Abstract
Cyclamen aldehyde is a fragrant substance with the scent of cyclamen or lily-of-the-valley. In this work, the desired cyclamen aldehyde was prepared by two-step synthesis. At the first step, aldol condensation of 4-isopropylbenzaldehyde and propanal was carried out. The influence of used catalyst (potassium hydroxide and sodium methoxide) and propanal amount were tested. Propanal was used in excess and it was added to the reaction mixture dropwise (to prevent its self-condensation to 2-methylpent-2-enal). Resulting mixture of 4-isopropylbenzaldehyde and forcyclamen aldehyde was hydrogenated using different Ru/C catalysts. The products detected in hydrogenation reaction mixture were: desired cyclamen aldehyde, cyclamen alcohol and forcyclamen alcohol. The influence of catalyst type and amount, reaction temperature and hydrogen pressure on the reaction course was tested. The highest yield (19.4 %) was obtained using pressure 10 MPa, temperature 110 °C and 2 wt% of catalyst Ru/C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
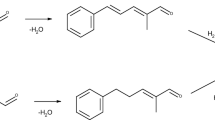
Cyclamen aldehyde (2-methyl-3-(4-iso-propylphenyl)propanal) is used as a fragrant substance for its fresh flower smell (cyclamen, lilac and violet). It is used as a surrogate of the currently utilized favorite and cheap analogue of cyclamen aldehyde, namely lily aldehyde (2-methyl-3-(4-tert-butylphenyl)propanal), which was classified as a possible mutagenic agent [1]. Lily aldehyde is frequently used in detergents, soaps, etc. Another use of cyclamen aldehyde is as an intermediate in fungicide synthesis [2]. Cyclamen aldehyde can be prepared by aldol condensation of 4-isopropylbenzaldehyde and propanal (Fig. 1), which is usually performed using inorganic basic catalyst (NaOH, KOH) [3, 4], but some research using heterogeneous catalyst are also available [5].
A product of aldol reaction, forcyclamen aldehyde (2-methyl-3-(4-iso-propylphenyl)prop-2-enal), can be hydrogenated using different metal catalysts, e.g., palladium on active carbon, or different metals (nickel, rhodium, palladium) on Al2O3 [5, 6]. Hydrogenation of forcyclamen aldehyde can give three possible products: 3-(4-isopropylphenyl)-2-methylprop-2-en-1-ol (forcyclamen alcohol), which can be isomerized to desired cyclamen aldehyde, e.g., using Fe(CO)5and UV irradiation [7], the desired cyclamen aldehyde or the undesired product of subsequent cyclamen aldehyde hydrogenation, cyclamen alcohol (3-(4-isopropylphenyl)-2-methylpropan-1-ol) (Fig. 2). Some syntheses leading to non-racemic cyclamen aldehyde have also been reported [8, 9].
Materials and methods
4-Isopropylbenzaldehyde (Acros), potassium hydroxide (Penta), sodium methoxide (Sigma Aldrich), methanol (Penta), N,N′-dimethylformamide (Penta), toluene (Penta), isopropylalcohol (Penta), diethylether (Penta), and different ruthenium catalysts (Johnson Matthey; Table 1) were purchased from commercial sources and used without purification. Propanal (Sigma Aldrich) was freshly distilled before reaction (b. p. 46 °C).
Aldol reaction
The flask was charged with the catalyst and the solvent (3 ml) and 4-isopropylbenzadehyde (1 ml, iPB) were added. The total amount of propanal was added dropwise to the mixture in four portions at 0, 30, 60 and 90 min of the reaction. Except for monitoring the influence of temperature, all experiments were performed at room temperature (25 °C). When a higher temperature was used, the flask was equipped with a condenser. Samples were neutralized by acetic acid, diluted with ethanol, centrifuged and analyzed. At the end of the reaction, the reaction mixture was also neutralized using acetic acid, washed with water, extracted using diethylether and evaporated.
