Abstract
Superadsorbent poly (acrylamide-co-itaconic acid)/nanoclay hydrogels were prepared by in situ free-radical polymerization of acrylamide and itaconic acid in an aqueous media with nanoclay as a crosslinker. The properties of nanocomposite hydrogels (NCHs) were characterized by FTIR spectroscopy, XRD patterns, and TGA thermal methods. Morphology of the samples was also examined by scanning electron microscopy. The swelling properties and cationic dye adsorption behaviors of the NCHs were also investigated. The adsorption behavior of the cationic dyes such as methylene green (MG), methylene blue (MB), and crystal violet (CV) were studied on the NCHs. The effects of the clay content of the hydrogel on its cationic dye uptake behavior were studied. The adsorption studies indicated that the rates of dye uptake by the NCHs increased in the following order: MG > MB > CV. The effect of time on the different dye adsorption by the NCH was nearly the same. The NCHs may be considered as a good candidate for environmental applications to retain more water and to remove dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymeric hydrogels, consisting of a three-dimensional polymer network and water filling at the interstitial space of the network, have attracted much attention of scientists as functional soft materials. To date, hydrogels have been used in many fields such as soft contact lenses, super adsorbent polymeric gels used in sanitary napkins and disposable diapers, carriers for protein and nucleic acid in gel electrophoresis, amendments in greening and agriculture, medical and food products, etc. Also, many functional, polymeric hydrogels have been investigated for their potential use in glucose-responsive, insulin-releasing gels [1], photoresponsive gels [2], enzyme carriers [3], separation devices [4], colloid crystals [5], and cell-cultivation substrates [6]. Hydrogels loaded with dispersed clays are a new class of composite materials which combine the elasticity and permeability of the gels with the high ability of the clays to adsorb different substances [7–9]. Many hydrogel composites such as polyacrylamide (PAAm) with bentonites [7, 10–12] or montmorillonites [13], polyacrylic acid [14] or poly (N-isopropylacrylamide) [15] with montmorillonites, etc., have been studied. Among the possible host materials, clays are natural, abundant, and inexpensive minerals that have a unique layered structure, high-mechanical strength, as well as high chemical resistance. It is well known that clays are good adsorbents for removing dye and other contaminants from the wastewater of the textile and dye industries. Because many types of clay can absorb/desorb organic molecules, they are widely used as carriers or supports for pharmaceuticals.
Recently, clay–polymer nanocomposites (NCs) have been the focus of much attention because of their excellent physical properties, such as their toughness, high modulus, heat resistance, transparency, and so on [16]. These properties are superior to those that would be expected by a simple additive rule. This is partially due to the strong interactions at the clay–polymer interface. Owing to the nature of the complex molecules of clay, a variety of chemical reactions can take place at its surface due to its high chemical reactivity, including reactions with a complex chemistry [17, 18].
For the removal of cationic dyes, polymer hydrogels and their composites have been employed because of their unique properties, such as their excellent dimensional and adsorption behaviors. Most of these hydrogels are prepared by the copolymerization of AAm with some kinds of acid, to endow them with the ability to remove cationic dyes from the water. The acid, which has negative charges, can interact with the cationic dye. Recently, some researchers used clay-filled hydrogel composites for the removal of cationic dyes [19–22]. To date, the clays used are all natural and all the hydrogels used for this purpose were crosslinked by an organic crosslinker. Recently, Haraguchi et al. [19] prepared nanoclay incorporated hydrogels using hectorite clay as a crosslinker in place of the traditional organic crosslinkers. It was found that the mechanical properties of the NC hydrogels improved with increasing hectorite content [20–22].
In recent years, a series of papers has been published by Guven [23, 24] and Katime et al. [25–30] who synthesized new hydrogels from the copolymers of acrylamide, itaconic acid and maleic acid, and showed that the use of even very small quantities of diprotic acid proved to impart remarkable properties to the hydrogels of starting monomers and/or homopolymers. However, none of them has been concerned with solvent effect on swelling–shrinking properties of these hydrogels.
