Abstract
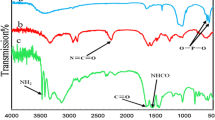
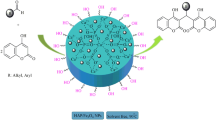
In this work, Fe2+ supported on hydroxyapatite-core–shell-γ-Fe2O3 nanoparticles (γ-Fe2O3–HAp-Fe2+ NPs) has been prepared and characterized by Fourier transform infrared spectroscopy, X-ray diffraction, transmission electron microscopy, scanning electron microscopy, and vibrating sample magnetometry spectra. Then, γ-Fe2O3–HAp-Fe2+ NPs were used as an efficient, reusable and heterogeneous nanocatalyst for one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes via the one-pot condensation reaction of arylaldehydes and 2-naphthol under solvent-free conditions in good to excellent yields. This procedure has a lot of advantages such as very easy reaction conditions, simplicity in operation, short reaction time, high yield, and green aspects by avoiding toxic conventional catalysts and solvents. Additionally, γ-Fe2O3–HAp-Fe2+ NPs were easily recycled from the reaction mixture and were reused seven times without any loss in activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The synthesis of xanthene and benzoxanthene compounds has received great attention from various pharmacological activities and organic chemists because of their broad spectrum of biological and pharmaceutical properties, such as anti-viral [1], antioxidant [2], anti-cancer [3], antibacterial and antifungal [4, 5], analgesic and anti-inflammatory [6, 7], cytotoxic [8], and anti-proliferative properties [9]. Moreover, xanthene and benzoxanthene derivatives can be used as sensitisers in dye-sensitised solar cells (DSSCs) [10–12], in the food industry as additives [13], in laser technologies [14], in photodynamic therapy [15], as fluorescent materials for the visualisation of biomolecules [16], and also as hole-transporting materials in organic light-emitting devices (OLEDs) [17]. Xanthenes and their derivatives are rare in natural plants [18, 19]. Blumeaxanthene (A), blumeaxanthene (B), and 3-isopropyl-9a-methyl-1,2,4a,9a-tetrahydroxanthene (C) are examples of natural xanthenes (Scheme 1). Compounds A and B have been used to treat gynecological disorders, and compound C has been used in traditional Indian medicine as an antidote for all snake venoms.
A number of methods have been developed and reported for the synthesis of xanthenes and benzoxanthenes. These methods include the cyclocondensation reaction between 2-tetralone and 2-hydroxyarylaldehydes under acidic conditions [20], the palladium-catalysed cyclisation of polycyclic aryltriflate esters [21], the reaction of alkylphenoxy magnesium halides with triethylorthoformate [22], and the reaction of the condensation of cyclic 1,3-diketones with aryl aldehydes catalysed by molybdate sulphonic acid [23]. Also, 14-aryl-14H-dibenzo[a,j]xanthene derivatives can be prepared by condensation of 2-naphthol with aldehydes in the presence of different catalysts, including Lewis acids and Brønsted acids. For example, carbon-based solid acid [24], functionalized mesoporous materials [25], cellulose sulfuric acid [26], P2O5/Al2O3 [27], amberlyst-15 [28], silica sulfuric acid [29], HClO4–SiO2 [30], heteropoly acid [31], montmorillonite K-10 [32], Yb(OTf)3 [33], phospho sulfonic acid (PSA) [34], boron sulfonic acid (BSA) [35], molecular iodine [36], and boric acid [37] have been used as catalysts for the synthesis of xanthenes. However, some of these methods suffer from drawbacks such as strongly acidic wastes, high cost, reagent toxicity, unsatisfactory yields, tedious work-up procedures, harsh conditions and nonrecyclable reagents. Recently, the application of nanoparticles (NPs) has received great attention from organic chemists because of their high surface area and unique magnetic properties. Moreover, they have a wide range of usage in various fields, such as data storage [38], environmental remediation [39], biology and medical applications [40], magnetic resonance imaging (MRI) [41], and magnetic fluids [42]. γ-Fe2O3–HAp-Fe2+NPs are important both environmentally and economically, because they can be conveniently handled, making the experimental procedure simple, low cost and eco-friendly. Also, the catalyst can be recovered from the reaction mixtures and reused. Recently, we used a magnetic NP with a high surface area, narrow pore size distribution and large pore volume as a recoverable heterogeneous catalyst in some organic transformations [43–49]. Therefore, in the current work, it seems that the major task of current research is developing more acceptable methods based on the use of Fe2+ supported on hydroxyapatite-core–shell-Fe2O3 (γ-Fe2O3–HAp-Fe2+) NPs as an efficient and reusable Lewis acid catalyst for the one-pot synthesis of 14-aryl-14H-dibenzo[a,j] xanthenes in good to excellent yields and in short reaction times under solvent-free conditions at 90 °C (Scheme 2). (Please see supporting information for experimental procedures for the synthesis of γ-Fe2O3–HAp-Fe2+ NPs, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), vibrating sample magnetometry (VSM ), and Fourier transform infrared spectroscopy, (FTIR)].
