41(6), 3447 (2015)
S. Zhaleh, N. Hazeri, M.T. Maghsoodlou, Res. Chem. Intermed. 42(7), 6381 (2016)
S.P. Bahekar, N.D. Dahake, P.B. Sarode, H.S. Chandak, Synlett 26(18), 2575 (2015)
A. Magyar, Z. Hell, Catal. Lett. 146, 1153 (2016)
Z.B. Xie, S.G. Zhang, G.F. Jiang, D.Z. Sun, Z.G. Le, Green. Chem. Lett. Rev. 8, 95 (2015)
A. Das Gupta, S. Samanta, A.K. Mallik, Org. Prep. Proc. Int. 47(5), 365 (2015)
M. Mohammadpur, A. Shaabani, A. Bazgir, Catal. Commun. 7, 843 (2006)
I.V. Kozhevnikov, Chem. Rev. 98, 171 (1998)
A.A. Jafari, H. Mahmoudi, B.F. Mirjalili, J. Iran. Chem. Soc. 8(3), 851 (2011)
E. Rafiee, F. Shahbazi, M. Joshaghani, F. Tork J. Mol. Catal. A Chem. 242, 129 (2005)
Y. Isumi, K. Hisano, T. Hida, Appl. Catal. A Gen. 181, 277 (1999)
E.F. Kozhevnikova, E. Rafiee, I.V. Kozhevnikov, Appl. Catal. A 260, 25 (2004)
G. Meuzelaar, L. Maat, R. Sheldon, I.V. Kozhevnikov, Catal. Lett. 45, 249 (1997)
G. Majid, A. Kobra, M.P. Hamed, S. Hamid Reza, Chin. J. Chem. 29(8), 1617 (2011)
Q.R. Zhang, B.L. Xu, Y.H. Wang, Chin. Chem. Lett. 18, 656 (2007)
X.S. Wang, K. Yang, J. Zhou, S.J. Tu, J. Comb. Chem. 12, 417 (2010)
M. Baghbanzadeh, P. Salehi, M. Dabiri, G. Kozehgary, Synthesis 2, 344 (2006)
P. Murthy, G. Krishna, C. Reddy, K. Prasad, Tetrahedron Lett. 53, 863 (2012)
A. Gharib, B.R. HashemipourKhorasani, M. Jahangir, M. Roshani, R. Safaee, Org. Chem. Int. 2013 (2013)
G. Yassaghi, A. Davoodnia, S. Allameh, A. Zare-Bidaki, N. Tavakoli-Hoseini, Bull. Korean Chem. Soc. 33(8), 2724 (2012)
M. Dabiri, P. Salehi, M. Baghbanzadeh, M.A. Zolfigol, M. Agheb, S. Heydari, Catal. Commun. 9, 785 (2008)
V.B. Ningdale, U.N. Chaudhary, K.A. Shaikh, Arch. Appl. Sci. Res. 5, 82 (2013)
S.B. Bharate, N. Mupparapu, S. Manda, J.B. Bharate, R. Mudududdla, R.R. Yadav, R.A. Vishwakarma, Arkivoc 8, 308 (2012)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material containing spectral data (1H NMR, 13C NMR and IR) of 2,3-dihydroquinazolin-4(1H)-one derivatives.
Rights and permissions
About this article
Cite this article
Alinezhad, H., Soleymani, E. & Zare, M. Facile method for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by SiO2–H3PW12O40 in water. Res Chem Intermed 43, 457–466 (2017). https://doi.org/10.1007/s11164-016-2634-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2634-4