Abstract
The nanosheets TiO2/g-C3N4 hybrid material with efficient visible-light photocatalytic activity was prepared by a facile solvothermal method. The as-prepared TiO2/g-C3N4 nanosheets composite was thoroughly characterized by X-ray diffraction, Fourier transform infrared spectroscopy, transmission electron microscopy, N2 adsorption–desorption analysis, UV–Vis diffuse reflectance spectroscopy, and X-ray photoelectron spectroscopy. As evaluated by the degradation of methylene blue under visible light irradiation, TiO2/g-C3N4 hybrid composites exhibit much higher photocatalytic activity than pristine g-C3N4 and TiO2, respectively. The significant enhancement in photodegradation activity over the TiO2/g-C3N4 photocatalyst can be ascribed to the combined effects of the nanosheet structure and subsequent efficient separation of photogenerated charge carriers. A tentative mechanism for the photodegradation process was proposed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the related fully condensed polymers known as graphitic carbon nitride (g-C3N4) has attracted considerable attention. Graphitic carbon nitride is the most stable allotrope, with a layered structure similar to graphene and has metal-free chemical compositions of C, N, and H [1]. Meanwhile, a narrow band gap semiconductor (Eg = 2.7 eV) in g-C3N4 is of growing interest as a photocatalyst and has been extensively studied in visible light-driven water splitting and degradation of organic pollutants [2–4]. However, the photocatalytic efficiency of single g-C3N4 is limited due to the high recombination probability of photogenerated electron–hole pairs [5, 6]. In order to improve photocatalytic activity, several efforts have been developed to improve the photocatalytic performance of g-C3N4 in terms of introducing heteroatoms [7–10], sensitized by dyes [11], coupled with semi-conductors or polymers [12–16], and controlling morphology [17–19]. Nevertheless, a simple development of highly efficient g-C3N4 remains a significant challenge.
To date, the combination of g-C3N4 and TiO2 into a heterostructure presents a feasible and inspiring route towards improved charge separation in the electron transfer process [20–25]. Yu et al. [26] synthesized g-C3N4-TiO2 nanocomposites by a facile calcination route using P25 and urea, resulting in higher photoactivity for the decomposition of HCHO than that of single-phase C3N4 and bare TiO2. The g-C3N4/TiO2 photocatalyst with extremely simple steps using cyanamide and anatase TiO2 as resources was reported by Lei et al. [27]. This photocatalyst shows prominent improvement in degradation of Acid Orange 7 dyes under both visible and UV light [27]. Colón et al. [28] reported the preparation of g-C3N4-TiO2 composites with different g-C3N4 loadings by simple impregnation, giving good photoactivity for the degradation of phenol under UV irradiation. Zhang et al. [29] has found that well-dispersed TiO2 nanocrystals with (001) facets prepared in situ on g-C3N4 through a facile solvothermal method exhibit higher efficiency for photocatalytic degradation of phenol as compared to pure g-C3N4 and TiO2 or mechanically mixed TiO2/g-C3N4. Therefore, combining TiO2 with g-C3N4 to form composite photocatalysts seems to be an effective approach to construct a heterojunction structure leading to enhanced photoactivity. In this work, TiO2 hybridized with g-C3N4 nanosheets were prepared by a facile approach. The TiO2 nanosheets prepared by solvothermal reaction were found to be well anchored on the surface of g-C3N4 nanosheets, endowing the hybrid with an interfacial junction. The as-prepared TiO2/g-C3N4 nanosheets composites have shown stronger photocatalytic performance than pure TiO2 nanosheets and g-C3N4.
Experimental sections
Catalyst preparation
The polymeric g-C3N4 was synthesized by calcination with melon as precursor. Typically, 12 g of melon was put into a crucible with a cover and then heated at 550 °C in a muffle furnace for 4 h with a heating rate of 10 °C min−1. The resultant yellow powder was washed with 1.0 M HCl and distilled water and then sonicated in methanol for 60 min, dried at 100 °C, and collected for further use.
