Abstract
Cassava is the third significant source of calories after rice and maize in tropical countries. The annual production of cassava crop is approximately 550 million metric tons (MMT) which generates about 350 MMT of cassava solid residues, including peel, bagasse, stem, rhizome, and leaves. Cassava peel, bagasse, stem, and rhizome can be exploited for solid, liquid and gaseous biofuels production. Biofuels production from cassava starch started in the 1970s and researchers are now extensively studying cassava residues like peel, bagasse, stem, rhizome, and leaves to unravel their applications in biofuels production. However, there are technical and economic challenges to overcome the problems existing in the production of biofuels from cassava-based residues. This review provides a comprehensive summary of the techniques used for biofuels production from various cassava-based residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The primary requirements for the human survival are food, clothing, and shelter (Bansal 2005). Now, the secondary requirements are essentially healthcare, energy, and environment (Dineshkumar et al. 2015). Energy is produced from transportation fuels, heat, and power (McKendry 2002) and fossil fuels are one of the major sources of energy (Shafiee and Topal 2009). Due to depletion of fossil fuels and instability in the organization of the petroleum exporting countries (OPEC), researchers are focusing on alternative renewable energy (McKendry 2002; Demirbas 2005; Banerjee et al. 2010). Many studies have revealed that the major share of renewable energy can be produced from biomass (Berndes et al. 2003; Demirbas 2005; Goldemberg 2007; Lund 2007). Biomass resources include a variety of materials which are broadly categorized as woody biomass, agricultural and agro-industrial residues, aquatic biomass, animal wastes, and commercial remains (Bhardwaj et al. 2015). All non-edible parts of plants, animals, domestic and industrial litters are the important sources of biomass energy since edible parts are used for food and feed supply (Casson et al. 2014). Agriculture is the pillar of sustainable development (Nurse 2006), and agro-industries produce, process, and package food on large-scale using modern methods and machinery. The residues generated from agro-industries constitute a great share in bio-energy production (Wilkinson and Rocha 2008). Food and agricultural organization (FAO) statistics have revealed that sugarcane, corn, rice, wheat, potato, soybean, cassava, tomato, banana, and onion are the ten valuable crops in terms of production in 2017 (FAO 2018). This article presents a comprehensive review on solid, liquid and gaseous biofuels production from the solid residues of cassava crops and processing industries.
2 Cassava
Cassava or tapioca or yuca or manioca (Manihot spp.) is a perennial shrub and belongs to the family of Euphorbiaceae that can be planted for producing edible starchy tubers (Nassar 2007). It is ranked as the sixth most important source of calories in the human diet (FAO 1999). It grows in soil with low nutrients and less rainfall (Cock 1982). The edible part of cassava is called tuber whilst the non-edible part contains leaves, stem or stalk, rhizome, peel, and bagasse or fibrous residue (Rymowicz et al. 2004). The biochemical composition of cassava-based residues is presented in Table 1. Whole cassava plant (Fig. 1a) contains 45% (w/w) tuber, 10% (w/w) rhizome, 35% (w/w) stem, and 10% (w/w) leaves (Ravindran 1993). Cassava plants grow denser as it grows taller (Fig. 1b). Rhizome is the portion between stem and tuber (Fig. 1a) (Pattiya et al. 2008). Tuber (Fig. 1c) typically contains 6% (w/w) peel, 15% (w/w) bagasse and 79% (w/w) starch (Sriroth 2001). Tuber contains two outer layers: thin brown periphery (periderm) and thick leather wrap (cortex) (Fig. 1d, e) and both layers constitute a peel (Sivamani and Baskar 2015). Also, tubers have central vascular fibre (bagasse) which contains high resistant starch (Fig. 1f) (Alves 2002). Other than peel and bagasse, the remaining part is edible starch (El-Sharkawy 2003). The leaves, petiole and tender stem constitute forage (Fig. 1g, h) (Ravindran 1993).
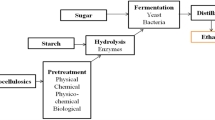
Among cassava producing countries, Nigeria is the highest producer with 54.83 million metric tons (MMT) in 2017 followed by Thailand (30.02 MMT) and Indonesia (23.44 MMT) (Fig. 2). Normally, cassava starch yields 150–200 kg per ton of tuber processed and 2–5 m3 of liquid waste (effluent) and about 200 kg of solid waste (peel and bagasse) are generated per ton of processed tuber (Sriroth 2001). Liquid waste is mainly used for biogas production and solid wastes are used for the production of various value-added products including biofuels (Ubalua 2007), while cassava starch is mainly used to produce a variety of food products (Jobling 2004).
Storage of cassava tubers after harvest is a major challenge restricting industrial scale production. The process of postharvest physiological damage starts in 1 day as soon as the cassava roots are dug up from the soil. So, cassava roots cannot be stored more than 1 month due to its high moisture content. Low protein level, high cyanide and rapid deterioration rate limit the utilization of cassava roots in industries (Uchechukwu-Agua et al. 2015).
3 Biofuels
Basically, biofuels are derived from biomass and they are classified into three types based on feedstock—first, second, and third generation biofuels (Fig. 3a). First generation biofuels are produced from edible feedstocks like edible oils, sugary, and starchy materials. Second generation biofuels are produced from non-edible feedstocks like non-edible oils, and lignocellulosic materials. Third generation biofuels are from algae. Based on the state of matter, biofuels are also classified into three types—solid, liquid and gaseous biofuels (Fig. 3b).
Pellets, briquettes, and biochar are considered as solid biofuels. To overcome the harvesting, handling, transportation and storage costs of less dense agricultural residues, they are condensed to produce small-sized high dense materials that can be used for combustion. Biomass briquettes are large sized high dense materials with a hole at the centre to allow the passage of air. Biochar is produced by the pyrolysis of biomass and used as a soil enhancer to make fertile. All solid biofuels are used as a substitute for coal and charcoal.
Biobutanol, biodiesel, bioethanol, bio-oil and synthetic diesel are common liquid biofuels. Biobutanol and bioethanol are produced by fermentation of sugars with microbes and used as a substitute for gasoline. Biodiesel is produced by transesterification of oils that can be an alternative to diesel. Synthetic diesel is produced by the integration of biomass gasification and the Fischer–Tropsch (BG–FT) synthesis or by catalytic depolymerization process (CDP). This also can be an alternative to diesel. Bio-oil is produced by the pyrolysis of biomass and used as a substitute for heavy and light end fuel oils.
Biogas and syngas are listed as the common gaseous biofuels. Biogas comprises mainly of methane and carbon dioxide can be produced by the anaerobic digestion of biomass with livestock waste. Syngas contains mainly of carbon monoxide and hydrogen can be generated by gasification. These biofuels can be a suitable alternative to natural gas. Biohydrogen is also considered as a gaseous biofuel that can be produced by fermentation of sugars with microbes. The schematic representation of the production of different biofuels from various cassava substrates is depicted in Fig. 4.
4 Cassava peel
Cassava peel is an industrial residue obtained during the processing of cassava tuber to starch. The steps involved in cassava processing are washing, peeling, cutting, brushing, screening, centrifuging, and drying (Sivamani and Baskar 2015). Cassava peel is not widely used for biofuels production because of its toxicity due to cyanogenic glycoside. Because of the detoxification effect in animals, the peel is commonly utilizing as an animal feed. The toxicity is observed in cassava due to the presence of cyanogenic glycosides, lotaustralin, and linamarin. They are converted to toxic prussic or hydrocyanic acid (HCN) when they are contacted with the linamarase enzyme, which is released when cassava cells rupture. The detoxification of cassava before biofuels production was performed either by soaking in clean water or by fermentation (Sivamani and Baskar 2015).