Experimental
Materials and methods
All chemicals were purchased from Merck or Fluka chemical companies. All the products are know compounds and were characterized by comparing the proton nuclear magnetic resonance (1H-NMR) and carbon-13 (13C)-NMR spectroscopic data and their melting point literature values. The uncorrected melting points of all compounds were measured in an open capillary in a paraffin bath. NMR chemical shifts are reported in (δ) ppm relative to tetramethylsilane (TMS, d = 0.00) with the residual solvent as an internal reference [deuterated chloroform (CDCl3), δ 7.26 ppm for 1H-NMR and δ 77.0 for 13C-NMR]. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constants (Hz) and integration. Mass spectrometry (MS) analyses were performed on a PerkinElmer Clarus 680 gas chromatograph (GC) equipped with a Clarus SQ 8T mass spectrometer. Infrared (IR) spectra were recorded on a Frontier FT-IR (Perkin Elmer) spectrometer using a potassium bromide (KBr) disk. The phases present in the magnetic materials were analyzed using a powder X-ray diffractometer, Philips (Holland), model X0 Pert with X´ Pert with CuKα1 radiation (λ = 1.5401 Å), and the X-ray generator was operated at 40 kV and 30 mA. Diffraction patterns were collected from 2 h = 20°–80°.
Preparation of γ-Fe2O3–HAp-Fe2+NPs
In this study, γ-Fe2O3–HAp-Fe2+ NPs were prepared in two steps. The iron oxide magnetic particles (IOMPs) were synthesized by a chemical co-precipitation technique of ferric and ferrous chlorides in aqueous solution. Solutions of FeCl3·6H2O (0.25 mol L−1) and FeCl2·4H2O (0.125 mol L−1) were mixed and precipitated with NH4OH solution (25 %) at pH 12, while stirring vigorously. The black suspension, which formed immediately, was maintained at 70 °C for approximately 1 h and washed several times with ultrapure water until the pH decreased to 7. IOMP/HAp was prepared by the impregnation method according to known procedures with some modifications [50]. Then hydroxyapatite-encapsulated γ-Fe2O3 (0.6 g) was introduced into 100 ml of distilled water containing 6.4 mmol of FeCl2·4H2O. The mixture was stirred (500 rpm) for 24 h, filtered, and washed several times with ethanol. The recovered solid was dried at 70 °C overnight (Scheme 3). The mean size and the surface morphology of the γ-Fe2O3–HAp-Fe2+ NPs were characterized by TEM, SEM, VSM, XRD and FTIR techniques [43].
Procedure for preparation of 14-aryl-14H-dibenzo[a,j]xanthenes 3a–t
To a mixture of aldehyde (1 mmol) and 2-naphthol (2 mmol), γ-Fe2O3–HAp-Fe2+ NPs (20 mg) were added and the mixture was inserted in an oil bath and heated at 90 °C for the appropriate time (Table 3). Completion of the reaction was indicated by thin-layer chromatography (TLC). After completion of the reaction, the reaction mixture was cooled to room temperature. The reaction mixture was dissolved in ethylacetate and the catalyst was separated by simple filtration. Excess solvent was removed under reduced pressure and the crude product was recrystallized with ethanol to afford the pure product in 81–95 % yield. All the products were known compounds and characterized by comparing Melting points, 1H-NMR 13C-NMR, and Mass spectra with those reported in the literature. The spectral data for some selected compounds are given below.