A 1.5 g amount of urea was dissolved in 10 mL of anhydrous ethanol, then 1.0 mL distilled water was added slowly with stirring for 30 min at room temperature (solution A). A certain amount of tetrabutyl titanate (TBT) was dissolved into 10 mL anhydrous ethanol (solution B). After that, solution A was added drop-wise to solution B under magnetic stirring. The resultant mixture was stirred at room temperature for 2 h until the transparent sol was obtained. One gram of g-C3N4 was added into the transparent sol, and the mixture was sonicated for 60 min to disperse completely g-C3N4. Subsequently, the mixture was transferred into a 50 mL Teflon-inner-liner stainless steel autoclave. The autoclave was kept at 150 °C for 16 h. Finally, the precipitate gained was washed with distilled water and dried at 100 °C for 24 h. The weight percentages of TiO2 to g-C3N4 were varied by varying the weight of TiO2, and the samples were presented as x % TiO2/g-C3N4, where x is the weight content of TiO2. For comparison, pure TiO2 was also prepared by the same procedure without adding g-C3N4, which is denoted as TiO2.
Catalyst characterization
The crystal structures of samples were determined by an X-ray diffactometer (a Bruker D8) with Cu Kα radiation (λ = 0.15418 nm). Thermogravimetric analysis was conducted on an NETZSCH STA 449F3 instrument. The experiment temperature ranged from 25 to 1000 °C at a constant heating rate of 10 °C/min in air. Optical properties were analyzed with a spectrophotometer (Shimadzu SolidSpec-3700DUV) and converted from reflection to absorbance by the standard Kubelka–Munk method. FT-IR measurements were performed using a Fourier transform infrared (FT-IR) spectrometer (Nicolet 670) only in the mid-infrared range of the instrument (400–4000 cm−1) for samples dispersed in KBr pellets in 1:99. X-ray photoelectron spectroscopy (XPS) was recorded with a Perkin Elmer PHI 5000C ESCA System with Al Kα radiation operated at 250 W. The shift of binding energy due to relative surface charging was corrected using the C 1s level at 284.6 eV as an internal standard. The nitrogen adsorption–desorption isotherms at 77 K were investigated using a nitrogen adsorption apparatus (QUADRASORB IQ, Quantachrome Instrument Corp). The morphology was observed using a scanning electron microscope(SEM, JSM5600LV, JEOL Ltd.) and JEM-100CX Transmission Electron Microscopy (TEM, JEOL, Japan). The photoelectrochemical measurements were carried out on a Shanghai Chenhua CHI-660D electrochemical system, using a conventional three-electrode cell. The counter and the reference electrodes were Pt wire and saturated calomel electrodes (SCE), respectively. The electrolyte solution was 0.5 M Na2SO4. The working electrodes were prepared as follows: 0.1 g of catalyst sample was mixed with 0.02 g PEG, and 0.5 mL distilled water was added to make slurry. The slurry was then injected onto a 1.0 cm × 1.0 cm ITO glass electrode, and these electrolytes were dried at 60 °C for 2 h and then calcined at 250 °C for 4 h.
Photocatalytic activity test
The photocatalytic activities of the samples were measured by the degradation of aqueous methylene blue under visible light. About 100 mg of a sample photocatalyst was first added to an aqueous solution of methylene blue (100 mL, 20 mg·L−1) in a beaker. The pH value of mixture was kept at 7.0. The solution was sonicated for 10 min and stirred for 1 h in the dark to reach the adsorption–desorption equilibrium. This is followed by irradiation of visible light obtained from 400 W Xe lamp with a cutoff filter (λ > 420 nm), and the average visible light intensity was 30 mW·cm−2. At certain time intervals, 3 mL aliquots were sampled and centrifuged to remove the photocatalyst particles. Then the filtrates were analyzed by recording variations in the absorption band maximum (663 nm) in the UV–Vis spectra of MB. The reproducibility was checked by repeating the measurements at least three times and was found to be within the acceptable limit (±5 %).