The aim of this study is to investigate the swelling properties and dye adsorption characteristics of nanoclay crosslinked poly (AAm-co-IA) hydrogels. Dynamic swelling studies are important for the swelling characterization of NCH systems. In view of our current interest in adsorption of water-soluble cationic dyes with nanoclay crosslinked polymers hydrogels [31], in this research work, authors have made an attempt to find a convenient method of removing water-soluble cationic dyes from aqueous solutions by adsorption on a novel polymeric adsorbent such as poly (AAm-co-IA)/Laponite clay hydrogels. The structure of the three different water-soluble cationic dyes used in this work, include methylene green (MG), methylene blue (MB), and crystal violet (CV), and are shown in Scheme 1.
Experimental
Materials
Acrylamide (AAm) and itaconic acid (IA) (chemically pure; Shanghai Fine Chemical Material Institute, Chemical Purity, Shanghai, China), ammonium persulfate (APS), and N,N,N’,N’-tetramethyldiamine (TEMED) (analytical reagent; Shanghai Chemical Reagent) were used as received. Laponite XLS (cation exchange capacity ¼ 104 mequiv/100 g), were obtained from KanTo Chemical Industries, Ltd., Tokyo, Japan and Rockwood Co. (Princeton, NJ, USA), respectively. Laponite RD was provided by Rockwood Additive Limited (surface area: 370 m2/g, bulk density: 1,000 kg/m3, chemical composition: SiO2 59.5 %, MgO 27.5 %, Li2O 0.8 %, Na2O 2.8 %, loss on ignition: 8.2 %). All solutions used in the experiments were prepared in deionized water. The cationic dyes namely, MG, MB, and CV (Merck, Darmstadt, Germany), were of analytical reagent grade and used as received. Deionized water was distilled for all experiments including the swelling experiments.
Preparation of poly (AAm-co-IA) nanocomposite hydrogels
NCHs were prepared using initial solutions consisting of comonomers (AAm and IA), crosslinker (clay), solvent (water), initiator (APS), and catalyst (TEMED). The molar ratio between AAm and IA was 1.74:0.26. The NCHs were prepared by the in situ free-radical polymerization of comonomers in the presence of the water-swollen inorganic clay without using any organic crosslinker [32]. First, a transparent aqueous solution consisting of water (27 ml), AAm (3 g), IA (0.22 g), and various amounts of inorganic clay (1–4.0 g) was prepared. The catalyst (TEMED, 24 µl) and subsequently, the aqueous solution of the initiator (0.05 g APS) were added to the former solution with stirring at freezing temperature. Then, the copolymerization of AAm and IA was allowed to proceed in a water bath at 25 °C for 48 h. The hydrogels were heat treated in an incubator at 60 °C for different duration (10–14 days).
Characterization
The UV–visible spectra of the dye solution were recorded using an Uvikon 923 Double Beam UV/VIS spectrophotometer. The absorption spectra of the dilute dye solution in water were recorded in the wavelength range of 300–1,000 nm using a 3-ml stopper silica cell with a path length of 10 mm. Distilled water was chosen as the reference. Optimized NCH was used to study XRD and scanning electron microscopy (SEM). FTIR spectra of samples were taken in KBr pellets using a Perkin–Elmer PE 1600 FTIR spectrophotometer. So, after purifying NCHs according to measurement of gel content, the dried powders were used to study instrumental analysis. The dried sample was coated with a thin layer of gold and imaged in an SEM instrument (Philips, XL30). One-dimensional, wide-angle X-ray diffraction patterns were obtained by using a Philips X’Pert MPD X-ray diffractometer with wavelength, k = 1.54 Å (Cu-Kα), at a tube voltage of 40 kV and tube current of 40 mA. Thermogravimetric analysis (TGA) of the NCHs was carried out on a 2100 thermoanalytical system, USA. The TGA scans were recorded in the temperature range of 25–600 °C with a heating rate of 10 °C/min in air media.
Swelling behavior of NCH
A gravimetric procedure was adopted to monitor the swelling behavior of the NCHs in the different aqueous dye solutions and water. In brief, a known weight of NCH was immersed in distilled water at 25 °C and taken out at regular intervals of time to measure the change in weight. The measurements were continued until a constant weight was reached for each sample. The swelling percentage (water uptake) (Q) was calculated with the following equation:
where W s is the mass of the swollen hydrogel at time t and W d is the mass of the original hydrogel (after drying).