4.1 Bioethanol production from cassava peel
Starch, sugars, cellulose, and hemicellulose are the carbohydrates present in cassava peel. Polysaccharides (Starch, cellulose, and hemicellulose) are hydrolyzed to monosaccharides (Glucose and xylose) and then fermented with bacteria or yeast for the bioethanol production. The factors affecting the bioethanol production from sugars are substrate concentration, inoculum size, reaction time, pH, reaction temperature, and agitation speed. The chemistry of bioethanol production from polysaccharide is shown in Eqs. (1, 2).
The mixed enzyme system was utilized for the hydrolysis of cassava peel (Srinorakutara et al. 2004). Cassava peel was mixed with cellulase and pectinase at 28 °C for 1 h followed by incubation with α-amylase at 100 °C for 2 h and glucoamylase at 60 °C for 4 h to obtain reducing sugars concentration of 122.4 g/L for substrate concentration of 100 g/L. Finally, ethanol concentration of 3.62% (w/v) was obtained after 24 h fermentation at initial reducing sugar concentration of 89.2 g/L. The bioethanol production from cassava peel was studied through acid hydrolysis and microbial fermentation (Yoonan et al. 2007). Here, cassava peel was hydrolyzed using sulphuric acid, hydrochloric acid, and acetic acid at 135 °C and 90 min. The sulphuric acid was found to be effective for cassava peel hydrolysis. Then, hydrolysate obtained after hydrolysis was found to contain 60.79 g of reducing sugars and 37.09 g of glucose per 100 g of cassava peel. Eventually, ethanol yield and productivity were obtained at 0.43 g/g and 0.51 g/L h respectively after 18 h of fermentation with Saccharomyces cerevisiae. Aspergillus niger from rotten cassava wastes was found to contain the highest amylase activity on starch agar and used S. cerevisiae for ethanol fermentation (Adesanya et al. 2008). Sugar concentration of 0.88 g/L was produced on the 7th day and ethanol concentration of 1.05% (v/v) was obtained on the 10th day. The feasibility of cassava peel for bioethanol production with a monoculture of Saccharomyces diastaticus 2047 and S. cerevisiae 7532, and co-culture of S. diastaticus 2047 and Candida tropicalis 5045 was analyzed (Kongkiattikajorn and Sornvoraweat 2011). The bioethanol yield of 0.4416 g/g cassava peel was achieved for co-culture fermentation after pretreatment with 0.1 M sulphuric acid.
The peel of crops of yam, potato, and cassava was investigated for bioethanol production (Akponah and Akpomie 2011). The peels were hydrolyzed using inorganic acids, α-amylase and A. niger. Then, S. cerevisiae was employed in the fermentation process. Cassava peel has generated highest of 250 mg/g of glucose with acid pretreatment and eventually, 17.52% (v/w) of ethanol was produced. The production of bioethanol from cassava and sweet potato peel by the hydrolysis process using Gloeophyllum sepiarium and Pleurotus streatus was studied (Oyeleke et al. 2012). Finally, Zymomonas mobilis and S. cerevisiae were compared for the fermentation process. Cassava peel has yielded 11.97 g/cc of ethanol at substrate quantity of 50 g whereas ethanol concentration of 6.5 g/cc was obtained with 50 g of sweet potato peel when co-culture was employed. The bioethanol was obtained from cassava peel by sulphuric acid hydrolysis followed by fermentation with S. cerevisiae (Abidin et al. 2014). The acid concentration, time of hydrolysis and fermentation conditions were optimized and 0.5 M H2SO4, 1 h and 4 days of hydrolysis and fermentation respectively were found to be the optimal conditions to produce 3.58% (v/v) of bioethanol. Also, the bioethanol was yielded from cassava peel with A. niger for hydrolysis and S. cerevisiae for the fermentation process (Adetunji et al. 2015). Bioethanol concentration of 8.5% (v/v) was produced for 20 g of cassava peel and pH of 6.71 after 7 days each for hydrolysis and fermentation processes. The bioethanol concentration from cassava (Manihot esculenta) peel was optimized through enzymatic hydrolysis and fermentation by Z. mobilis MTCC 2427 using response surface methodology (RSM) (Sivamani and Baskar 2015). Ethanol concentration of 35.010 g/L (83% of theoretical yield) was achieved under optimized conditions of 69.82 g/L of substrate concentration, 24.74% (v/v) of α-amylase concentration and 5.22% (v/v) of simultaneous saccharification and fermentation (SSF) mixture.
The mixed feedstock of cassava peel and waste was evaluated to maximize the bioethanol yield with co-culture of Saccharomycopsis fibuligera NCIM 3161 and Z. mobilis MTCC 92 using one factor at a time (OFAT) method (Sivamani et al. 2015). Ethanol concentration of 26.46 g/L (93.75% of theoretical yield) was obtained in single step fermentation at optimum conditions of cassava peel-to-waste ratio of 4, substrate concentration of 50 g/L, pH of 4.5, temperature of 37 °C, reaction time of 120 h, inoculum size of 10% v/v and agitation speed of 100 rpm. The wild cassava (Manihot glaziovii) was utilized for the production of bioethanol followed by biogas (Moshi et al. 2015). Alkali, enzyme and alkali-enzyme sequential pretreatment methods were attempted and alkali-enzyme method improved combined ethanol and methane yield by 1.2–1.3 fold. Also, the cassava peel was utilized to maximize the bioethanol yield with co-culture of S. fibuligera NCIM 3161 and Z. mobilis MTCC 92 using OFAT method (Pandian et al. 2016). The maximum bioethanol concentration was obtained at an optimum substrate concentration of 70 g/L.
The bioethanol was obtained from cassava and yam peel through hydrolysis by G. seplarium and P. ostreatus and fermentation by Z. mobilis and S. cerevisiae (Adiotomre 2015). The ethanol concentrations of 26.5, 42.6 and 55.2 g/cc were achieved for 20, 35 and 50 g of cassava peel respectively. The potential of Bacillus cereus GBPS9 was studied for the bioethanol production from sugarcane bagasse and cassava peel (Ezebuiro et al. 2015). Peel was hydrolyzed with acid, alkali, and steam explosion pretreatments followed by the fermentation. Here, acid pretreatment was found to be effective for cassava peel and carbohydrate and lignin contents have reduced from 69.6 and 13.9 to 80.4 and 4.8 respectively and achieved ethanol concentration of 17.8 g/L. The production of ethanol from three cultivars of cassava (TME 0505, TME 419 and TME 4779) was studied in single step fermentation for 21 days using five various microbial inoculants (Rhizopus nigricans and S. cerevisiae, A. niger and S. cerevisiae, R. nigricans, A. niger and S. cerevisiae, R. nigricans, Spirogyra africana and S. cerevisiae, and A. niger, S. africana and S. cerevisiae) (Chibuzor et al. 2016). TME 4779 has produced the maximum bioethanol concentration of 14.46 ± 2.08 g/cc using mixed inoculant of R. nigricans, S. africana, and S. cerevisiae. The summary of bioethanol production from cassava peel is presented in Table 2.