Results and discussion
XRD analysis
Figure 1 shows the powder XRD patterns of C3N4, TiO2, and TiO2/g-C3N4 composite. Two pronounced peaks were found in g-C3N4 at 27.4° and 13.1°, which can be indexed to (002) and (001) diffraction planes of the graphite-like carbon nitride [5, 21] and correspond to the characteristic interplanar staking peak of aromatic systems and the inter-layer structural packing, respectively. For TiO2 sample, its diffraction peaks are in good agreement with the anatase phase of TiO2 (JCPDS no. 21-1272, space group: I41/amd (141)) [30–32]. The TiO2/g-C3N4 composite presents a two-phase composition: g-C3N4 and TiO2, indicating that the hybridization of TiO2 with g-C3N4 did not change its structure. Additionally, it showed that the intensity of diffraction peaks of g-C3N4 decrease with the increasing of loading of TiO2.
FT-IR analysis
Figure 2 shows the Fourier transform infrared (FT-IR) spectra of C3N4, TiO2 and TiO2/g-C3N4 composite, respectively. In the spectrum of g-C3N4, the absorption band near 1640 cm−1 is attributed to C–N stretching, and the three at 1240, 1320, and 1403 cm−1 to aromatic C–N stretching [33, 34]. The band near 807 cm−1 corresponds to the out-of-plane bending vibration of the triazine units [2, 35]. A broad band near 3140 cm−1 corresponds to the stretching modes of terminal NH2 or NH groups at the defect sites of the aromatic ring [36]. As to the spectrum of pure TiO2, the weak absorption band centered at 443 cm−1 is attributed to the Ti-O-Ti stretching vibration [37]. As expected, all the TiO2/g-C3N4 samples show typical peaks of both compounds, confirming a successful combination of g-C3N4 and TiO2.
Microstructure analysis
Figure 3 shows the SEM images of TiO2, g-C3N4 and TiO2/g-C3N4 composites. From Fig. 3a, it can be seen that the obvious agglomeration of nanoparticles of TiO2. Also, the g-C3N4 sample shows obvious two-dimensional lamellar structures with wrinkle and irregular folding structures (seen from Fig. 3b). The images of 20 %TiO2/g-C3N4 and 40 %TiO2/g-C3N4 in Fig. 3c, d resemble a quite distinct morphology from TiO2 combined with layer-like texture of g-C3N4. This indicates that TiO2 was successfully deposited on the surface of g-C3N4. To confirm further the chemical composition of the as-prepared samples, the EDS map of 40 %TiO2/g-C3N4 was taken as shown in Fig. 3e. As can be obviously observed, C, N, O, and Ti elements is found, which verifies that the sample is composed of the four elements above.
The TEM images of g-C3N4, TiO2 and 40 %TiO2/g-C3N4 are shown in Fig. 4. As shown in Fig. 4a, tremella-like g-C3N4 is composed of a number of micro-scale layers, coinciding with the SEM observation. From Fig. 4b, TiO2 is composed of a lot of thinner nanosheets. It was reported that TiO2 with nanosheets structure could be synthesized by the solvothermal method when tetrabutyl titanate (TBT) is mixed with ethanol in the presence of acid [38]. Our results were similar to the literature reported [38]. For 40 %TiO2/g-C3N4 sample (Fig. 4c), TiO2 nanosheets were combined with g-C3N4 nanosheets. It can be observed from Fig. 4d that clear lattice fringes for the identification of crystallographic spacing, and the fringe spacing of ca. 0.35 nm matches well with the anatase TiO2 (101) plane. During solvothermal treatment, the nanosheet TiO2 assembled on the surface of g-C3N4 nanosheet to achieve a minimum surface energy [39]. This type of interfacial contact would facilitate the transfer of photo-generated charge carriers between g-C3N4 and TiO2 when used as a heterogeneous photocatalyst.