14-(Phenyl)14H-dibenzo[a,j]xanthenes (3a)
Pale yellow solid; 1H-NMR (300 MHz, CDCl3): δ 5.39 (1H, s, CH), 7.28–7.31 (d, 2H, J = 8.5 Hz, Ar–H), 6.68–6.74 (4H, m, Ar–H), 5.89–6.80 (11H, m, Ar–H); 13C-NMR (75 MHz, CDCl3): δ 38.03, 117.31, 118.01, 122.68, 124.23, 126.37, 126.78, 128.25, 128.47, 128.79, 128.85, 131.04, 131.44, 144.98, 148.71; MS (EI), m/z: 358 (M+), 282, 281, 252, 250, 179.
14-(2-Nitrophenyl)-14H-dibenzo[a,j]xanthenes (3b)
Yellow solid; 1H-NMR (300 MHz, CDCl3): δ 5.91 (1H, s, CH), 6.08–6.43 (8H, m, Ar–H), 6.48–6.51 (2H, m, Ar–H), 6.68–6.72 (4H, m, Ar–H), 7.41–7.44 (2H, d, J = 8.53, Ar–H); 13C-NMR (75 MHz, CDCl3): δ 32.5, 117.5, 118.0, 122.5, 124.6, 124.9, 127.3, 127.5, 128.7, 129.4, 130.9, 131.7, 132.2, 134.1, 140.8, 147.0, 149.3; MS (EI), m/z: 403 (M+), 282, 281, 252, 250, 178, 141.
14-(4-Bromo)-14H-dibenzo[a,j]xanthenes (3e)
Pink solid; 1H-NMR (300 MHz, CDCl3): δ 7.19–7.22 (m, 2H), 6.16–6.75 (m, 4H), 6.15–6.75 (m, 10H), 5.34 (s, 1H); 13C-NMR (75 MHz, CDCl3): δ 37.18, 115.18, 115.47, 117.07, 118.03, 122.46, 124.33, 126.88, 128.89, 129.56, 129.67, 131.04, 131.26, 140.80, 148.68; MS (EI), m/z: 438, 436 (M+), 282, 281, 252, 250, 179.
14-(4-Methyl)-14H-dibenzo[a,j]xanthenes (3f)
White solid; 1H-NMR (300 MHz, CDCl3): δ 7.32–7.29 (m, 2H), 6.74–6.67 (m, 4H), 6.51–6.15 (m, 8H), 5.87–5.85 (m, 2H), 5.36 (s, 1H), 1.04 (s, 3H); 13C-NMR (75 MHz, CDCl3): δ 20.91, 37.63, 117.44, 118.01, 122.71, 124.21, 126.76, 128.11, 128.79, 129.18, 131.07, 131.45, 135.90, 142.13, 148.67; MS (EI), m/z: 372 (M+), 282, 281, 252, 250, 186.
14-(4-methoxyphenyl)-14H-dibenzo[a,j]xanthenes (3g)
Pink solid; 1H-NMR (300 MHz, CDCl3): δ 2.49 (3H, s, OMe), 5.36 (1H, s, CH), 5.59–5.61 (2H, d, J = 8.66 Hz, Ar–H), 6.32–6.45 (6H, m, Ar–H), 6.49–6.55 (2H, m, Ar–H), 6.69–6.76 (4H, m, Ar–H), 7.31–7.33 (2H, d, J = 8.48, Ar–H); 13C-NMR (75 MHz, CDCl3): δ 37.17, 55.05, 113.89, 117.58, 118.06, 122.77, 124.28, 126.81, 128.80, 128.87, 129.23, 131.13, 131.47, 137.45, 148.69, 157.89; MS (EI), m/z: 388 (M+), 282, 281, 252, 250, 194, 187.
14-(4-Nitrophenyl)-14H-dibenzo[a,j]xanthenes (3i)
Pale yellow solid; 1H-NMR (300 MHz, CDCl3): δ 5.44 (1H, s, CH), 6.33–6.57 (8H, m, Ar–H), 6.71–6.76 (4H, m, Ar–H), 6.85–6.89 (2H, d, Ar–H), 7.15–7.18 (2H, J = 8.3, Ar–H); 13C-NMR (75 MHz, CDCl3): δ 37.88, 115.75, 118.06, 122.04, 123.86, 124.58, 127.19, 128.96, 129.06, 129.59, 131.07, 146.27, 148.75, 152.01; MS (EI), m/z: 388 (M+), 282, 281, 252, 250, 178, 177.