4.2 Biogas production from cassava peel
Carbohydrates, lipids, and proteins present in cassava peel leads to the production of biogas. The livestock wastes act as the source of inoculum. Carbon, hydrogen, and oxygen produce methane and carbon dioxide, whereas nitrogen and sulphur produce ammonia and hydrogen sulphide respectively. The factors affecting the biogas production are substrate concentration, inoculum loading, carbon to nitrogen (C/N) ratio, time, pH, temperature, hydraulic retention time, and agitation speed. The chemistry behind the biogas production is shown in Eq. (3).
The production of methane and cyanide removal from cassava peel was studied together (Cuzin and Labat 1992). During methanogenesis, in a plug flow digester, the linamarin was detoxified by linamarase and β-cyanoalanine synthase and leads to the formation of hydrogen cyanide. The same research group has studied the production of biogas from cassava peel (Cuzin et al. 1992). The high starch, C/N ratio, and cyanogenic glucosides are present in cassava peel reduce the methane production. Plug flow digester has overcome the drawbacks and yielded 0.661 m3 biogas/kg volatile solids (VS) with a loading rate of 3.6 kg VS/m3 days. A digester with a capacity of 88 m3 is required to produce methane from 1 ton of cassava peel on a dry basis from energy saving calculations. Methanobacterium congolense sp. nov., a gram-positive bacterium that was isolated and utilized for the production of biogas from cassava peel in an anaerobic digester (Cuzin et al. 2001). The doubling time under optimal growth conditions was 7.5 h and the DNA base composition was 39.5 mol% G + C. Methane and carbon-dioxide production were increased in the presence of 2-propanol, 2-butanol or cyclopentanol as hydrogen donors, temperature range from 37 to 42 °C, and pH of 7.2. The feasibility of cassava tuber, cassava peel, palm kernel cake, and palm kernel shells was studied for the production of methane at 35 °C for 30 days in 2 L batch digester (Jekayinfa and Scholz 2013). The highest biogas of 0.66 m3/kg VS was yielded for cassava-based residues and the highest methane of 0.32 m3/kg VS was obtained for palm kernel cake.
The effect of wood ash was investigated as a seeding material on biogas production from a mixture of pig waste and cassava peel in a laboratory anaerobic digester for 45 days (Adeyanju 2008). A mixture containing 200 g of pig waste, 200 g of cassava peel and 1200 mL of water were seeded with wood-ash produced the highest biogas volume of 2345 mL. The productivity of biogas from cassava peel and livestock waste (poultry, piggery and cattle waste) was investigated by mixing peel and waste of different ratios (1:1, 2:1, 3:1 and 4:1 by mass) (Adelekan and Bamgboye 2009). The factorial design for 3 × 4 trials was performed with a retention period of 30 days in a batch anaerobic digester within mesophilic temperature range and the maximum biogas yield of 35 L biogas/kg total solids (TS) was obtained for piggery waste in the ratio of 1:1. The biogas production from a mixture of cassava peel and livestock waste (cow dung, poultry droppings, and swine dung) was evaluated in the ratio of 1:1 in 50 L digester (Ofoefule and Uzodinma 2009). The swine dung has produced the highest biogas yield of 169.6 L for the total mass of slurry; however, the biogas production was initiated from the 11th day. On the other hand, cow dung started biogas production from the 9th day onwards. The biogas from cow dung, spent grain/cow dung mixture and cassava peel/rice husk mixture was examined in 971 L digester at mesophilic temperature (Ezekoye and Ezekoye 2009). Spent grain and cow dung were mixed in the proportion of 70:30 and cassava peel and rice husk were mixed in the ratio of 50:50. The cow dung spent grain/cow dung mixture and cassava peel/rice husk were mixed with water in the ratio of 1:2, 1:2 and 1:5 respectively in the batch fixed dome plastic biodigester. The production rate is higher for cow dung whereas biogas yield (3.839 m3) is higher for spent grain/cow dung mixture.
The significance of retention time on biogas production was studied from poultry droppings and cassava peel in polyethylene digester (Ezekoye et al. 2011). The cumulative biogas yield of 1.508 and 1.179 m3 was obtained for poultry droppings and cassava peel after 42 and 79 days at 34 and 33 °C respectively. The potential of water hyacinth (Eichhornia crassipes) and cassava (Manihot esculentum) peel for biogas production were investigated using locally-designed and modified digesters with and without starter culture (Asikong et al. 2012). The biogas yield of 650 and 635 mL was obtained with water hyacinth and mixture of water hyacinth and cassava peel respectively after 30 days of digestion with the starter culture. The mixture of residues (cow dung, cassava peel, and cowpea) was employed for the production of biogas with water in the ratios of 1:2, 1:5 and 1:5 respectively in 45 L metallic biogas digester at the ambient mesophilic temperature and neutral pH for 30 days (Ukpai and Nnabuchi 2012). Biogas produced by mixture cowpea and water have the highest methane content of 76.2% (v/v) and cow dung in water has produced the highest cumulative biogas yield of 124.3 L for the total mass of slurry. The effect of pig dung was studied on crop wastes such as bean husks, peel of yam, cassava and plantain for biogas production in the ratio of 4:1 on the wet basis for 16 days (Okareh et al. 2012). During digestion, pH was varied from 5.2 to 7.1, temperature variation from 26 to 34 °C within mesophilic range and biogas yield were varied from 85.5 to 314.5 mm H2O.
A mixture of wastes (cowpea, cassava peel, and cow dung) was analyzed on daily basis for the production of gas with water at a retention period of 30 days in 45 L metallic biogas digester (Nnabuchi and Ukpai 2012). The peel showed the highest production rate of 8.3 L per day at the mesophilic temperature of 37 °C. Also, the mixture of cassava (Manihot esculentus) peel and pig dung was investigated with different ratios of 1:1 (B1), 3:7 (B2) and 1:9 (B3) (Oparaku et al. 2013). B2 has yielded the highest biogas production of 78.5 L. The mixture of flour and peel of wild non-edible cassava (Manihot glaziovii) was characterized and evaluated for the simultaneous production of biogas and bioethanol (Moshi et al. 2015). The biochemical analysis of cassava flour and peel reveals the composition as 77–81% (w/w) of starch, 3–16% of structural carbohydrates and 2–8% of total crude protein. Finally, fermentation was catalyzed by yeast that yielded the bioethanol concentration of up to 85 g/L (89% fermentation efficiency), 5–13 and 11–14 MJ/kg VS energy yield were obtained for bioethanol and methane respectively. Also, 15–23 MJ/kg VS energy was yielded for the co-production of bioethanol and biogas from the peel.
The effect of temperature was examined on biogas production from cow dung, cassava peel and cowpea peel for 30 days (Ukpai et al. 2015). The optimum temperature was found to be 35 °C for biogas yield. The temperature above 50 °C has killed the bacteria and thereby curtails the biogas production. The effect of urea on biogas production was investigated from cassava peel in 1 L digester (Nkodi et al. 2016). Cassava peel (42.7 g) was mixed with 750 mL of water and different concentrations of urea (0, 0.01, 0.03, 0.04 and 0.05% (w/v). Then, the anaerobic digestion was performed for 14 days under mesophilic temperature. Finally, the cumulative biogas yield reveals that the cassava peel with 0.01% (w/v) urea produced the highest biogas of 80.79 L/kg TS. The effect of carbon to nitrogen ratio (C/N) on biogas production was investigated from multiple substrates (vegetable waste, cow dung, cassava peel, yam peel, sweet potato peel, beans waste, rice waste and plantain waste) (Orhorhoro et al. 2016). The results revealed that the substrates with C/N ratio between 20 and 30 produced more biogas i.e. beans waste with C/N ratio from 24 to 30 has produced the highest biogas yield of 0.216 kg/kg beans waste.