TG-DSC and BET analysis
To understand the TiO2 contents in the final products, thermogravimetric analysis was performed from room temperature to 1000 °C at a heating rate of 10 °C min−1 under air conditions. As shown in Fig. 5a and b, in the case of g-C3N4, a rapid weight loss region occurring from about 680 °C is observed, which can be assigned to the combustion of g-C3N4. It was reported that the tri-s-triazine ring structure and the high temperature condensation make g-C3N4 have a high thermal stability up to 600 °C [35]. The weight of the 20 %TiO2/g-C3N4 sample decreases rapidly in the temperature range 450–600 °C, indicating that the combustion of g-C3N4 occurred in this temperature range. However, it can be seen that a rapid weight loss temperature occurs at 564 °C, which is lower than single g-C3N4. This indicates the easy combustion of g-C3N4 when TiO2 deposited on the surface of g-C3N4. The contents of TiO2 in the composites of TiO2/g-C3N4 could be easily derived from the weight remainder after heating the samples over 1000 °C. TiO2 contents in 20 %TiO2/g-C3N4 sample were determined to be 14.35 %. TiO2 contents in other TiO2/g-C3N4 samples are listed in Table 1.
Figure 6 shows the nitrogen adsorption–desorption isotherms for 40 %TiO2/g-C3N4 at 77 K. The 40 %TiO2/g-C3N4 sample yields type IV isotherms, and an H3-type hysteresis loop, indicating the presence of slit-like pores and the formation of large mesopores and macropores [26, 40]. The pore distributions (inset in Fig. 6) are very broad, further indicating the existence of mesopores and macropores. The BET surface areas of TiO2/g-C3N4 with different loading of TiO2 are listed in Table 1. It is found that pure TiO2 (144 m2/g) has a larger surface area than that of g-C3N4(26.3 m2/g). When g-C3N4 nanosheets are facet coupled with TiO2 nanosheets, the surface areas of TiO2/g-C3N4 hybrids are increased with the increased content of TiO2.
UV–Vis DRS
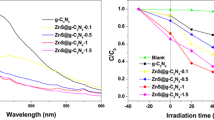
The optical absorption properties of g-C3N4, TiO2 , and TiO2/g-C3N4 hybrids with different TiO2 loading are investigated by UV–Vis DRS spectra in Fig. 7. The absorption onset of g-C3N4 is found at about 460 nm, corresponding to band gap energy of 2.7 eV. This result is consistent with other groups’ work [12, 41]. Meanwhile, the band gap of TiO2 is estimated to be about 3.2 eV with the absorption edge of 384 nm from its UV–Vis DRS spectrum in Fig. 7. After hybridization with TiO2, the absorbance of TiO2/g-C3N4 is extended to the visible region due to the presence of g-C3N4. The absorption edge of the hybrid are at 450 nm corresponding to band gap energy of 2.8 eV. It was also seen that the absorbance onset of the hybrid materials are unchanged; however, the absorption intensity of UV region increase with the loading amount of TiO2 increasing.
XPS analysis
XPS analysis was carried out to analyze further the surface chemical composition of the as-prepared samples and to identify the chemical status of the Ti, C, O, and N elements in the samples. Figure 8a illustrates XPS survey spectra of g-C3N4, TiO2, and 40 %TiO2/g-C3N4 samples and elements of C, N, O, Ti were detected. As seen in Fig. 8b, a signal deconvolution after Gaussian curve fitting three peaks centered at 284.6, 285.1, 287.5 eV can be observed for C 1 s of g-C3N4. The peak located at 284.6 eV can be assigned to the C–C or adventitious carbon. The peak at 287.5 eV can be ascribed to the N–C=N group of g-C3N4 and the peak at 285.7 eV can be attributed to C–O species from incompletely reacted oxygen-containing intermediates formed in the heating process [5, 12, 26]. For the 40 %TiO2/g-C3N4 sample, in comparison with that of pure g-C3N4, the peak at 285.7 eV is attributed to C-O species positive shift to 285.8 eV. This can be ascribed to the hybridization of TiO2 and g-C3N4. Figure 8c shows the N 1s spectrum of g-C3N4 and 40 %TiO2/g-C3N4 sample. The N 1s peak of g-C3N4 can be deconvoluted into two fitted peaks at 399.3 and 400.2 eV. The two peaks found can be ascribed to the sp2-hybridized nitrogen (C=N–C) at 399.3 eV and tertiary nitrogen (N–C3) at 400.2 eV, respectively [42]. The average C/N ratio calculated in our case is 0.72, in accordance with values reported for g-C3N4 obtained this way [2]. Figure 8d displays the XPS Ti 2p spectra of TiO2 and 40 %TiO2/g-C3N4 samples. For TiO2 sample, two peaks for the Ti 2p spectra were observed at 458.4 eV and 464.1 eV, assigned to Ti 2p3/2 and Ti 2p1/2, in good agreement with the presence of Ti(IV) [43]. With respect to the 40 %TiO2/g-C3N4 sample, the binding energy of Ti 2p3/2 and 2p1/2 is located at around 458.8 eV and 464.6 eV, respectively, positively shifted by 0.4 eV, suggesting the possible interaction between g-C3N4 and TiO2.