14-(2-Chlorophenyl)-14H-dibenzo[a,j]xanthenes (3p)
White solid; 1H-NMR (300 MHz, CDCl3): δ 5.69 (1H,s, CH), 5.81–5.82 (2H, d, Ar–H), 6.27–6.40 (5H, m, Ar–H), 6.50 (2H, d, Ar–H), 6.55–6.67 (4H, m, Ar–H), 7.63–7.66 (2H, J = 8.5, d, Ar–H); 13C-NMR (75 MHz, CDCl3): δ 34.63, 118.02, 118.11, 123.46, 124.44, 126.93, 127.87–127.94, 128.66, 129.08, 129.60, 130.13, 130.89, 131.76, 131.81, 143.57, 148.95; MS (EI), m/z: 394, 392 (M+), 282, 281, 252, 250, 178, 177.
Results and discussion
In this study, initially the Fe2+ supported on hydroxyapatite-core–shell-γ-Fe2O3 NPs were prepared according to the reported procedures (Scheme 3). Then, they were fully characterized by TEMSEM, XRD, VSM, and FTIR techniques.
Herein, we wish to report for the condensation of 2-naphthol and various aromatic aldehydes in the presence of γ-Fe2O3–HAp-Fe2+ NPs as a green, reusable and Lewis acid catalyst for the preparation of 14-aryl-14H-dibenzo[a,j]xanthenes derivatives under solvent-free conditions (Scheme 2).
To optimize the reaction conditions for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives, the reaction between 2-naphthol and benzaldehyde under solvent-free conditions was selected as a model reaction to provide compound 3a (Table 4, entry 1). At first, we examined the catalytic activity of various acids in a model reaction (Table 1). As it can be seen in Table 1, the high yield and short reaction time of compound 3a was obtained when γ-Fe2O3–HAp-Fe2+ was utilized as catalyst (Table 1, entry 6).
In the next step, the model reaction was tested using different amounts of γ-Fe2O3–HAp-Fe2+ at the various temperatures. The results are summarized in Table 2. It can be seen in Table 2 that this reaction was strongly influenced by the amount of catalyst and temperature. The best results were obtained using 20 mg of catalyst in 15 min at 90 °C. (Table 2, entry 4). Accordingly, 20 mg of catalyst was selected for use in these reactions due to its low concentration, which resulted in excellent yields and short reaction times. Moreover, the product yield was not changed by increasing the amount of the catalyst and increasing the reaction temperature. In the next part, using these optimized reaction conditions, other benzaldehydes (3b–3t) were examined in the presence of γ-Fe2O3–HAp-Fe2+NPs.
It was found that all the reactions proceeded smoothly to give the corresponding 14-aryl-14H-dibenzo[a,j]xanthene derivatives in good to excellent yields and short reaction times (Table 3). The results given in Table 3 show that, in all cases, aromatic aldehydes containing both electron-donating and electron-withdrawing groups reacted under solvent-free conditions with 2-naphthol and within a short reaction time (10–35 min) to generate 14-aryl-14H-dibenzo[a,j]xanthene derivatives with good to excellent yields (81–95 %). Ortho-substituted aromatic aldehydes, however, did not react as smoothly, likely because of steric hindrance, and longer reaction times were required to get the corresponding products in good yields (Table 2, entries 2 and 15–16).
