Abstract
In this study, as a low-cost bio-sorbent prepared from agricultural waste, Platanus orientalis leaf powder (POLP) was used for the removal of Cu(II) ions from aqueous solutions. Morphology of the adsorbent surface and the nature of the possible POLP and Cu(II) interactions were examined by SEM micrograph and FTIR technique, respectively. The influence of various operating parameters such as temperature, pH, contact time, and initial concentration of Cu(II) ions on the leaf powder adsorption capacity was investigated. The adsorption was investigated using different isotherms and kinetic models. The results showed that the adsorption isotherm data were fitted well to the Langmuir isotherm and adsorption kinetics followed the pseudo-second-order model. The thermodynamic parameters, such as ΔG°, ΔH°, and ΔS°, were also evaluated. The calculated parameters indicated that the adsorption process was spontaneous and endothermic in nature. The adsorption capacity of Cu(II) onto POLP was found to be as high as 49.94 mg g−1, which is higher than the adsorption capacity of many recently studied potential adsorbents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals such as copper, nickel, chromium, and zinc are widely used in various industries [1]. These are stable and persistent in the environment, and thus the removal of these metals has become a significant issue. Although Cu(II) is a necessary microelement for life, an excessive amount of this metal produces many toxic and harmful effects for living organisms [2, 3]. Therefore, it is necessary to remove Cu(II) and other harmful heavy metals from effluents [1].
The common methods for the removal of heavy metals from wastewater are chemical precipitation [4], biological process [5], membrane process [6], electrochemical treatment [7], ion exchange [8], solvent extraction [9, 10], ultra filtration [11], and adsorption [12–19]. However, applications of these methods have been limited in comparison with adsorption, because of high capital and operation costs, and ineffective means of disposal of resulting sludge [20].
Several different-origin adsorbents have been used for Cu(II) removal from industrial effluents [21, 22]. Currently, the cost of processes has become an important issue, so finding a low-cost and efficient adsorbent can affect the total cost of the process. Natural adsorbents such as algae, bentonite, wheat straw, and pine bark have the ability to be an effective Cu(II) adsorbent [1, 3, 15, 21, 23, 24].
Platanus orientalis is a large, deciduous tree, with a native range spreading from southeastern Europe to India, including Iran and Turkey [25]. In this study, the use of powdered leaf of P. orientalis as an adsorbent was tested for Cu(II) removal from aqueous solutions. The effects of contact time, pH, initial Cu(II) concentration and temperature on the adsorption were investigated. Kinetics, isotherms, and thermodynamic studies related to the process were also performed.
Materials and methods
Preparation of adsorbent
Platanus orientalis leaves were collected from the locally available plant in Mellat Park, Tehran, Iran. The leaves were washed several times with distilled water to remove the dust and other impurities from their surfaces. Leaves were dried at room temperature. The dried leaves ground in a domestic grinder and sieved. The size of the POLP particle is about 200 µm. The color of the sieved powder was removed by boiling the aqueous leaf powder at 100 °C for 2 h. The resulting dried P. orientalis leaves powder (POLP) was kept in a glass bottle for use in adsorption studies.
Characterization of POLP
FTIR study was carried out on an FTIR spectrometer (the Perkin Elmer Spectrum RX1) to analyze the functional groups, using potassium bromide (KBr). The specific surface area was measured on a NOVA Station B surface area analyzer using the Brunauer–Emmett–Teller (BET) method. In addition, the average pore diameter and total pore volume were also determined. Morphology of adsorbents was investigated by VEGA\\TESCAN scanning electron microscope operated at 30-kV accelerated voltage.