4.3 Bio-oil production from cassava peel
Pyrolysis is the process of heating biomass in the absence of air. The products of pyrolysis include biochar, bio-oil, and volatiles consisting of methane, hydrogen, carbon monoxide and carbon dioxide. The factors affecting pyrolysis production distribution are biomass type, pyrolysis temperature, primary condensation temperature, heating rate, particle size, moisture content, pyrolyzer type, inert gas flow rate, and time. Low, intermediate and high temperatures favour the formation of biochar, bio-oil, and volatiles respectively.
The chemistry behind the bio-oil production is as follows:

The bio-oil was produced through the pyrolysis of cassava peel in a fixed bed tubular reactor at temperatures ranging from 400 to 600 °C at a heating rate of 20 °C/min (Ki et al. 2013). The analysis of bio-oil for chemical composition by gas chromatography-mass spectrometry (GC–MS) revealed that the highest bio-oil yield of 51.2% was obtained at 525 °C with gross calorific value (GCV) of 27.43 MJ/kg. The mechanistic model showed that the mean squared error for bio-oil, biochar and pyrogas were 16.24 (R2 = 0.95), 13.37 (R2 = 0.96) and 0.49 (R2 = 0.99) respectively. The bio-oil, pyrogas, and biochar were produced from cassava peel through pyrolysis in a fixed bed reactor by varying temperatures (400, 500, 600, and 700 °C) for 20 min (Okekunle et al. 2016). The highest yield of 44, 34, and 64% was obtained for bio-oil, biochar and pyrogas at 500, 400 and 700 °C respectively.
5 Cassava stem
Cassava stem is an agricultural residue obtained from the fields after harvest. It is formed by nodes and internodes. The node is the junction where leaf and stem join together whereas the internode is the part between nodes. The plant grows from 7 to 8 viable nodes. The mature stems can have up to 30 nodes and yield about 10 planting materials. Eventually, excessive stems are underutilized for burning (Alves 2002). The stem has good potential to use for biofuels production because of its lignocellulosic content and non-toxicity. The stem can be used for the production of bioethanol and bio-oil.
5.1 Bioethanol production from cassava stem
Cassava stem is rich in cellulose, hemicellulose, and lignin. Hemicellulose solubilizes faster in dilute inorganic acids than cellulose at high pressure and temperature. Hemicellulose and lignin are soluble in alkali whereas cellulose is not. Thus, the prime step in the conversion of lignocellulosic materials to bioethanol is the pretreatment. The pretreatment methods used for cassava-based residues are summarized in Table 3. In the pretreatment step, hemicellulose solubilizes without disturbing the cellulignin (mixture of cellulose and lignin) fractions. The enzymatic hydrolysis converts cellulose to cellobiose by cellulase, and then to glucose by cellobiase. Finally, co-fermentation of monomers (sugars) from hemicellulose and cellulose produces bioethanol. The success of bioethanol production from lignocellulosic materials depends solely on the selection of pretreatment and fermentation methods.
The optimization of temperature, time and acid concentration of dilute hydrochloric acid pretreatment conditions was studied for bioethanol production from cassava stem using RSM (Han et al. 2011). The temperature of 177 °C, 10 min, and 0.14 M was found to be optimum for pretreatment at substrate concentration of 30 g/L followed by enzymatic hydrolysis with 20 FPU/g cellulase and 30 CbU/g β-glucosidase lead to 70% yield in saccharification. The fermentation of hydrolysate with S. cerevisiae yielded 7.55 g/L ethanol (89.6% of theoretical yield). The direct fermentation was studied for bioethanol production from 100 mesh tapioca stem through dilute acid and alkali pretreatments followed by fermentation with Fusarium oxysporum in a batch reactor using RSM based optimization parameters (Magesh et al. 2011b). The results showed that ethanol concentration of 8.64 g/L was obtained at an optimum substrate concentration of 33 g/L, pH 5.52 and temperature of 30 °C. The SSF for bioethanol production from tapioca stem was studied through sequential dilute acid and alkali pretreatments followed by saccharification with cellulase and fermentation with S. cerevisiae in a fermentor (Magesh et al. 2011a). The results showed that ethanol concentration of 13.6 g/L was achieved at an optimum substrate concentration of 50 g/L, pH 5, the temperature of 35 °C and particle size of 100 mesh size. The viability of utilizing stem, leaves, peel, and the root of cassava was examined for the production of bioethanol through acid, alkaline and enzymatic hydrolysis followed by fermentation with S. cerevisiae (Nuwamanya et al. 2012). The alkaline hydrolysis produced the better result in saccharification except for stem. The maximum ethanol yields of 55.8 and 52.4 mL were obtained for root and stem respectively.
The potential of cassava stalk was investigated for bioethanol production by dilute acid pretreatment followed by separate hydrolysis and fermentation (SHF) and SSF with S. cerevisiae TISTR5048, S. cerevisiae KM1195, S. cerevisiae KM7253 and co-culture of S. cerevisiae TISTR5048 and Candida tropicalis TISTR5045 (Sovorawet and Kongkiattikajorn 2012). Ethanol produced with the highest concentration of 6.23 g/L for SSF with co-culture. The effect of inoculum size and activity of the enzyme on ethanol production from alkali pretreated cassava stem through SHF and non-pretreated cassava stem through SSF in Erlenmeyer flask and 5 L bioreactor at 38 °C, 4 pH and solid-to-liquid ratio of 1:10 using accellerase 1500 enzyme and ethanol red yeast (Castaño Peláez et al. 2013). At optimal inoculum size of 1.59 g/L and enzyme activity of 13.3 FPU/g, ethanol concentrations of 14.7 and 11.5 g/L were obtained in the Erlenmeyer flask and bioreactor respectively after 72 h for SSF. The cassava stem was pretreated through two steps (sodium chlorite followed by sodium hydroxide) for lignin destruction, optimized hydrolysis using sulphuric acid by RSM, and finally fermented with S.cerevisiae TISTR 5048 (Klinpratoom et al. 2015). The pretreatment showed 91.44% of delignification and 78.18% of hemicellulose solubilized. RSM results revealed that 0.6737 g of reducing sugars was obtained per g of stem at optimum values of 0.2 M, 16.70 mL/g and 90 min for acid concentration, acid volume to substrate and time at 121 °C and 13.52 g/L of ethanol (84.41% of theoretical yield) during batch fermentation.