Hydrogenation
Hydrogenations were performed in an autoclave (volume 160 ml; Parr). A mixture containing 4-isopropylbenzaldehyde (cca 10 wt%) and forcyclamen aldehyde (FCA, ca. 90 wt%) was used for the hydrogenation. Autoclave was charged with an appropriate amount of catalyst (active metal Ru; Table 1), 10 g of a mixture of 4-isopropylbenzadehyde and forcyclamen aldehyde and 90 ml of solvent, isopropylalcohol. Samples taken during the reaction were centrifuged and analyzed.
Both samples from the aldol reaction and hydrogenation were analyzed using a Shimadzu GC 17A chromatograph fitted with nonpolar column ZB-5 (60 m, 0.32 mm diameter, 0.25 μm film) and FID. Composition of reaction mixture was also monitored using GC-MS Shimadzu 2010 fitted with nonpolar column.
Results and discussion
Aldol condensation
Reaction course
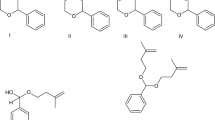
All results were compared after 180 min of the reaction (after 180 min from the reaction starting, the maximal 4-isopropylbenzaldehyde conversion was reached in all cases; Fig. 3). In all cases, selectivity decreased with conversion, which was caused by formation of side products (Fig. 4a, b). The formation of these side products is also the reason for stopping the conversion of iPB because the propanal was consumed by the side reactions. The side products identified in the reaction mixture were the products of the subsequent aldol reaction between forcyclamen aldehyde and propanal [2,4-dimethyl-3-hydroxy-5-(4-isopropylphenyl)pent-4-enal(III.), 2,4-dimethyl-5-(4-isopropylphenyl)pent-2,4-dienal(IV)]. The product of aldolization [3-hydroxy-2-methyl-3(4-isopropylphenyl)propanal(I.)] between propanal and 4-isopropylbenzaldehyde was also present in the reaction mixture (content lower than 2 %), and was directly dehydrated to create the desired forcyclamen aldehyde (II).
Due to the fact that iPB and propanal were used in the molar ratio 1:1, no subsequent reactions took place and total conversion of iPB was not reached because there was no other reactant in the reaction mixture. In Fig. 4b, the selectivity as a function of iPB conversion is illustrated. The apparent formation of by-products not before 40 % conversion is given by the detection limit of GC (5 %).
Influence of catalyst type and amount
Two types of basic catalysts were studied: 36 wt% water solution of potassium hydroxide (Fig. 5a) and 33 wt% methanolic solution of sodium methoxide (Fig. 5b). Ther heterogeneous system containing potassium hydroxide in water was tested for comparison and because this synthesis type was used in the literature [4]. Sodium methoxide seems to be a more active catalyst than potassium hydroxide (concerning the same molar ratio to substrate). The higher activity of sodium methoxide was caused by its higher basicity and the use of the homogeneous reaction system compared to potassium hydroxide (two-phase system). Considering the same 4-isopropylbenzaldehyde conversion (conversion of propanal was not calculated because this reaction compound was in stoichiometric excess), the selectivity was almost the same. Using larger amounts of the catalyst in the case of sodium methoxide compared with the molar ratio substrate:catalyst 1:0.05 led to the formation of larger amounts of side products, while the highest conversion remained the same (96 %). TOF at 10 min of the reaction was also calculated (Table 2). TOF was evaluated as the initial rate of the reaction. Usually, the increase of reaction rate with increasing catalyst amount is observed (that is the case of molar ratios 1:0.03 and 1:0.05; in these two cases, the formation of side products is suppressed). In the case of the studied reaction with increasing catalyst amount (twice in the case 1:0.05–1:0.5), the autocondensation of propanal started to be the preferred reaction due to the high amount of catalyst.