Adsorption of cationic dye
For the adsorption kinetics, the adsorptions of cationic dye onto the poly (AAm-co-IA)/clay NCHs were examined in batch mode. NCHs were immersed in 100 ml of dye solutions at 25 °C with 30 mg l−1. During the adsorption process, a small amount of the dye solution was withdrawn from the system at regular intervals of time and the dye concentrations were measured using a UV–visible spectrophotometer. The amount of the dye adsorbed by the hydrogel at time t, Q (mg g−1 dried hydrogel), was calculated using the following relation:
where C i and C e are the initial and the equilibrium concentrations of cationic dyes (mg ml−1), V is the total volume of dye solutions (ml), and m is the mass of the dry NCH(g). As mentioned before, NCHs were subjected to heat treatment at 60 °C for 10–14 day. So, the effects of the heat treatment of the NCHs on their dye uptake behavior were monitored. The removal efficiency (RE %) of the dye was calculated using the following relation:
Partitioning of dissolved constituents between an aqueous phase and the adsorbents in water and sediments has commonly been described by an empirical partition coefficient that simply relates the total concentration of the dissolved species to the total concentration of the adsorbed species.
where K d is the empirical partition coefficient at equilibrium.
Results and discussion
Synthesis and characterization
The proposed mechanism of the grafting copolymerization reaction is presented in Scheme 2. Superabsorbent copolymers of acrylamide (Am) and itaconic acid (ItA) were prepared by free-radical polymerization of the monomers in aqueous solution using sodium persulfate (SPS) and N,N,N′,N′-tetramethylenediamine (TMEDA) as initiating system. Clay was used as crosslinking agent. The polymerization was initiated first by the reaction of SPS and TMEDA to form a complex that produces more free radicals leading to high efficiency of initiation as illustrated in Scheme 2. Figure 1 shows the appearances of NC gels synthesized in glass vessels. It was found that the shape of the resulting hydrogel was the same as that of the vessel used. The hydrogels were alternately moved into water at 25 °C.
Instrumental analyses of poly (AAm-co-IA)/clay NCHs
FT-IR spectra
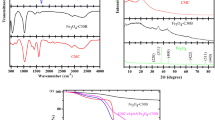
Figure 2 shows the FT-IR spectra of NCHs and the nanoclay. The spectra of the NCHs contained all bands of the clay. This was also demonstrated by the vibrations of the –COOH and CONH2 groups at 1,674 and 1,457 cm−1 and by the vibrations of the –OH and –NH groups at 3,436 cm−1. As shown in Fig. 1, the bands at 1,007, 653, 538, and 471 cm−1 are assigned to Si–O stretching, Al–O was stretching and M–O stretching (M is multivalent metal).
XRD analyses
The XRD patterns of clay and NCHs containing 1.0, 2.0, 3.0, and 4.0 g were studied and shown in Fig. 3. The profiles were collected from 2 to 10° (2θ). It is suggested that polymer intercalated into the gallery of clay. The XRD pattern of pure clay showed the main diffraction peak of at 2θ = 7–8, which disappeared in the diffraction profiles of NCHs. It can be concluded that the interlayer distance of clay was changed and the clay layers are completely exfoliated and uniformly dispersed in organic network.
Thermal characterization
The thermogravimetric curves of the prepared NCHs contain 1.0 and 4.0 g clay were studied up to 600 °C and depicted in Fig. 4. Three main decomposition regions of the samples were revealed. The first is in the range for 1.0 g clay from 28.26 to 192.72 °C. This weight loss can be attributed to the moisture in the samples. The values of weight loss for NCH is 4.78 wt% in this stage. As shown in Fig. 3, the weight loss of NCH increase to 17.52 wt% at 289.37 °C, and 46.70 wt% at 600 °C. For NCH containing 4.0 g clay, weight loss is 7.00 wt% at 239.76 °C, 15.00 wt% at 372.43 °C, and 25.25 wt% at 600 °C. As shown in Fig. 4, by increasing the clay content, thermal stability of the NCHs was clearly improved. These observations have indicated that grafting has improved the thermal stability of polymer, and thermal stability was found to be dependent on the degree of grafting onto polymer.
Scanning electron microscopy
One of the most important properties of NCs that can be considered is hydrogel microstructure morphology. Figure 5 shows the surface morphology of clay NCH containing 3.0 g clay. According to these images, the NCH surface is completely different from the one of clay, which shows uniform dispersion of clay in organic network.