Photocurrent measurements
The photocurrent is widely regarded as the most efficient evidence for demonstrating the separation of electrons and holes in the composite photocatalysts [44, 45]. The relationship is commonly recognized as the higher the photocurrent, the higher the electron–hole separation efficiency, and thus the higher the photocatalytic activity. Figure 9 shows the photocurrent–time curves of TiO2, g-C3N4, and theTiO2/g-C3N4 hybrid materials with five on–off intermittent irradiation cycles. The 40 %TiO2/g-C3N4 sample presents higher photocurrent intensity, thus it can have a lower rate of electrons and holes recombination.
Photocatalytic activity
The photoinduced degradation of MB is carried out under visible light illumination in aqueous TiO2/g-C3N4 suspensions. Figure 10 displays the changes of the MB concentration versus the reaction time over the TiO2/g-C3N4 catalysts and the corresponding first-order kinetics plot by the equation of ln(C0/C) = k−1t, where C0 and C are the MB concentrations in solution at times 0 and t, respectively, and k−1 is the apparent first-order rate constant. As it can be observed in Fig. 10a, TiO2 presents a rather low degradation rate, which can be attributed to the self-sensitization of MB. At the same time, g-C3N4 also shows lower MB degradation after 3 h of reaction. Although g-C3N4 sample shows strong absorption of visible light, its low degradation efficiency may be due to the poor surface areas. However, the incorporation of different amounts of TiO2 clearly enhances the photocatalytic activity of g-C3N4 (Fig. 9b). It is easy to find that the photocatalytic activity increases with the amount of TiO2 increasing. The 40 %TiO2/g-C3N4 sample exhibits a maximum value; the degradation rate constant k−1 is 0.014 min−1 which is above 7 times as high as that of TiO2 (0.002 min−1) and 10 times than that of g-C3N4 (0.0012 min−1).