Influence of propanal amount
The influence of propanal amount was examined using two ratios: 4-isopropylbenzadehyde:sodium methoxide of 1:0.1 (Fig. 6a) and 1:0.05 (Fig. 6b). From these figures, it can be seen that the results are moreover in the range of measurement error. The propanal amount significantly influenced the content of propanal autocondensation product in the reaction mixture, but the main factor influencing the 4-isopropylbenzaldehyde conversion and selectivity was the amount of catalyst discussed above. However, TOF values were also calculated (Table 3), and they showed that the excess of propanal increased the reaction rate at the beginning of the reaction.
Influence of the solvent
The influence of the solvent type was monitored (Fig. 7). Besides methanol, three different solvents with different polarities were used. With the higher polarity of the solvent, 4-isopropylaldehyde conversion increased. When a nonpolar solvent, toluene, was used, more side products (high boiling) originated in the reaction mixture. This means that the polarity of the solvent influences the formation of 2-methylpent-2-enal in the reaction mixture. TOF calculations (Table 4) showed that other solvent properties (e.g., basicity that can work synergically with the catalyst) influenced the reaction course. Using N,N′-dimethylformamide, more propanal autocondensation product originated in the reaction mixture.
Hydrogenation
Hydrogenation of forcyclamen aldehyde results in the desired cyclamen aldehyde (Fig. 8), which is subsequently hydrogenated to the undesired cyclamen alcohol. Another by-product is forcyclamen alcohol. Hydrogenation was performed using the reaction mixture containing ca. 10 % of 4-isopropylbenzaldehyde and 90 % of forcyclamen aldehyde obtained in the previous step. Different ruthenium catalysts were studied: Type 97 (Ru/C, ruthenium content 4.62 %, moisture 55 wt%), Type 698 (Ru/SiO2, ruthenium content 4.84 %, moisture 2 %) and Type 600 (Ru/C, ruthenium content 4.9 % moisture 50 wt%).
Reaction course
The reaction course of hydrogenation is recorded in Fig. 8. With forcyclamen aldehyde, the amount was decreasing until 180 min of the reaction. Cyclamen aldehyde acted as an intermediate, with maximum content around 70 % conversion of forcyclamen aldehyde. Cyclamen aldehyde was continuously hydrogenated to cyclamen alcohol. Forcyclamen alcohol was also identified in the reaction mixture in a small amount because it is directly hydrogenated to cyclamen alcohol.
Influence of catalyst type
Using the three types of catalyst led to almost the same results: 20–23 % relative concentration of cyclamen aldehyde at 300 min of the reaction under the same reaction conditions (Table 5, rows 7, 8, 9). For the comparison, the two characteristic values (Table 5) were chosen: the time to reach 40 % forcyclamen aldehyde conversion and the possible maximum yield (maximum selectivity × matching 4-isopropylbenzaldehyde conversion in percent). It can be seen that the better results were obtained using the ruthenium catalyst supported on SiO2; the catalyst with the supporting active carbon containing about 50 wt% of moisture seems to be the more active because the time for 40 % conversion was lower and so was the possible maximal yield. The low yield means that the desired cyclamen aldehyde is very rapidly subsequently hydrogenated to the undesired cyclamen alcohol. For the following experiments, the catalyst Type 600 was chosen due to the industry requirements.
Influence of temperature
Using lower temperatures (80, 100 °C), the rate of subsequent hydrogenation to cyclamen alcohol was significantly lower. The reaction rate was higher using higher temperatures (Table 5, rows 6, 9, 10, 11). The maximal yield was also higher using higher temperatures. This was caused by the higher rate of C=C double bond hydrogenation in the case of FCA compared with the rate of hydrogenation of the C=O double bond. The difference between these hydrogenation rates was more significant using the higher temperatures.
Influence of pressure
The influence of three different pressures on the reaction course was monitored (Table 5, rows 10, 13, 14). With the increasing pressure, the reaction rate also increased. The existence of nonlinear dependence of yield on the pressure could be explained by the fact that, at low pressure as in the case of low temperature, the hydrogenation rate of the C=C double bond and the C=O double bond are not so different as in the case of higher pressures. But at even higher pressure (15 MPa), the amount of hydrogen in the reaction mixture increased and also increased the rate of C=O hydrogenation.