Adsorption experiments
A stock solution (500 mg l−1) of Cu(II) was prepared by dissolving proper amount of CuSO4·5H2O (Merck) in double-distilled water. Batch adsorption experiments were carried out to investigate the effect of initial Cu(II) concentration, contact time, pH, and temperature on the adsorption of Cu(II) on POLP by varying the parameters under study and keeping other parameters constant. In each experiment, 50 mg of POLP was added to 50 ml of Cu(II) solution, and the pH was adjusted by using 0.1 M NaOH (98 %, Merck) or 0.1 M HCl (Merck). The resulting solution was agitated at 125 rpm on a stirrer at constant temperature and centrifuged at 5,000 rpm for 15 min. The Cu(II) concentration in supernatant solution was determined at characteristic wavelength of 620 nm [26], by a double-beam UV–visible spectrophotometer (Shimadzu UV-1800). Each adsorption sample was analyzed twice. Then the amounts of Cu(II) adsorbed per unit mass of adsorbent were calculated from the differences between the initial and final Cu(II) concentrations in solution by the following equation:
where q t (mg g−1) is the removal amount of Cu(II) at equilibrium, C 0 and C e (mg l−1), initial and final concentration of Cu(II) solution, V (l), the volume of Cu(II) solution used, M (g), the weight of the adsorbent used in the present study.
Results and discussion
Characterization
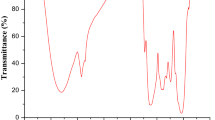
The FTIR technique is an important tool to identify functional groups, which are capable of adsorbing metals. FTIR spectra of POLP, before and after adsorption of Cu(II), are shown in Fig. 1. The broad absorption peaks observed at 3,428 cm−1 correspond to the O–H stretching vibration of alcohols, phenols, and carboxylic acids as in pectin, cellulose, and lignin, thus showing the presence of “free” hydroxyl groups on the POLP surface [27]. The peak located at 2,924 cm−1 was assigned to the stretching vibration of C–H bond in methyl group. The peak at 1,612 cm−1 was characteristic of carbonyl group stretching. The peak at 1,056 cm−1 was assigned to C–O–C stretching of cellulose present in PLOP [28].Characteristic changes observed in the FTIR spectrum of PLOP after adsorption of Cu(II) indicated that some peaks were shifted or disappeared, and new peaks were also detected. Changes in vibration wavenumbers of the adsorbate as a result of adsorption can be due to changes in the bond strength. Bands shifting to lower wavenumbers indicates bond weakening, while a shift to higher wavenumbers indicates an increase in bond strength [29].
The surface area and total pore volume of the POLP are calculated by the standard Brunauer–Emmett–Teller (BET) method and Horvath–Kawazoe (HK) method. The average pore diameter, surface area, and total pore volume were 14.16 nm, 15.44 m2 g−1, and 6.377 × 10−3 cm3 g−1, respectively.
Figure 2 shows the SEM micrographs of POLP. As observed, POLP has an irregular, rough, and porous surface that provides suitable binding sites for the Cu(II) molecule.
Removal of Cu(II)
Effect of solution pH on Cu(II) adsorption
The effect of pH on the Cu(II) adsorption on POLP is shown in Fig. 3. Due to the precipitation of Cu(II) at pH >6, the pH range of 2–6 was chosen. The solution pH affects not only the surface charge of the adsorbent but also the degree of ionization and speciation of the metal in solution. The Cu(II) uptake was found to increase with increasing pH, and it increased from 42.00 to 49.94 mg g−1 for the increase in pH from 2 to 6. Metals give positively charged ions when dissolved in water. Thus, in acidic mediums (lower pH), the positively charged surface of sorbent tends to oppose the adsorption of the cationic adsorbate [25].
Effect of contact time on Cu(II) adsorption
The effect of contact time on the adsorption process is shown in Fig. 4. As observed, the equilibrium was reached after 180 min. The adsorption process could be described as a two-stage kinetic behavior, which was a rapid initial adsorption, then followed by a slow rate. It is obvious that the Cu(II) adsorption rate is rapid at the first 40 min, indicating a high affinity between the Cu(II) molecules and the POLP surface due to fact that initially all the adsorbent sites are vacant and the solute concentration gradient is very high. The decrease in the adsorption rate toward the end of experiment is due to a decrease in the number of vacant sites of adsorbent and metal concentrations [30]. The decreased adsorption rate indicates the possible monolayer formation of metal on the adsorbent surface [27].