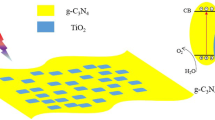
The as-prepared nanosheet 40 %TiO2/g-C3N4 hybrid photocatalyst showed excellent photocatalytic performance on the degradation of MB under visible light irradiation. Based on the characterization of structure and properties, the efficient photocatalytic activity should be mainly ascribed to the following reasons. Firstly, the structure characterizations have proven that the TiO2/g-C3N4 photocatalyst is a two-phase composite. Considering that the composite presents much higher photocatalytic activity than the component phase alone, it is reasonable that there might have synergetic effect between TiO2 and g-C3N4. To evaluate further the synergetic effect on the photocatalytic activity of the g-C3N4 and TiO2, g-C3N4 (0.0056 g) and TiO2 (0.044 g) were mechanically mixed with respect to the degradation of MB under visible light irradiation. The mechanically mixed sample degraded 23 % of the MB for 180 min of visible light irradiation. Its photocatalytic activity is much lower than that of the heterostructure sample 40 %TiO2/g-C3N4. This result suggests that the heterostructure between TiO2 and g-C3N4 plays a major role in the enhancement of photocatalytic activity. Actually, many researchers have attributed the effect to the efficient charge transfer at the interface of two semiconductors, which would result in an effective photoexcited electron–hole separation and, consequently, enhance the photocatalytic activity [26, 27]. The scheme (Fig. 11) for electron–hole separation and transport at the light-driven heterostructure TiO2/g-C3N4 photocatalyst as well as the MB degradation mechanism are discussed as follows. According to the relative research, the valence band (VB) potentials and the conduction band (CB) edge potentials of a semiconductor at the point of zero charge can be theoretically predicted [45]:
where X represents the electronegativity of the semiconductor, which is the geometric mean of the electronegativity of the constituent atoms, the X values for the g-C3N4 and TiO2 materials are 4.72 eV and 5.81 eV; Ec is the energy of free electrons on the hydrogen scale (here about 4.5 eV), Eg is the band gap energy of the semiconductor. According to our results of UV–Vis DRS, the Eg of g-C3N4 and TiO2 were estimated at 2.70 eV and 3.20 eV, respectively. Therefore, the CB and VB edge potentials of g-C3N4 were determined at −1.13 eV and +1.57 eV, respectively. The CB and VB edge potentials of TiO2 were calculated at −0.29 eV and +2.91 eV, respectively. As shown in Fig. 11.
Under visible light irradiation, g-C3N4 absorbed visible light and excited. The photogenerated electrons first transferred from valence band of g-C3N4 to conduction band. Since the CB edge potential of g-C3N4 (−1.13 eV) was more negative than that of TiO2 (-0.29 eV), the photoinduced electrons on g-C3N4 particle surfaces transferred easily to TiO2. Then, the electrons stored in the CB of TiO2 are trapped by O2 near the surface of TiO2 to formed superoxide radicals ·O2 − produces HOO· radicals, which can transform into H2O2 by further trapping electrons, and finally into ·OH radicals for the further photocatalytic degradation of MB [46]. Meanwhile, the photogenerated holes in TiO2, which have a high oxidation potential (+2.91 V vs. SHE), diffuse to the surfaces, and they directly degrade MB adsorbed or react with surface-bound H2O to produce hydroxyl radical species, ·OH. However, the valence band level of g-C3N4 (1.57 eV) is not sufficient for direct oxidation of H2O (2.27 eV) molecules through oxidation process [22]. Therefore, the photogenerated holes in g-C3N4 and some photogenerated holes in TiO2 transferred from the VB of TiO2 (+2.91 V vs. SHE) to the VB of g-C3N4 (+1.57 V vs. SHE) could directly degrade the MB.
As a result, the photocatalytic activity of TiO2/g-C3N4 hybrid is much higher than that of pure g-C3N4 or TiO2. However, with the content of TiO2 in TiO2/g-C3N4 being in excess, numerous photo-induced electrons and holes would recombine easily on the surface of TiO2. Therefore, 40 %TiO2/g-C3N4 sample exhibited the best photocatalytic activity among these different TiO2/g-C3N4 photocatalysts. The result of photocurrent measurement suggests that 40 %TiO2/g-C3N4 composite holds stronger ability in separation of electron–hole pairs than other samples, which is in good agreement with the mechanism suggested above.
Additionally, the thinner nanosheet structure of TiO2/g-C3N4 is benefit to suppressing recombination of electrons and holes. Since the structure of each thinner nanosheet determines that the distance of the photogenerated electrons and holes move from the bulk to the particle surface is much shorter than the spherical particles done, thus the photogenerated electrons and holes can transport to the TiO2 surface in a short time. Also, bulk recombination is reduced and the electrons and holes can be applied for photocatalytic degradation of MB efficiently.