The temperature, time and solid concentration were optimized for thermohydrolysis pretreatment for bioethanol production from cassava stem and peel by RSM (Kouteu Nanssou et al. 2016). The optimal conditions were found to be 223.9 °C, 52.1 min and 10.7% (w/v) to produce 22.82 g/L of reducing sugars from cassava stem. The temperature of 224.1 °C, 50.3 min, and 11.48% (w/v) solids concentration were found to be optimum for reducing sugars (31.29 g/L) release from cassava peel. After thermohydrolysis, a part of the sample was subjected to direct fermentation with Rhizopus spp. and another part of the sample was hydrolyzed with accellerase 1500 enzyme followed by fermentation with S. cerevisiae. The highest bioethanol concentration of 6.15 g/L was achieved for stem by direct fermentation with Rhizopus spp. The feasibility of ethanol production was studied from the three varieties of cassava stem based on three locations in China at five different harvesting times (Wei et al. 2015). The rich content of starch and soluble sugars in stem made it suitable for bioethanol production. The effect of popping pretreatment was investigated on bioethanol production from mixed feedstocks (coffee husk + cassava stem, coffee husk + coconut coir, coffee husk + coconut coir and coffee husk + cassava stem + coconut coir) (Nguyen et al. 2017). The SHF and SSF were also compared for bioethanol fermentation. The highest fermentable sugar of 53.66 g/L was achieved for pretreated cassava stem + coconut coir mixture. The maximum ethanol concentration of 11.1 mg/mL was obtained from SSF of pretreated cassava stem + coconut coir mixture.
5.2 Bio-oil and biogas production from cassava stem
The biochar yield was investigated from cassava stem and rhizome through slow pyrolysis at different temperatures and heating rates (Noor et al. 2012). The maximum biochar yield of 35.85% (w/w) on a dry basis was achieved for cassava stem at 400 °C and 5 °C/min. Pattiya and co-workers have studied the fast pyrolysis of cassava stalk in comparison with a rhizome (Pattiya 2011b; Pattiya et al. 2012; Pattiya and Suttibak 2012). The viability of utilizing sugar-free cassava stem (SFCS) was attempted for biogas production (Zhu et al. 2015). The biogas production of 153.3 Nm3 was achieved per mg of dry mass SFCS. The cassava stem powder was employed as an additive for fuel pellet production from the mixture spruce and pine (Larsson et al. 2015). The edible potato starch and inedible cassava stem powder were utilized as additives and both showed low emissions of dust and CO.
6 Cassava rhizome
Cassava rhizome is an agricultural residue obtained from fields after harvest. The rhizome has no applications except firewood that leads to environmental concerns. Thus, rhizome can be utilized as a solid residue for the production of biofuels because of its lignocellulosic nature.
6.1 Bio-oil production from cassava rhizome
Cassava rhizome was employed for catalytic fast pyrolysis at 500 °C using pyrolysis coupled with GC–MS (Py–GC–MS) technique with zeolites (Pattiya et al. 2007, 2008, 2010), metal oxide (Pattiya et al. 2007, 2010), natural (Pattiya et al. 2007, 2010), alumino-silicate mesoporous (Pattiya et al. 2008) and proprietary commercial (Pattiya et al. 2007, 2008, 2010). The pyrolysis products were analyzed by the principal component analysis (PCA) from chromatographic peak areas. The results showed that the zeolite, commercial and few metal oxide catalysts were found to reduce oxygenated lignin derivatives thereby improving the viscosity of bio-oil. Also, it enhances the formation of aromatic hydrocarbons and phenols, lower carboxylic acids. The kinetics of pyrolysis of lignin (alcell, Asian, etek, organosolv, Klason from cassava rhizome, beech, cassava stalk, mixed softwood, and willow) was investigated using thermogravimetric analysis (TGA) (Jiang et al. 2010). The activation energy, reaction order and frequency factor were calculated and found that activation energy depends on biomass species and lignin separation method, reaction order slightly depend on biomass species and strongly on lignin separation method, and frequency factor is independent on both. The agricultural residues from plantations of Thailand were characterized to design and apply thermochemical conversion processes for bioenergy production (Pattiya 2011a). Also, the properties and yield of pyrolysis products from cassava stalk and rhizome were investigated by conducting pyrolysis experiments in fluidized bed reactor optimizing pyrolysis temperature (Pattiya 2011b). The results have revealed that 62 and 65% (w/w) yield of bio-oil were obtained for stalk and rhizome respectively on a dry basis at optimum pyrolysis temperature from 475 to 510 °C. The bio-oil from rhizome showed the improved quality than stalk in terms of HHV, lower oxygen content and better storage stability.
The effect of sugarcane and cassava residues, pyrolysis temperature, condensation temperature, nitrogen flow rate and time was studied on pyrolysis product distribution in a laboratory scale-free fall reactor (Pattiya et al. 2012). The results have revealed that the cassava stalk produced a bio-oil yield of 70% (w/w) at the pyrolysis temperature of 70 °C, condensation temperature of 10 °C, and nitrogen flow rate of 1.5 L/min. The cassava stalk and rhizome were compared for fast pyrolysis in a fluidized bed reactor combined with hot vapour filter (Pattiya and Suttibak 2012). The results showed that rhizome produced the highest bio-oil yield of 69.1% (w/w) at the pyrolysis temperature of 475 °C for particle size ranging from 250 to 425 μm. The incorporation of the filter has reduced the yield by 6–7% (w/w) and improved the bio-oil quality. The effect of temperature on fast pyrolysis of cassava rhizome was reported in a fluidized bed reactor combined with hot vapour filter (Suttibak et al. 2012). The bio-oil yielded the maximum of 63.23% (w/w) at 472 °C with moisture, total solids, ash, density, pH and HHV of 18% (w/w), 0.8% (w/w), < 0.01% (w/w), 1.1 g/mL, 3.1 and 26.9 MJ/kg respectively. The economic feasibility of biofuel was studied in Thailand by utilizing different agricultural residues including cassava rhizome (Thanarak 2012). The feasibility studies included the calculation of the cost of raw fuel, collection and processing cost, transportation costs, electricity prices, prices of agricultural products, the price level of agricultural waste, fuel prices, employment and business on producing bioenergy. The effect of pyrolysis temperature, particle size, nitrogen flow rate and pressure, and sand as the heat transfer medium was examined on pyrolysis of cassava rhizome in counter-rotating twin screw reactor (Sirijanusorn et al. 2013). The bio-oil yield of 50% (w/w) was achieved at the pyrolysis temperature of 550 °C, the particle size of 0.250–0.425 mm, nitrogen flow rate and pressure of 4 L/min and 2 bar respectively and the addition of heat transfer medium has increased the bio-oil yield.
6.2 Bioethanol production from cassava rhizome
The utility of computer-aided process engineering/process systems engineering (CAPE/PSE) tools were employed for the bioethanol production from cassava rhizome (Mangnimit et al. 2013). PRO/II simulator, SustainPro, SimaPro and ECON tools were used for process simulation, sustainability analysis, life cycle assessment and economic analysis respectively. A sustainable process design with wastewater recovery by membranes and combustion of lignin with heat integration was shown to be effective for the production of bioethanol from rhizome because of eco-friendliness, conservation of water and energy, and the highest profit. The sustainable process design was investigated for the production of bioethanol from cassava rhizome (Mangnimit et al. 2013). The final design includes 39 unit operations with ethanol production capacity of 150,000 L/day. The simulation on bioethanol production from cassava rhizome was performed for sustainable process design using LCSoft, a life cycle assessment (LCA) software, SustainPro, a process sustainability analysis tool and ECON, an economic analysis tool (Kalakul et al. 2014).