In order to show the merit of the present work, the advantages of γ-Fe2O3–HAp-Fe2+NPs were compared (reaction condition, time and yield) with some homogeneous and heterogeneous catalysts for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes, and the results are presented in Table 4. Based on previous research using cellulose sulfuric acid (Table 4, entry 1), Polyvinylpolypyrrolidone-bound boron trifluoride (PVPP-BF3 ; Table 4, entry 5), zinc oxide NP (Table 4, entry 6), and p-Toluene sulfunic acid (Table 4, entry 8) for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes derivatives, which requires long reaction time and high temperature to complete the reaction. In the recent years, Khaksar and et al. reported the synthesis of xanthene derivatives using Pentafluorophenyl Ammonium Triflate (Table 4, entry 9). This method requires long reaction time and the use of hazardous organic solvents such as toluene to complete the reaction. In the 2011, Rahmatpour and et al. reported using Polystyrene-supported aluminium chloride (Table 4, entry 10) and in the 2005, Khosropour and et al. using p-toluene sulfunic acid (Table 4, entry 7) for the one-pot synthesis of 14-aryl-14H-dibenzo[a,j]xanthenes derivatives. This methods requires long reaction times, and requires harsh reaction conditions like use of reflux conditions and also use hazardous organic solvents such as dichloromethane. As shown in Table 4, γ-Fe2O3–HAp-Fe2+ NPs can act as effective catalyst with respect to reaction times, amount of the catalyst, and yields of the obtained products. Thus, the present protocol with γ-Fe2O3–HAp-Fe2+ NPs catalysts is convincingly superior to the some reported catalytic methods.
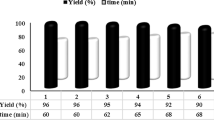
The reusability of catalysts is of major importance in green chemistry and also is of major importance for large scale operations and from an industrial point of view. Thus, the recovery and reusability of γ-Fe2O3–HAp-Fe2+ NPs was investigated. Catalyst reusability was also examined for the preparation of 14-(phenyl)14H-dibenzo[a,j]xanthenes (3a) from the reaction between 2-naphthol with benzaldehyde as a model reaction. When the reaction was completed (monitored by TLC), ethylacetate was added until the solid crude product was dissolved. Then, γ-Fe2O3–HAp-Fe2+ NPs as the catalyst were isolated from the reaction mixture by simple filtration and reused again after washing with ethylacetate. The recovered catalyst was reused in seven similar subsequent runs. For each of the runs, the product yield was 95, 95, 93, 91, 91, 90 and 88 %, respectively, which verifies that the activity of the catalyst remained unchanged throughout these seven runs (Fig. 1).
The suggested mechanism for the γ-Fe2O3–HAp-Fe2+ NP-catalyzed transformation is shown in Scheme 4. According to the mechanism, γ-Fe2O3–HAp-Fe2+ NPs readily catalyzed the in situ formation of 14-aryl-14H-dibenzo[a,j]xanthenes 9. The activated aromatic aldehyde 1 reacted with one molecule of 2-naphthol 2 to provide intermediate 3, which can be regarded as a fast Knoevenagel addition. Then the active methylene of the second molecule of 2-naphthol reacted with intermediate 3 via conjugate Michael addition to produce the intermediate 7, which underwent intramolecular cyclodehydration to give the 14-aryl-14H-dibenzo[a,j]xanthenes 9.
Conclusion
We have synthesized Fe2+ supported on hydroxyapatite-core–shell-γ-Fe2O3 NPs as a green and non-toxic nanocatalyst, and successfully used the compound as a catalyst for one-pot condensation of arylaldehydes with 2-naphthol. Some important advantages of this work are high yield, ease of product isolation, non-use of hazardous organic solvents, short reaction times, use of various substrates, and ease of catalyst recovery and reuse, which make it a useful, attractive, and green strategy for the preparation of xanthene derivatives.