Conclusions
In summary, we have successfully prepared the nanosheet TiO2/g-C3N4 composites by a facile solvothermal method. It was found that the nanosheets TiO2 was covered on the surface of g-C3N4. The influence of TiO2 ratio on the resulting photocatalytic activity of TiO2/g-C3N4 nanosheets has been investigated in detail by photocatalytic degradation of methylene blue under visible light irradiation. It was found that TiO2/g-C3N4 with 40 wt% TiO2 exhibited the best photocatalytic activity toward photocatalytic degradation of MB, about 7 times and 10 times higher than that of g-C3N4 and TiO2, respectively. The significantly improved photocatalytic activity of the nanocomposite is mainly due to the nanosheets' structure and superior electron transport property. This work demonstrates that TiO2/g-C3N4 nanosheets is a very promising candidate for the development of high-performance photocatalysts with high visible-light photocatalytic activity that could find potential applications in water treatment and dye sensitized solar cells.
References
X.C. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J.M. Carlsson, K. Domen, M. Antonietti, Nat. Mater. 76, 8 (2009)
X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu, M. Antonietti, J. Am. Chem. Soc. 1680, 131 (2009)
S.B. Yang, Y.J. Gong, J.S. Zhang, L. Zhan, L.L. Ma, Z.Y. Fang, R. Vajtai, X.C. Wang, P.M. Ajayan, Adv. Mater. 2452, 25 (2013)
Y. Zheng, J. Liu, J. Liang, M. Jaroniec, S.Z. Qiao, Energy Environ. Sci. 6717, 5 (2012)
S.C. Yan, Z.S. Li, Z.G. Zou, Langmuir 10397, 25 (2009)
M. Zhang, J. Xu, R. Zong, Y. Zhu, Appl. Catal. B 229, 147 (2014)
L. Ge, C.C. Han, J. Liu, Y.F. Li, Appl. Catal. A 215, 409–410 (2011)
G. Liu, P. Niu, C.H. Sun, S.C. Smith, Z.G. Chen, G.Q. Lu, H.M. Cheng, J. Am. Chem. Soc. 11642, 132 (2010)
Y.J. Zhang, T. Mori, J.H. Ye, M. Antonietti, J. Am. Chem. Soc. 6294, 132 (2010)
Y. Wang, Y. Di, M. Antonietti, H.R. Li, X.F. Chen, X.C. Wang, Chem. Mater. 5119, 22 (2010)
Y.B. Wang, J.D. Hong, W. Zhang, R. Xu, Catal. Sci. Technol. 1703, 3 (2013)
S.C. Yan, S.B. Lv, Z.S. Li, Z.G. Zou, Dalton Trans. 1488, 39 (2010)
L. Ge, C.C. Han, J. Liu, Appl. Catal. B Environ. 100, 108–109 (2011)
J. Sun, J. Song, M.A. Gondal, S. Shi, Z. Lu, Q. Xu, X. Chang, D. Xiang, K. Shen, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1789-0
Y.J. Wang, R. Shi, J. Lin, Y.F. Zhu, Energy Environ. Sci. 2922, 4 (2011)
S. Chu, Y. Wang, Y. Guo, J.Y. Feng, C.C. Wang, W.J. Luo, X.X. Fan, Z.G. Zou, ACS Catal. 912, 3 (2013)
F. Goettmann, A. Fischer, M. Antonietti, A. Thomas, Angew. Chem. Int. Ed. 4467, 45 (2006)
Y.J. Zhang, A. Thomas, M. Antonietti, X.C. Wang, J. Am. Chem. Soc. 50, 131 (2009)
Y.W. Zhang, J.H. Liu, G. Wu, W. Chen, Nanoscale 5300, 4 (2012)
X. Zhou, F. Peng, H. Wang, H. Yu, Y. Fang, Appl. Catal. B Environ. 718, 142–143 (2013)
L. Gu, J.Y. Wang, Z.J. Zou, X.J. Han, J. Hazard. Mater. 216, 268 (2014)