Effect of comonomers ratio on swelling capacity
The swelling capacity of NCH as a function of the comonomers ratio is illustrated in Fig. 6. It is observed that the absorbency is substantially increased with an increased in the IA/AAm ratio and then decreased. The initial increment in swelling values can be attributed to increase in ionic groups existing in copolymer chains along with increase of IA in the hydrogel, which allows polymer coils to expand more easily. The swelling-loss after the maximum may be originated from reducing the hydrogel strength and increasing sol content because of the higher amount of IA [33]. As shown in Fig. 5, an IA/AAm ratio of 0.149 provides the best value of swelling.
Effect of clay content on swelling behavior
Figure 7 shows the equilibrium swelling ratio of NCHs in distilled water. As is well known, the equilibrium swelling ratio of a hydrogel depends on the hydrophilicity of the polymer chains and the physical structure of the hydrogels. Maximum swelling (116 g/g) was obtained at 3.0 g of nanoclay. When clay is present in the sorption system, they compete and are preferably coordinated with –NH2 and –CO2H groups of polymer chains [34]. When the clay content in NCH is low, separation of chains takes place easily, and subsequently the osmotic pressure on the inside of the nanocomposite is increased. Enhancement of osmotic pressure in hydrogel causes an increase in water absorbency of hydrogel [35]. On the other hand, when the content of clay is higher than 3.0 g, clay counter ions remain in the local volume around the clay particles or between the plates and do not contribute to the total osmotic pressure inside the hydrogel [36]. Also, it has been reported that clay can act as a crosslinker in hydrogel systems. It is clear that by increasing the clay content, the crosslinking density increases and results in low swelling capacity [37–39].
Water retention capacity
It is important to investigate the water retention ability of a composite hydrogel in view of practical applications. Figure 8 shows the water retention ability of swollen NCHs on at a temperature of 80 °C. According to the literature [35], the water in a hydrogel can be classified into bound water, half-bound water, and free water. Compared to bound water and half-bound water, the free water in a hydrogel has high mobility and can easily be lost. The percentages of bound water and half-bound water content in swollen gel are related to the number of hydrophilic groups (–CO2H and –CONH2) in a unit volume in hydrogel. As shown in Fig. 7, NCH with 1.0, 2.0, 3.0, and 4.0 g clay lost its whole absorbed water approximately within 8, 10, 11, and 12 h, respectively. These observations depict that inclusion of nanoclay into hydrogel composition can improve its water retention property under heating because of increasing hydrophilic groups within the hydrogel.
Effect of nanoclay content on cationic dye adsorption
For the adsorption studies of dyes, the NCHs were placed in aqueous solutions of the cationic dyes consisting of MG, MB, and CV and allowed to equilibrate for 24 h. At the end of the 6-h period, the NCHs in the solution of all three dyes showed dark colorations of the original dye solutions, which confirm the cationic dyes adsorption by the NCHs. The mechanism of dye adsorption by the hydrogel is mainly due to the ion exchangeability and the physical interactions such as dipole–dipole interactions and hydrogen bond formation between the dye molecules and hydrogels. As is well known, the modified Laponite clay contains anionic charges on its surface and, after the incorporation of the clay into poly (AAm-co-IA), the number of anionic groups in the nanocomposite are increased. Therefore, the NCHs have many anionic groups that allow for the increased interaction between the cationic groups of the dyes and anionic groups of the NCHs.
Figure 9 shows the adsorption of the three different dyes onto the NCHs with different amounts of nanoclay. The equilibrium dye uptake behavior of hydrogels may be influenced by the size, chemical structure, polarity, and solubility parameter of dyes, and the nature of hydrogels. In Fig. 9, it is clearly seen that the dye uptake behavior differs for the three dyes, which have different molecule structures and consequently different hydrogel–dye interactions. Organic cations can bind to the NCHs in different ways. Through ion exchange, the cations (metal ions) on the hydrogel surface can be exchanged by the dye cations, and this results in a neutral complex between the dye cation and the negative site present on the hydrogel surface. As can be seen in Fig. 9, at low nanoclay content, clay is easily ionized and dispersed into the polymer gels, thus improving the hydrophilicity of the hydrogel, which favors the adsorption of cationic dyes. At a higher amount of nanoclay, more clay particles act as an additional network point, then the reaction occurs between –OH groups on the surface of nanoclay and functional groups of polymer, and accordingly, the crosslink density of the hydrogel becomes larger. In addition, the amount of ionic groups like –COOH−, –NH2, and –OH groups decreases, which is also an important reason for the decrease in adsorption capacity for dyes due to reduced electrostatic attraction.