6.3 Synthetic diesel and briquettes production from cassava rhizome
The feasibility of utilizing available biomass in Thailand was theoretically discussed to produce synthetic diesel through the integration of biomass gasification and the Fischer–Tropsch synthesis (BG–FT) and the catalytic depolymerization processes (CDP) (Laohalidanond et al. 2006). A total of eleven biomass species were selected for the study and cassava rhizome was one among them. Based on the product yield and process economics, CDP was found to be effective and produced synthetic fuel from rhizome with higher heating value (HHV) and product yield was found to be 7451 kJ/kg and 0.14 L/kg respectively. The cassava rhizome was utilized to produce fuel briquettes with binders at different ratios (Sen et al. 2016). The rhizome bound with molasses, starch gel and concentrated slop in the ratios of 6:4 and 7:3 yielded briquettes with density, compressive strength, impact resistance index, and calorific value in the range of 0.69–0.91 g/cc, 8.51–14.94 kg/cm2, 153.7–416.7 and 21,670–24,367 kJ/kg respectively.
7 Cassava leaves
Cassava leaves are agricultural residues obtained during plant growth and after harvest. Generally, tubers are harvested from 8 to 24 months. The pruning is the process of removal of unwanted leaves that can be done from 4th month onwards to enhance the yield of tubers. From young to mature leaves, the crude protein, and carbohydrate contents started to decrease whilst the lipid, crude fibre, ash, and lignin contents increase. The most common use of leaves is animal feed because of its cyanide toxicity. After the removal of toxic materials, leaves can be used for biofuels production. However, leaves are not equally potential substrate like peel, bagasse, stem, and rhizome for biofuels production because of low carbohydrate and high protein content. The production of bioethanol was investigated from cassava leaves and pulp through hydrolysis by α-amylase from barley, followed by fermentation with S. cerevisiae (Anbuselvi and Balamurugan 2013). The highest ethanol concentration of 5.89% (v/v) was achieved from leaves.
8 Cassava bagasse
Cassava bagasse is an industrial residue obtained during the processing of cassava tuber to starch. During the screening process, the fibrous part of cassava is separated from starch and the leftover is called bagasse. The cassava bagasse was mentioned in literature by different names like cassava fibrous residue or cassava fibrous waste or cassava grate waste or cassava waste or cassava pulp. The bagasse is the most widely used residue for the production of value-added bioproducts. Since the biotechnological potential of cassava bagasse was sufficiently reviewed, there is no need to discuss on biofuels production from bagasse (Pandey and Soccol 2000; Pandey et al. 2000; Pandey 2003, 2004; Wang and Yang 2007; Nigam and Pandey 2009; Singhania et al. 2009; Mussatto and Teixeira 2010; Edama et al. 2014; Zhang et al. 2016; Li et al. 2017).
9 Conclusion
Basically, residues generated from the cassava fields after harvesting and from cassava processing industries were proved as a potential feedstock for the production of biofuels. The biofuels technology, a sustainable alternative to petrochemical engineering, provides environmental, economic and sustainable merits, with advancements in industrial biotechnology offers employment. The cassava peel has been utilized to produce biochar, biobutanol, bioethanol, biogas, and syngas with good yield at the laboratory scale. The cassava stem has been utilized for fuel pellets, briquettes, biochar, bioethanol, and bio-oil production. The cassava rhizome has been utilized for the production of fuel pellets, briquettes, biochar, and bio-oil. The cassava leaves are recalcitrant residue for biofuels production. Finally, cassava bagasse has been utilized to produce biochar, bioethanol, biobutanol, biogas, and syngas. The scale-up of processes at the industrial level is the crucial step in the commercialization of biofuels. The production of biofuels supports the demand of petrofuels to effect the maximum utilization of cassava residues.
References
Abidin Z, Saraswati E, Naid T (2014) Bioethanol production from waste of the cassava peel (Manihot esculenta) by acid hydrolysis and fermentation process. Int J PharmTech Res 6:1209–1212
Adelekan BA, Bamgboye AI (2009) Comparison of biogas productivity of cassava peels mixed in selected ratios with major livestock waste types. Afr J Agric Res 4:571–577
Adesanya OA, Oluyemi KA, Josiah SJ et al (2008) Ethanol production by Saccharomyces cerevisiae from cassava peel hydrolysate. Internet J Microbiol 5:25–35
Adetunji OR, Youdeowei PK, Kolawole OO (2015) Production of bioethanol from cassava peel. In: Proceedings from international conference on renewable energy and power held at Atlanta, Georgia
Adeyanju AA (2008) Effect of seeding of wood-ash on biogas production using pig waste and cassava peels. J Eng Appl Sci 3:242–245
Adiotomre KO (2015) Production of bioethanol as an alternative source of fuel using cassava and yam peels as raw materials. Int J Innov Res Sci Eng Technol 3:28–44
Akponah E, Akpomie OO (2011) Analysis of the suitability of yam, potato and cassava root peels for bioethanol production using Saccharomyces cerevisiae. Int Res J Microbiol 2:393–398
Alves AAC (2002) Cassava botany and physiology. Cassava Biol Prod Util. https://doi.org/10.1079/9780851995243.0067
Anbuselvi S, Balamurugan T (2013) Study on ethanol production from cassava leaves and pulp using S. cerevisiae. Res J Pharm Biol Chem Sci 4:1755–1761
Asikong BE, Epoke J, Eja EM, Antai EE (2012) Potentials of biogas generation by combination of cassava peels (CP) and poultry droppings (PD) in cross river state—Nigeria. Niger J Microbiol 26:2543–2552
Banerjee S, Mudliar S, Sen R et al (2010) Commercializing lignocellulosic bioethanol: technology bottlenecks and possible remedies. Biofuels Bioprod Biorefining 4:77–93. https://doi.org/10.1002/bbb
Bansal P (2005) Evolving sustainably: a longitudinal study of corporate sustainable development. Strateg Manag J 26:197–218. https://doi.org/10.1002/smj.441
Berndes G, Hoogwijk M, Van Den Broek R (2003) The contribution of biomass in the future global energy supply: a review of 17 studies. Biomass Bioenergy 25:1–28. https://doi.org/10.1016/S0961-9534(02)00185-X
Bhardwaj AK, Zenone T, Chen J (eds) (2015) Sustainable biofuels an ecological assessment of future energy. Walter Gruyter GmbH Co KG, Berlin
Casson A, Muliastra YIKD, Obidzinski K (2014) Large-scale plantations, bioenergy developments and land use change in Indonesia. CIFOR Working Paper No. 170. CIFOR 170. https://doi.org/10.17528/cifor/005434
Castaño Peláez H, Reales Alfaro J, Zapata Montoya J (2013) Simultaneous saccharification and fermentation of cassava stems. Dyna 80:97–104