Effect of temperature on Cu(II) adsorption
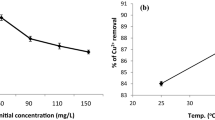
To determine the effect of temperature, adsorption experiments were carried out at 25, 40, 50, and 60 °C, respectively. As shown in Fig. 5, the adsorption capacity slightly enhances with the increase in temperature. The maximum adsorption capacity increases from 49.88 to 49.94 mg g−1 with the increase in temperature from 25 to 60 °C, which illustrates that the adsorption process of Cu(II) onto POLP is endothermic.
Adsorption kinetics
To evaluate the kinetic mechanism of the adsorption process, the pseudo-first-order, pseudo-second-order, and intraparticle diffusion models were applied to fit the experimental data.
The pseudo-first-order rate model is defined as the following equation [31]:
where k 1 (min−1) is the Lagergren rate constant of pseudo-first order, q t (mg g−1) and q e (mg g−1) are the amounts of Cu(II) adsorbed at time t (min) and equilibrium. As observed in Fig. 6a, the values of k 1 and q e were determined from the intercept and the slope of the plot of log (q e − q t ) versus t.
The pseudo-second-order rate model is as follows [31]:
where k 2 (g mg−1 min−1) is the rate constant of pseudo-second order. As observed in Fig. 6b, the values of k 2 and q e were determined from the intercept and the slope of the plot of t/q t versus t.
The initial adsorption rate, h [mg (g−1 min−1)] is frequently used as a measure of adsorption rate. For the pseudo-second-order equation, h can be determined by the following equation [30]:
The adsorbate species are probably transported from the bulk into the solid phase through an intraparticle diffusion. The possibility of intraparticle diffusion, which is often the rate-limiting step in many adsorption processes, was explored by using the intraparticle diffusion model [32]:
where C is the intercept and k id is the intraparticle diffusion rate constant (mg g−1 min−1/2), which was determined from the intercept and the slope of the plot of q t versus t 1/2 (Fig. 6c).
The kinetic data were analyzed by the kinetic equation mentioned above, and evaluated parameters are summarized in Table 1. The values of correlation coefficient (R 2) obtained from pseudo-second-order kinetics (0.9968) were higher than those from pseudo-first-order kinetics (0.9522) and intraparticle diffusion model (0.9370). The values of q e,2cal were closer to experimentally obtained (q e,exp), and thus pseudo-second-order kinetics could reasonably describe the Cu(II) adsorption on POLP.
Adsorption isotherms
Figure 7 shows the POLP adsorption capacity with different Cu(II) concentrations at equilibrium. The adsorption capacity increases with the increasing Cu(II) equilibrium concentration and then reached the saturation plateau. This trend may be due to the extent of a driving force of concentration gradients with the increase of the Cu(II) concentration and then, due to the amount construction of POLP, the adsorption tended to be a saturated state [31].
The adsorption parameters were evaluated using Langmuir, Freundlich, and Temkin isotherm models. The Langmuir isotherm supposing monolayer sorption onto a surface with a finite number of identical sites. The equation of the Langmuir isotherm is as follows [27]:
where q e is metal concentrations onto the sorbent (mg g−1) at equilibrium and q m is maximum adsorption capacity (mg g−1). C e is metal concentration at equilibrium in solution (mg l−1). K L is Langmuir constant (L mg−1) related to the affinity of binding sites and the free energy of sorption. A straight line was obtained when 1/q e was plotted against 1/C e. The values of q m and K L were calculated from the slopes and intercepts.
The empirical Freundlich equation for adsorption on a heterogeneous surface is commonly represented by [33]:
where K F (((mg g−1)/(mg L−1)1/n)) and n are the Freundlich constants, indicating the sorption capacity and the sorption intensity of the system, respectively. These parameters were determined from the plot of ln q e versus ln C e.
The enthalpy of the adsorption and the adsorbent–adsorbate interactions were evaluated by using the Temkin isotherm model. The Temkin isotherm equation in linear form is shown as [30]:
where T is the absolute temperature in Kelvin. R is gas universal constant [8.314 J (mol−1 K−1)], b T (J mol−1) is the Temkin constant related to enthalpy of adsorption and K T (L g−1) is equilibrium binding constant. The Temkin constants b T and K T are calculated from the slope and intercept of q e versus ln C e.