The calculated dye RE % and empirical partition coefficient at the equilibrium (Kd) values for the NCHs with different nanoclay contents are given in Figs. 10 and 11. From Fig. 10, it can be seen that the RE % for MG is above 92 %. This result indicates that the synthesized NCHs are efficient materials for the removal of MG from water. This may be due to the greater physical interaction of the MG dye toward the NCHs than the other dyes probably because of higher interaction of the –NO2 group of MG dye with –CO2H and –NH2 groups in NCHs due to hydrogen bond. The K d values are increased for MG in the range of 9.38–11.93 that this results show an similar trends to the RE % data (Fig. 11). These results show that MG adsorption is higher than CV and MB and in continue we chose MG as a model dye for another research.
Effect of contact time on cationic dye adsorption
It is important to determine the contact time effect on sorption capacity of an adsorbent for a given initial adsorbate concentration. From Fig. 12, it can be observed that the adsorption rate of MG is faster than MB and CV due to the better interaction of MG dye with adsorbent because of structural reasons as explained in previous section. The experimental data showed that more than 80 % of MG removal, 67 % of MB removal, and 19 % of CV removal were achieved by the prepared NCHs within the initial 1 h. Table 1 shows the RE % and K d values of the NCHs for MG, MB, and CV cationic dyes. It was noticed that in 24 h there was approximately 11 % improvement in the dye adsorption for MG, 9 % improvement for MB, and 317 % improvement for CV. A drastic increase in the K d values was also noticed for 24 h of the NCHs. These results show that time direct effect of the adsorption dyes. Also, the result of the equilibrium swelling is parallel to the result of the dye uptake speed. This may be because the swelling ability of the hydrogel can influence the movement of the dye molecules, so as to influence the interaction between the dye molecules and nanoclay.
Effect of dye on the swelling behavior of NCHs
The swelling capacity of nanocomposite in 30 ppm of MG, MB, and CV dye solutions was studied (Fig. 13). As can be seen, swelling increased with the increase of time until 24 h for all three dyes. Maximum swelling is for CV (102.54) and obtained at 24 h. It is observed that the absorbency is substantially increased from 3.75 g/g for 1 h to 102.54 g/g for 24 h. These results show that when increasing the dye adsorption, swelling decreased because of functional groups interaction through NCHs with dye molecule cause to less osmotic pressure. The adsorption of MG by NCH is more than other dyes and cause to less swelling of NCH in water.
Conclusions
On the basis of the XRD measurements, it can be concluded that in the NCHs the clay is extensively exfoliated, and the clay platelets are dispersed uniformly throughout the sample. The effects of the clay contents on the dyes uptake behavior were studied. There is optimal clay content in the NCHs for the effective removal of cationic dyes. The values of the equilibrium swelling degree (qe) in the dye solutions follow the order: MG > MB > CV. A significant improvement in the dye uptake speed, RE %, and empirical partition coefficient (K d) values for the 24 h time NC hydrogels was noticed. Thus, NCHs can be used as an adsorbent for the treatment of wastewater containing pollutants such as cationic dyes, which is an important problem for the textile industry.