H.J. Yan, H.X. Yang, J. Alloys Compd. 26, 509 (2011)
X. Wang, W.Y. Yang, F.T. Li, Y.B. Xue, R.H. Liu, Y.J. Hao, Ind. Eng. Chem. Res. 17140, 52 (2013)
Z. Huang, Q. Sun, K. Lv, Z. Zhang, M. Li, B. Li, Appl. Catal. B Environ. 420, 164 (2015)
J. Lei, Y. Chen, L. Wang, Y. Liu, J. Zhang, J. Mater. Sci. 3467, 50 (2015)
J.G. Yu, S.H. Wang, J.X. Low, W. Xiao, Phys. Chem. Chem. Phys. 16883, 15 (2013)
J. Lei, Y. Chen, F. Shen, L. Wang, Y. Liu, J. Zhang, J. Alloys Compd. 328, 631 (2015)
C. Miranda, H. Mansilla, J. Yánez, S. Obregón, G. Colón, J. Photochem. Photobiol. A 16, 253 (2013)
H. Li, L. Zhou, L. Wang, Y. Liu, J. Lei, J. Zhang, Phys. Chem. Chem. Phys. 17406, 17 (2015)
J. Chen, S. Qin, Y. Liu, F. Xin, X. Yin, Res. Chem. Intermed. 637, 40 (2014)
Y. You, Z. Jiang, Y. Zhang, C. Ge, R. Li, Res. Chem. Intermed. 3079, 41 (2015)
K. Zhao, Z. Wu, R. Tang, Y. Jiang, Y. Lu, Res. Chem. Intermed. 4405, 41 (2015)
S.W. Bian, Z. Ma, W.G. Song, J. Phys. Chem. C 8668, 113 (2009)
L. An, G. Wang, Y. Cheng, L. Zhao, F. Gao, Y. Tian, Res. Chem. Intermed. (2014). doi:10.1007/s11164-014-1836-x
A. Eshaghi, S. Hayeripour, A. Eshaghi, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2161-8
F. Dong, L. Wu, Y. Sun, M. Fu, Z. Wu, S.C. Lee, J. Mater. Chem. 15171, 21 (2011)
J. Yu, H. Yu, B. Cheng, X. Zhao, J. Yu, W. Ho, J. Phys. Chem. B 13871, 107 (2003)
W. Wang, Y. Ni, C. Lu, Z. Xu, Mater. Lett. 161, 111 (2013)
C. Han, Y. Wang, Y. Lei, B. Wang, N. Wu, Q. Shi, Q. Li, Nano Res. 1199, 8 (2015)
Z. Liu, X. Chen, Y. Wang, Q. Tao, Res. Chem. Intermed. 1683, 41 (2015)
K. Sridharan, E. Jang, T.J. Park, Appl. Catal. B Environ. 718, 142–143 (2013)
K. Nagaveni, M.S. Hegde, N. Ravishankar, G.N. Subbanna, G. Madras, Langmuir 2900, 20 (2004)
M. Kong, Y.Z. Li, X. Chen, T.T. Tian, P.F. Fang, F. Zheng, X.J. Zhao, J. Am. Chem. Soc. 16414, 133 (2011)
X. Lü, J. Shen, D. Fan, J. Wang, Z. Cui, J. Xie, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-1953-1
K. Dai, L. Lu, C. Liang, Q. Liu, G. Zhu, Appl. Catal. B Environ. 331, 156–157 (2014)
A. Fujishima, T.N. Rao, D.A. Tryk, J. Photochem. Photobiol. C 1, 1 (2000)
Acknowledgments
This work has been supported by China Postdoctoral Science Foundation (2013M542415); National Nature Science Foundation of China (41305112); Youth Foundation of Xinjiang Uygur Autonomous Region (2013211B37); the “Cross-Cooperation Program for Creative Research Teams” of Chinese Academy of Sciences, and “Western Light” Program of Chinese Academy of Sciences (XBBS201126); Research projects of Education Department in Xinjiang Uygur Autonomous Region (XJEDU2011I56).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, Y., Tao, L., Zhao, J. et al. TiO2/g-C3N4 nanosheets hybrid photocatalyst with enhanced photocatalytic activity under visible light irradiation. Res Chem Intermed 42, 3609–3624 (2016). https://doi.org/10.1007/s11164-015-2234-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2234-8