References
J.M. Jamison, K. Krabill, A. Hatwalkar, E. Jamison, C.C. Tsai, Cell Biol Int Rep 14, 1075 (1990)
T. Nishiyama, K. Sakita, T. Fuchigami, T. Fukui, Polym Degrad Stab 62, 529 (1998)
Y.B. Song, Y.H. Yang, J. You, B. Liu, L.J. Wu, Y.L. Hou, W.J. Wang, J.X. Zhu, Chem Pharm Bull 61, 167 (2013)
S. Limsuwan, E.N. Trip, T.R.H.M. Kouwen, S. Piersma, A. Hiranrat, W. Mahabusarakam, S.P. Voravuthikunchai, J.M. van Dijl, O. Kayser, Phytomedicine 16, 645 (2009)
J.J. Omolo, M.M. Johnson, S.F. van Vuuren, C.B. de Koning, Bioorg Med Chem Lett 21, 7085 (2011)
H.N. Hafez, M.I. Hegab, I.S. Ahmed-Farag, A.B.A. El-Gazzar, Bioorg Med Chem Lett 18, 4538 (2008)
A.G. Banerjee, L.P. Kothapalli, P.A. Sharma, A.B. Thomas, R.K. Nanda, S.K. Shrivastava, V.V. Khatanglekar, Arab. J. Chem. (2011). doi:10.1016/j.arabjc.2011.06.001
A.K. Bhattacharya, K.C. Rana, M. Mujahid, I. Sehar, A.K. Saxena, Bioorg Med Chem Lett 19, 5590 (2009)
A. Kumar, S. Sharma, R.A. Maurya, J.J. Sarkar, Comb Chem 12, 20 (2010)
K. Hara, T. Horiguchi, T. Kinoshita, K. Sayama, H. Sugihara, H. Arakawa, Sol Energy Mater Sol Cells 64, 115 (2000)
B. Pradhan, S.K. Batabyal, A.J. Pal, Sol Energy Mater Sol Cells 91, 769 (2007)
G.D. Sharma, P. Balraju, M. Kumar, M.S. Roy, Mater Sci Eng, B 162, 32 (2009)
H. Qi, B.W. Zhu, N. Abe, Y. Shin, Y. Murata, Y. Nakamura, Food Chem Toxicol 50, 1841 (2012)
S. De, S. Das, A. Girigoswami, Spectrochim Acta, Part A 61, 1821 (2005)
C.C. Chang, Y.T. Yang, J.C. Yang, H.D. Wu, T. Tsai, Dyes Pigm 79, 170 (2008)
S.A. Hilderbrand, R. Weissleder, Tetrahedron Lett 48, 4383 (2007)
Z.Z. Chu, D. Wang, C. Zhang, F.H. Wang, H.W. Wu, Z.B. Lv, S.C. Hou, X. Fan, D.C. Zou, Synth Met 162, 614 (2012)
D. Thangadurai, N. Ramesh, M.B. Viswanathan, D.X. Prasad, Fitoterapia 72, 92 (2001)
L. Huang, T. Lei, C.W. Lin, X.C. Kuang, H.Y. Chen, H. Zhou, Fitoterapia 81, 389 (2010)
A. Jha, J. Beal, Tetrahedron Lett 45, 8999 (2004)
J.Q. Wang, R.G. Harvey, Tetrahedron 58, 5927 (2002)
G. Casiraghi, G. Casnati, M. Cornia, Tetrahedron Lett 14, 679 (1973)
B. Karami, Z. Zare, K. Eskandari, Chem Pap 67, 145 (2013)
V. Mirkhani, M. Moghadam, S. Tangestaninejad, I. Mohammadpoor-Baltork, M. Mahdavi, Synth Commun 39, 4328 (2009)
J. Mondal, M. Nandi, A. Modak, A. Bhaumik, J Mol Catal A: Chem 363–364, 254 (2012)
J. Venu-Madhav, Y. Thirupathi-Reddy, P. Narsimha-Reddy, J Mol Catal A: Chem 304, 85 (2009)
A. Zarei, A.R. Hajipour, L. Khazdooz, Dyes Pigm 85, 133 (2010)
S. Ko, C.F. Yao, Tetrahedron Lett 47, 8827 (2006)
H.R. Shaterian, M. Ghashang, A. Hassankhani, Dyes Pigm 76, 564 (2008)
M.A. Bigdeli, M.M. Heravi, G.H. Mahdavinia, J Mol Catal A: Chem 275, 25 (2007)
M.M. Amini, N. Seyyedhamzeh, A. Bazigir, Appl. Catal. A Gen. 23, 242 (2007)
A. Shari, S. Abaee, A. Tavakkoli, M. Mirzaei, A. Zolfagharei, Synth Commun 38, 2958 (2008)
W. Su, D. Yang, C. Jin, B. Zhang, Tetrahedron Lett 49, 3391 (2008)
S. Rezayati, Z. Erfani, R. Hajinasiri, Chem Pap 69, 536 (2015)