The adsorption equilibrium data were evaluated by the aforementioned isotherm equations, and corresponding calculated parameters are tabulated in Table 2. As observed, the value of R 2 obtained from the Langmuir isotherm equation (0.9802) was higher than that from the Freundlich (0.6986) and Temkin (0.8567) isotherm equations. This fact indicated that the Langmuir isotherm model, which assumes a monolayer coverage and uniform activity distribution on the sorbent surface, could well describe the Cu(II) adsorption on POLP.
Adsorption thermodynamic
Thermodynamic parameters such as change in standard free energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°) evaluated for Cu(II) adsorption onto POLP. These parameters were calculated using the following equations [34]:
The values of ΔH° and ΔS° were calculated from the slope and intercept of the plot of ln K D versus 1/T (Fig. 8). Standard Gibbs free energy change of sorption (ΔG°) was evaluated using Eq. (10).
The calculated thermodynamic parameters are tabulated in Table 3. The negative Gibbs free energy change (ΔG°) and the positive value of ΔH°, indicate that the adsorption process is spontaneous and endothermic, respectively. Further, the positive value of entropy change (ΔS°) reflects the random nature of the process at the solid–solution interface and the affinity of POLP for Cu(II) adsorption [27, 34].
Comparison of various adsorbents
Table 4 compares the adsorption capacities of the POLP in this work with some different adsorbents previously used for Cu(II) removal. As observed, the adsorption capacities of the POLP (q m) are much higher than that of many others previously reported adsorbents, indicating the promising application of POLP to be an effective adsorbent for removing Cu(II).
Conclusions
In this study, P. orientalis leaf powder appears as a prospective adsorbent for the Cu(II) removal from aqueous solution. The optimum parameters for the maximum adsorption were temperature of 333 K, contact time of 180 min, and pH of 6. The kinetics of Cu(II) adsorption on POLP followed the pseudo-second-order rate model. Equilibrium data fitted very well with the Langmuir isotherm equation. Maximum adsorption capacity was found to be 49.94 mg g−1. The negative ΔG° value indicated that the adsorption was feasible and spontaneous. The positive ΔH° and ΔS° values described the endothermic nature of the adsorption and the affinity of POLP for Cu(II) adsorption, respectively.
References
X. Tang, Q. Zhang, Z. Liu, K. Pan, Y. Dong, Y. Li, J. Mol. Liq. 191, 73–78 (2014)
Z. Özlem Kocabaş-Ataklı, Y. Yürüm, Chem. Eng. J. 225, 625–635 (2013)
N. Kawasaki, H. Tominaga, F. Ogata, K. Kakehi, Chem. Eng. J. 157, 249–253 (2010)
Y.-H. Kim, T. Ogata, Y. Nakano, Water Res. 41, 3043–3050 (2007)
F.A.A. Al-Rub, M.H. El-Naas, I. Ashour, M. Al-Marzouqi, Process Biochem. 41, 457–464 (2006)
M. Peydayesh, G. Esfandyari, T. Mohammadi, E. Alamdari, Chem. Pap. 67, 389–397 (2013)
N. Adhoum, L. Monser, N. Bellakhal, J.-E. Belgaied, J. Hazard. Mater. 112, 207–213 (2004)
A. Shafaei, F.Z. Ashtiani, T. Kaghazchi, Chem. Eng. J. 133, 311–316 (2007)
M. Machado, E. Soares, H.V.M. Soares, Environ. Sci. Pollut. Res. 18, 1279–1285 (2011)