Chibuzor O, Uyoh EA, Igile G (2016) Bioethanol production from cassava peels using different microbial inoculants. Afr J Biotechnol 15:1608–1612. https://doi.org/10.5897/AJB2016.15391
Cock JH (1982) Cassava: a basic energy source in the tropics. Science 218:755–762. https://doi.org/10.1126/science.7134971
Cuzin N, Labat M (1992) Reduction of cyanide levels during anaerobic digestion of cassava. Int J Food Sci Technol 27:329–336. https://doi.org/10.1111/j.1365-2621.1992.tb02034.x
Cuzin N, Farinet JL, Segretain C, Labat M (1992) Methanogenic fermentation of cassava peel using a pilot plug flow digester. Bioresour Technol 41:259–264. https://doi.org/10.1016/0960-8524(92)90011-L
Cuzin N, Ouattara AS, Labat M, Garcia JL (2001) Methanobacterium congolense sp. nov., from a methanogenic fermentation of cassava peel. Int J Syst Evol Microbiol 51:489–493. https://doi.org/10.1099/00207713-51-2-489
Demirbas A (2005) Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues. Prog Energy Combust Sci 31:171–192. https://doi.org/10.1016/j.pecs.2005.02.002
Dineshkumar R, Dash SK, Sen R (2015) Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv 5:73381–73394. https://doi.org/10.1039/C5RA09306F
Djuma’ali DA, Sumarno SN et al (2011) Cassava pulp as a biofuel feedstock of an enzymatic hydrolysis process. Makara Tecknologi 15:183–192. https://doi.org/10.7454/mst.v15i2.938
Edama NA, Sulaiman A, Abd.Rahim NS (2014) Enzymatic saccharification of Tapioca processing wastes into biosugars through immobilization technology. Biofuel Res J 1:2–6. https://doi.org/10.18331/BRJ2015.1.1.3
El-Sharkawy MA (2003) Cassava biology and physiology. Plant Mol Biol 53:621–641. https://doi.org/10.1007/s11103-005-2270-7
Ezebuiro V, Ogugbue CJ, Oruwari B, Ire FS (2015) Bioethanol production by an ethanol-tolerant Bacillus cereus strain GBPS9 using sugarcane bagasse and cassava peels as feedstocks. J Biotechnol Biomater. https://doi.org/10.4172/2155-952X.1000213
Ezekoye VA, Ezekoye BA (2009) Characterization and storage of biogas produced from the anaerobic digestion of cow dung, spent grains/cow dung, and cassava peels/rice husk. Pacific J Sci Technol 10:898–904
Ezekoye VA, Ezekoye BA, Offor PO (2011) Effect of retention time on biogas production from poultry droppings and cassava peels. Niger J Biotechnol 22:53–59
FAO (1999) FAO. www.apps.fao.org/lim500/nph-wrap.pl?FS.CropsAndProducts&Domain=FS&servlet=1%3E. Accessed 5 May 2014
FAO (2018) FAO. https://web.archive.org/web/20110713020710/ http://faostat.fao.org/site/339/default.aspx. Accessed 13 Mar 2018
Goldemberg J (2007) Ethanol for a sustainable energy future. Science 315:808–810. https://doi.org/10.1126/science.1137013
Han M, Kim Y, Kim Y et al (2011) Bioethanol production from optimized pretreatment of cassava stem. Korean J Chem Eng 28:119–125. https://doi.org/10.1007/s11814-010-0330-4
Hermiati E, Azuma J, Mangunwidjaja D et al (2011) Hydrolysis of carbohydrates in cassava pulp and tapioca flour under microwave irradiation. Indones J Chem 11:238–245
Jekayinfa SO, Scholz V (2013) Laboratory scale preparation of biogas from cassava tubers, cassava peels, and palm kernel oil residues. Energy Sources Part A Recover Util Environ Eff 35:2022–2032. https://doi.org/10.1080/15567036.2010.532190
Jiang G, Nowakowski DJ, Bridgwater AV (2010) A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta 498:61–66. https://doi.org/10.1016/j.tca.2009.10.003
Jobling S (2004) Improving starch for food and industrial applications. Curr Opin Plant Biol 7:210–218. https://doi.org/10.1016/j.pbi.2003.12.001
Johnson R, Padmaja G (2011) Utilization of cassava fibrous residue for the production of glucose and high fructose syrup. Ind Biotechnol 7:448–455. https://doi.org/10.1089/ind.2011.0015
Kalakul S, Malakul P, Siemanond K, Gani R (2014) Integration of life cycle assessment software with tools for economic and sustainability analyses and process simulation for sustainable process design. J Clean Prod 71:98–109. https://doi.org/10.1016/j.jclepro.2014.01.022
Ki OL, Kurniawan A, Lin CX et al (2013) Bio-oil from cassava peel: a potential renewable energy source. Bioresour Technol 145:157–161. https://doi.org/10.1016/j.biortech.2013.01.122
Klinpratoom B, Ontanee A, Ruangviriyachai C (2015) Improvement of cassava stem hydrolysis by two-stage chemical pretreatment for high yield cellulosic ethanol production. Korean J Chem Eng 32:413–423. https://doi.org/10.1007/s11814-014-0235-8
Kongkiattikajorn J (2013) Production of glucoamylase from Saccharomycopsis fibuligera sp. and hydrolysis of cassava peels for alcohol production. Int J Comput Internet Manag 21:1–7
Kongkiattikajorn J, Sornvoraweat B (2011) Comparative study of bioethanol production from cassava peels by monoculture and co-culture of yeast. Kasetsart J Nat Sci 45:268–274
Kouteu Nanssou PA, Jiokap Nono Y, Kapseu C (2016) Pretreatment of cassava stems and peelings by thermohydrolysis to enhance hydrolysis yield of cellulose in bioethanol production process. Renew Energy 97:252–265. https://doi.org/10.1016/j.renene.2016.05.050
Laohalidanond K, Heil J, Wirtgen C (2006) The production of synthetic diesel from biomass. KMITL Sci Technol 6:35–45
Larsson S, Lockneus O, Xiong S, Samuelsson R (2015) Cassava stem powder as an additive in biomass fuel pellet production. Energy Fuels 29:5902–5908. https://doi.org/10.1021/acs.energyfuels.5b01418
Li S, Cui Y, Zhou Y et al (2017) The industrial applications of cassava: current status, opportunities and prospects. J Sci Food Agric. https://doi.org/10.1002/jsfa.8287
Lund H (2007) Renewable energy strategies for sustainable development. Energy 32:912–919. https://doi.org/10.1016/j.energy.2006.10.017
Magesh A, Preetha B, Viruthagiri T (2011a) Simultaneous Saccharification and fermentation of tapioca stem var. 226 white rose to ethanol by cellulase enzyme and Saccharomyces cerevisiae. Int J ChemTech Res 3:1821–1829
Magesh A, Preetha B, Viruthagiri T (2011b) Statistical optimization of process variables for direct fermentation of 226 white rose tapioca stem to ethanol by Fusarium oxysporum. Int J Chem Mol Nucl Mater Metall Eng 5:226–231
Mangnimit S, Malakul P, Gani R (2013) Sustainable process design of biofuels: bioethanol production from cassava rhizome. In: Proceedings of the 6th international conference on process systems engineering (PSE ASIA) vol 25, pp 1–6
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Moshi AP, Temu SG, Nges IA et al (2015) Combined production of bioethanol and biogas from peels of wild cassava Manihot glaziovii. Chem Eng J 279:297–306. https://doi.org/10.1016/j.cej.2015.05.006
Mussatto SI, Teixeira JA (2010) Lignocellulose as raw material in fermentation processes. Curr Res Technol Educ Top Appl Microbiol Microb Biotechnol (Méndez-Vilas, A, Ed) 2:897–907. https://doi.org/10.1016/j.jrras.2014.02.003