References
A. Matsumoto, R. Yoshida, K. Kataoka, Biomacromolecules 5, 1038 (2004)
R. Akashi, H. Tsutusi, A. Komura, Adv. Mater. 14, 1808 (2002)
P.S. Stayton, T. Shimoboji, C. Long, A. Chilkoti, G. Chen, J.M. Harris, A.S. Hoffman, Nature 378, 472 (1995)
W. Cai, E.C. Anderson, R.B. Gupta, Ind. Eng. Chem. Res. 40, 2283 (2001)
T. Hellweg, C.D. Dewhurst, E. Bruckner, K. Kratz, W. Eimer, Colloid Polym. Sci. 278, 972 (2000)
M. Yamato, T. Okano, Mater. Today 7, 42 (2004)
S.G. Starodoubtsev, A.A. Ryabova, A.T. Dembo, K.A. Dembo, I.I. Aliev, A.M. Wasserman, A.R. Khoklov, Macromolecules 35, 6362 (2002)
P.C. LeBaron, Z. Wang, T.J. Pinnavaia, Appl. Clay Sci. 15, 11 (1999)
K. Kabiri, M.J. Zohuriaan-Mehr, Polym. Adv. Technol. 14, 438 (2003)
O.V. Evsikova, S.G. Starodoubtsev, A.R. Khoklov, Polym. Sci. Ser. A. 44, 6362 (2002)
S.G. Starodoubtsev, N.A. Churochkina, A.R. Khoklov, Langmuir 16, 1529 (2000)
D. Gao, R.B. Heimann, M.C. Williams, L.T. Wardhaugh, M. Muhammad, J. Mater. Sci. 34, 1543 (1999)
D. Gao, R.B. Heimann, J. Lerchner, J. Seidel, G. Wolf, J. Mater. Sci. 36, 4567 (2001)
J. Wu, J. Lin, G. Li, C. Wei, Polym. Int. 50, 1050 (2001)
X. Xia, J. Yih, A.D. D’Souza, Z. Hu, Polymer 44, 3389 (2003)
B.M. Novak, Adv. Mater. 5, 422 (1993)
H. Van Olphen, Clay Colloid Chemistry, 2nd edn. (Wiley, New York, 1977)
T.J. Pinnavaia, G.W. Beall, Polymer-Clay Nanocomposites (Wiley, England, 2000)
K. Haraguchi, H.J. Li, K. Matsuda, T. Takehisa, E. Elliott, Macromolecules 38, 3482 (2005)
D. Saraydın, E. Karadag, O. Guven, J. Appl. Polym. Sci. 79, 1809 (2001)
S. Duran, D. Solpan, O. Guven, Nucl. Instrum. Meth. B. 151, 196 (1999)
K. Erdener, B.U. Omer, S. Dursun, Eur. Polym. J. 38, 2133 (2002)
E. Karadag, D. Saraydın, O. Guven, J. Appl. Polym. Sci. 61, 2367 (1996)
M. Sen, A. Yakar, O. Guven, Polymer 40, 2969 (1999)
J.R. Quintana, N.E. Valderraten, I. Katime, J. Appl. Polym. Sci. 85, 2540 (2002)
E. Valles, D. Durando, I. Katima, E. Mendizabal, J.E. Puing, Polym. Bull. 44, 109 (2000)
I. Katime, N. Valderruten, J.R. Quintana, Polym. Int. 50, 869 (2001)
G. Bagheri Marandi, H. Hosseinzadeh, Polym. Compos. 15, 395 (2007)
G.R. Mahdavinia, G.B. Marandi, A. Pourjavadi, G. Kiani, J. Appl. Polym. Sci. 118, 2989 (2010)
G. Bagheri Marandi, G.R. Mahdavinia, S. Ghafarym, J. Appl. Polym. Sci. 120, 1170 (2011)
Y.M. Mohan, K. Sudhakar, P.S.K. Murthy, K.M. Raju, Int. J. Polym. Mater. 55, 513 (2006)
H. El-Hamshary, Eur. Polym. J. 43, 4830 (2007)
S.K. Bajpai, M. Bhowmik, J. Macromol. Sci. Part A Pure Appl. Chem. 48, 108 (2011)
J. Li, A.Wang Zhang, J. Appl. Polym. Sci. 103, 37 (2007)
X. Xia, J.D. Yih, N.A. Souza, Z. Hu, Polymer 44, 3389 (2003)
L. Liu, Y. Yu, B. Zhang, J. Liu, H. Zhang, RSC. Adv. 3, 13756 (2013)
J. Alaie, E. Vashegani-Farahani, A. Rajmatpour, M.A. Semsarzadeh, Eur. Polym. J. 44, 2024 (2008)
K. Xu, J. Wang, S. Xiang, Q. Chen, W. Zhang, P. Wang, Appl. Clay Sci. 38, 139 (2007)
K. Haragachi, T. Takehisa, Adv. Mater. 14, 1120 (2002)
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Islamic Azad University of Karaj.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marandi, G.B., Baharloui, M., Kurdtabar, M. et al. Hydrogel with high laponite content as nanoclay: swelling and cationic dye adsorption properties. Res Chem Intermed 41, 7043–7058 (2015). https://doi.org/10.1007/s11164-014-1797-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1797-0