H. Moghanian, A. Mobinikhaledi, M. Deinavizadeh, Res Chem Intermed 41, 4387 (2015)
M.A. Pasha, V.P. Jayashankara, Bioorg Med Chem Lett 17, 621 (2007)
S. Rezayati, R. Hajinasiri, Z. Erfani, S. Rezayati, S. Afshari Sharif-Abad, Iran. J. Catal. 4, 157 (2014)
T. Hyeon, Chem. Commun. 927 (2003). doi:10.1039/B207789B
A. Lu, W. Schmidt, N. Matoussevitch, H. Bonnemann, B. Spliethoff, B. Tesche, E. Bill, W. Kiefer, F. Schuth, Angew Chem 116, 4403 (2004)
A.K. Gupta, M. Gupta, Biomaterials 26, 3995 (2005)
S.I. Park, J.H. Kim, J.H. Lim, C.O. Kim, Curr Appl Phys 8, 706 (2008)
S. Chikazumi, S. Taketomi, M. Ukita, M. Mizukami, H. Miyajima, M. Setogawa, Y. Kurihara, J Magn Magn Mater 65, 245 (1987)
E. Rezaee Nezhada, Z. Abbasi, S. Sajjadifar, Sci. Iran. C 22, 903 (2015)
S. Sajjadifar, Z. Abbasi, E. Rezaee Nezhad, M. Rahimi-Moghaddam, S. Karimian, S. Miri, J Iran Chem Soc 11, 335 (2014)
E. Rezaee Nezhada, Z. Abbasi, S. Sajjadifar, S. Rezayati, J. Sci. I. R. Iran 25, 127 (2014)
S. Rezayati, R. Hajinasiri, Z. Erfani, Res Chem Intermed (2015). doi:10.1007/s11164-015-2168-1
S. Sajjadifar, S. Rezayati, Chem Pap 68, 531 (2014)
S. Rezayati, S. Sajjadifar, J. Sci. I. R. Iran 25, 329 (2014)
S. Rezayati, F. Sheikholeslami-Farahani, F. Rostami-Charati, S. Afshari Sharif Abad, Res Chem Intermed (2015). doi:10.1007/s11164-015-2261-5
K. Donadel, D.V. Marcosand, C.M. Mauro, Ana. Acad. Bras. Cienc. 81, 179 (2009)
F.Q. Ding, L.T. An, J.P. Zou, Chin J Chem 25, 645 (2007)
R. Kumar, G.C. Nandi, R.K. Verma, M.S. Singh, Tetrahedron Lett 51, 442 (2010)
B.F. Mirjalili, A. Bamoniri, A. Akbari, N. Taghavinia, J Iran Chem Soc 8, S129 (2011)
H. Naeimi, Z.S. Nazifi, Appl Catal A 477, 132 (2014)
S. Rostamizadeh, N. Shadjou, A.M. Amani, S. Balalaie, Chin Chem Lett 19, 1151 (2008)
J.P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf, R. Lacroix, Eur J Med Chem 13, 67 (1978)
M. Mokhtary, S. Refahati, Dyes Pigm 99, 378 (2013)
D. Prasad, M. Nath, Catal. Sci. Technol. 2, 93 (2012)
A.A. Bartolomeu, M.L. Menezes, L.C. Filho, Chem Pap 68, 1593 (2014)
G.B. Dharma-Rao, M.P. Kaushik, A.K. Halve, Tetrahedron Lett 53, 2741 (2012)
A.R. Khosropour, M.M. Khodaei, H. Moghannian, Synlett. 955 (2005). doi:10.1055/s-2005-864837
S. Khaksar, N. Behzadi, Chin J Catal 33, 982 (2012)
B. Maleki, S. Barzegar, Z. Sepehr, M. Kermanian, R. Tayebee, J Iran Chem Soc 9, 757 (2012)
Acknowledgments
The authors gratefully acknowledge partial support of this study by the Payame Noor University (PNU) of Ilam, I.R. Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haeri, H.S., Rezayati, S., Nezhad, E.R. et al. Fe2+ supported on hydroxyapatite-core–shell-γ-Fe2O3 nanoparticles: Efficient and recyclable green catalyst for the synthesis of 14-aryl-14H-dibenzo[a,j]xanthene derivatives. Res Chem Intermed 42, 4773–4784 (2016). https://doi.org/10.1007/s11164-015-2318-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2318-5