D. Bakircioglu, Environ. Sci. Pollut. Res. 19, 2428–2437 (2012)
E. Samper, M. Rodríguez, M.A. De la Rubia, D. Prats, Sep. Purif. Technol. 65, 337–342 (2009)
K.G. Bhattacharyya, S.S. Gupta, Adv. Colloid Interface Sci. 140, 114–131 (2008)
R. Shyam, J.K. Puri, H. Kaur, R. Amutha, A. Kapila, J. Mol. Liq. 178, 31–36 (2013)
S. Babel, T.A. Kurniawan, J. Hazard. Mater. 97, 219–243 (2003)
W.S. Wan Ngah, M.A.K.M. Hanafiah, Bioresour. Technol. 99, 3935–3948 (2008)
A. Dubey, A. Mishra, S. Singhal, Int. J. Sci. Environ. Technol. 11, 1043–1050 (2014)
H.Z. Mousavi, S.R. Seyedi, Int. J. Sci. Environ. Technol. 8, 195–202 (2011)
G. Ebrahimzadeh Rajaei, H. Aghaie, K. Zare, M. Aghaie, Res. Chem. Intermed. 39, 3579–3594 (2013)
J.-x. Yu, L.-y. Wang, R.-a. Chi, Y.-f. Zhang, Z.-g. Xu, J. Guo, Res. Chem. Intermed. 39, 3775–3790 (2013)
X. Tong, R. Xu, J. Environ. Sci. 25, 652–658 (2013)
Y. Liu, Q. Cao, F. Luo, J. Chen, J. Hazard. Mater. 163, 931–938 (2009)
K.Y. Ho, G. McKay, K.L. Yeung, Langmuir 19, 3019–3024 (2003)
Z. Kong, X. Li, J. Tian, J. Yang, S. Sun, J. Environ. Manag. 134, 109–116 (2014)
F.-M. Pellera, A. Giannis, D. Kalderis, K. Anastasiadou, R. Stegmann, J.-Y. Wang, E. Gidarakos, J. Environ. Manag. 96, 35–42 (2012)
Ş. Sert, C. Kütahyali, S. İnan, Z. Talip, B. Çetinkaya, M. Eral, Hydrometallurgy 90, 13–18 (2008)
B. Roig, F. Theraulaz, O. Thomas, in Mineral Constituents (Chapter 5), ed. by O. Thomas, C. Burgess (Elsevier, Amsterdam, 2007), pp. 115–144
D. Pathania, S. Sharma, P. Singh, Arab. J. Chem. (2013). doi:10.1016/j.arabjc.2013.04.021
N. Gupta, A.K. Kushwaha, M.C. Chattopadhyaya, Arab. J. Chem. (2011). doi:10.1016/j.arabjc.2011.07.021
A.D. Dwivedi, S.P. Dubey, K. Gopal, M. Sillanpää, Desalination 267, 25–33 (2011)
Y. Liu, Y. Kang, B. Mu, A. Wang, Chem. Eng. J. 237, 403–410 (2014)
J. Zhang, D. Cai, G. Zhang, C. Cai, C. Zhang, G. Qiu, K. Zheng, Z. Wu, Appl. Clay Sci. 83–84, 137–143 (2013)
Y. Bulut, H. Aydın, Desalination 194, 259–267 (2006)
Y. Li, Q. Du, T. Liu, J. Sun, Y. Wang, S. Wu, Z. Wang, Y. Xia, L. Xia, Carbohydr. Polym. 95, 501–507 (2013)
M. Ghaedi, M.D. Ghazanfarkhani, S. Khodadoust, N. Sohrabi, M. Oftade, J. Ind. Eng. Chem. 20, 2548–2560 (2014)
S. Lan, X. Wu, L. Li, M. Li, F. Guo, S. Gan, Colloids Surf. A 425, 42–50 (2013)
M.R. Lasheen, N.S. Ammar, H.S. Ibrahim, Solid State Sci. 14, 202–210 (2012)
Y.-S. Ho, Water Res. 37, 2323–2330 (2003)
T.A. Kurniawan, G.Y.S. Chan, W.-H. Lo, S. Babel, Sci. Total Environ. 366, 409–426 (2006)
K.K. Krishnani, X. Meng, C. Christodoulatos, V.M. Boddu, J. Hazard. Mater. 153, 1222–1234 (2008)
B. Yu, Y. Zhang, A. Shukla, S.S. Shukla, K.L. Dorris, J. Hazard. Mater. 80, 33–42 (2000)
Acknowledgments
The authors would like to thank Iran National Science Foundation for providing the financial support for this project (Project Number: 92034913).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abadian, S., Rahbar-Kelishami, A., Norouzbeigi, R. et al. Cu(II) adsorption onto Platanus orientalis leaf powder: kinetic, isotherm, and thermodynamic studies. Res Chem Intermed 41, 7669–7681 (2015). https://doi.org/10.1007/s11164-014-1851-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1851-y