Influence of catalyst amount
Different amounts of catalyst were used to perform the reaction. The supposed trend in the reaction rate was detected (Table 5, rows 1, 5, 13, 15). With increasing catalyst amount, the time for 40 % conversion decreased which means a higher reaction rate. The influence on the selectivity is not so apparent, but with higher catalysts amounts (2 and 4 %), higher yields could be obtained.
From the results, it was obvious that the main influence on the yield was the amount of hydrogen in the reaction. The amount of hydrogen was given by the temperature (with increasing temperature, the solubility of hydrogen in the reaction mixture increased) and by the pressure.
Conclusions
The optimal reaction conditions for the maximum yield of forcyclamen aldehyde were found. The product may be used for the preparation of cyclamen aldehyde which is a desirable compound in the fragrance industry. Two basic catalysts were studied for the first step (forcyclamen aldehyde preparation) of the reaction and three Ru-based hydrogenation catalysts were tested in the second step (cyclamen aldehyde preparation). The best reaction conditions were found as follows:
Aldol reaction: The best result (92 % selectivity to forcyclamen aldehyde, 90 % conversion of 4-isopropylbenzaldehyde, i.e. 82,8 % yield of desired product) was obtained using the following reaction conditions: molar ratio 4-isopropylbenzadehyde:sodium methoxide:propanal 1:0.05:1, 25 °C, solvent methanol, 180 min of reaction. Sodium methoxide was preferred as a catalyst toward potassium hydroxide, because of a higher reaction rate using the same amount of catalyst. Atthe the the the higher temperatures, the formation of undesired products occurred, and a higher molar excess of propanal led only to the increase of the reaction rate and production of 2-methylpent-2-enal.
Hydrogenation: The desired product, cyclamen aldehyde, was subsequently hydrogenated to cyclamen alcohol, and therefore coefficient maximal selectivity to cyclamen aldehyde × 4-isopropylbenzaldehyde conversion was taken as the most important criteria. The highest selectivity (51 % at the 4-isopropylbenzaldehyde conversion 38 %, i.e. yield 19.4 %) was obtained at 30 min of reaction using the following conditions: 120 °C, 5 MPa and 2 wt% of catalyst Type 600.
References
U. Huchel, et at. DE102009001570A1, Henkel (2010)
Ullmann’s Encyclopedia of Industrial Chemistry, Aldehydes, Araliphatic (Wiley-VCH, New York, 2013)
A. Knorr, A. Weissenborn, US1844013, Winthrop chemical company (1932)
W.C. Meuly, US2102965A, E. I. du Pont de Nemours Company (1937)
S.Y. Shiaou, A. Ko, J. Chin. Chem. Soc. 53, 1539 (2006)
A.M. Pak, Zh. Prikl. Khim. 59(5), 1135–1138 (1986)
H. Cherkaouia, M. Soufiaouib, R. Grée. Tetrahedron. 57(2), 2379–2383 (2001)
D. Limnios, C.G. Kokotos, RSC Adv. 3, 4496–4499 (2013)
V. Beghetto, U. Matteolia, A. Scrivantia, M. Bertoldinia, Sci. at Ca’ Foscari 1, 20–24 (2012)
J. Catalán, C. Díaz, V. Lopéz, P.Pérez, J.G. de Paz, J.G. Rodrigíguez, Liebigs Ann. 1785–1794 (1996)
Acknowledgment
Word of thanks to the Ministry of education, youth and sports for financial support from specific university research (MSMT No 20/2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vrbková, E., Skýpala, T., Vyskočilová, E. et al. Cyclamen aldehyde synthesis: aldol condensation followed by hydrogenation over ruthenium catalyst. Res Chem Intermed 41, 9195–9205 (2015). https://doi.org/10.1007/s11164-015-1948-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-1948-y