Nassar NMA (2007) Wild and indigenous cassava, Manihot esculenta Crantz diversity: an untapped genetic resource. Genet Resour Crop Evol 54:1523–1530. https://doi.org/10.1007/s10722-006-9144-y
Nguyen QA, Yang J, Bae HJ (2017) Bioethanol production from individual and mixed agricultural biomass residues. Ind Crops Prod 95:718–725. https://doi.org/10.1016/j.indcrop.2016.11.040
Nigam PSN, Pandey A (eds) (2009) Biotechnology for agro-industrial residues utilisation: utilisation of agro-residues. Springer Science & Business Media
Nkodi TM, Taba KM, Kayembe S et al (2016) Biogas production by co-digestion of cassava peels with urea. Int J Sci Eng Technol 55:139–141
Nnabuchi MN, Ukpai PA (2012) Comparative study of biogas production from cow dung, cow pea and cassava peeling using 45 l biogas digester. Prime Res Med 2:89–93
Noor NM, Shariff A, Abdullah N (2012) Slow pyrolysis of cassava wastes for biochar production and characterization. Iran J Energy Environ 3:60–65. https://doi.org/10.5829/idosi.ijee.2012.03.05.10
Nurse K (2006) Culture as the fourth pillar of sustainable development. Small States Econ Rev Basic Stat 11:28–40. https://doi.org/10.1177/026327690007002004
Nuwamanya E, Chiwona-karltun L, Kawuki RS, Baguma Y (2012) Bio-ethanol production from non-food parts of CASSAVA (Manihot esculenta Crantz). Ambio 41:262–270. https://doi.org/10.1007/s13280-011-0183-z
Ofoefule AU, Uzodinma EO (2009) Biogas production from blends of cassava (Manihot utilissima) peels with some animal wastes. Int J Phys Sci 4(1):Ofoef:398–Ofoef:402
Okareh OT, Adeolu AT, Shittu OI (2012) Enrichment of pig dung with selected crop wastes for the production of biogas. Int J Microbiol 3:258–263
Okekunle PO, Itabiyi OE, Adetola SO et al (2016) Biofuel production by pyrolysis of cassava peel in a fixed bed reactor. Int J Energy Clean Environ 17:57–65
Oparaku NF, Ofomatah AC, Okoroigwe EC (2013) Biodigestion of cassava peels blended with pig dung for methane generation. Afr J Biotechnol 12:5956–5961. https://doi.org/10.5897/AJB2013.12938
Orhorhoro OW, Orhorhoro EK, Ebunilo PO (2016) Analysis of the effect of carbon/nitrogen (C/N) ratio on the performance of biogas yields for non-uniform multiple feed stock availability and composition in Nigeria. Int J Innov Sci Eng Technol 3:119–126
Oyeleke SB, Dauda BEN, Oyewole OA, Okoliegbe IN, Ojebode T (2012) Production of bioethanol from cassava and sweet potato peels. Adv Environ Biol 6:241–245
Pandey A (2003) Solid-state fermentation. Biochem Eng J 13:81–84. https://doi.org/10.1016/S1369-703X(02)00121-3
Pandey A (2004) Concise encyclopedia of bioresource technology (No. C/620.803 C6). New York: Food Products Press
Pandey A, Soccol CR (2000) Economic utilization of crop residues for value addition: a futuristic approach. J Sci Ind Res 59:12–22
Pandey A, Soccol CR, Nigam P, Soccol VT (2000) Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresour Technol 74:69–80. https://doi.org/10.1016/S0960-8524(99)00142-X
Pandian CA, Suganya C, Sivamani S, Baskar R (2016) Saccharification and single step fermentation of cassava peel by mixed culture of Saccharomycopsis fibuligera NCIM 3161 and Zymomonas mobilis MTCC 92. Am J Biomass Bioenergy 5:57–64. https://doi.org/10.7726/ajbb.2016.1005
Patle S, Lal B (2008) Investigation of the potential of agro-industrial material as low-cost substrate for ethanol production by using Candida tropicalis and Zymomonas mobilis. Biomass Bioenergy 32:596–602. https://doi.org/10.1016/j.biombioe.2007.12.008
Pattiya A (2011a) Thermochemical characterization of agricultural wastes from thai cassava plantations. Energy Sources Part A Recover Util Environ Eff 33:691–701. https://doi.org/10.1080/15567030903228922
Pattiya A (2011b) Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidised-bed reactor. Bioresour Technol 102:1959–1967. https://doi.org/10.1016/j.biortech.2010.08.117
Pattiya A, Suttibak S (2012) Production of bio-oil via fast pyrolysis of agricultural residues from cassava plantations in a fluidised-bed reactor with a hot vapour filtration unit. J Anal Appl Pyrolysis 95:227–235. https://doi.org/10.1016/j.jaap.2012.02.010
Pattiya A, Titiloye JO, Bridgwater AV (2007) Catalytic fast pyrolysis of cassava rhizome in a micro-reactor. Asian J Energy Environ 8:211–228
Pattiya A, Titiloye JO, Bridgwater AV (2008) Fast pyrolysis of cassava rhizome in the presence of catalysts. J Anal Appl Pyrolysis 81:72–79. https://doi.org/10.1016/j.jaap.2007.09.002
Pattiya A, Titiloye JO, Bridgwater AV (2010) Evaluation of catalytic pyrolysis of cassava rhizome by principal component analysis. Fuel 89:244–253. https://doi.org/10.1016/j.fuel.2009.07.003
Pattiya A, Sukkasi S, Goodwin V (2012) Fast pyrolysis of sugarcane and cassava residues in a free-fall reactor. Energy 44:1067–1077. https://doi.org/10.1016/j.energy.2012.04.035
Raman N, Pothiraj C (2008) Screening of Zymomonas mobilis and Saccharomyces cerevisiae strains for ethanol production from cassava waste. Rasayan J Chem 1:537–541
Rattanachomsri U, Tanapongpipat S, Eurwilaichitr L, Champreda V (2009) Simultaneous non-thermal saccharification of cassava pulp by multi-enzyme activity and ethanol fermentation by Candida tropicalis. J Biosci Bioeng 107:488–493. https://doi.org/10.1016/j.jbiosc.2008.12.024
Ravindran V (1993) Cassava leaves as animal feed: potential and limitations. J Sci Food Agric 61:141–150. https://doi.org/10.1002/jsfa.2740610202
Ray RC, Mohapatra S, Panda S, Kar S (2008) Solid substrate fermentation of cassava fibrous residue for production of α-amylase, lactic acid and ethanol. J Environ Biol 29:111–115. https://doi.org/10.1016/0167-7799(85)90092-7
Rymowicz W, Kopec W, Stevens C (2004) Primary production of raw materials. In: Stevens C, Verhé R (eds) Renewable bioresources: scope and modification for non-food applications. Wiley, Chichester, UK
Sanette M, Tando YN (2013) Cassava as feedstock for ethanol production in South Africa. Afr J Biotechnol 12:4975–4983. https://doi.org/10.5897/AJB12.861
Sen R, Wiwatpanyaporn S, Annachhatre AP (2016) Influence of binders on physical properties of fuel briquettes produced from cassava rhizome waste. Int J Environ Waste Manag 17:158–175
Shafiee S, Topal E (2009) When will fossil fuel reserves be diminished? Energy Policy 37:181–189. https://doi.org/10.1016/j.enpol.2008.08.016
Singhania RR, Patel AK, Soccol CR, Pandey A (2009) Recent advances in solid-state fermentation. Biochem Eng J 44:13–18. https://doi.org/10.1016/j.bej.2008.10.019
Sirijanusorn S, Sriprateep K, Pattiya A (2013) Pyrolysis of cassava rhizome in a counter-rotating twin screw reactor unit. Bioresour Technol 139:343–348. https://doi.org/10.1016/j.biortech.2013.04.024
Sivamani S, Baskar R (2015) Optimization of bioethanol production from cassava peel using statistical experimental design. Environ Prog Sustain Energy 34:567–574. https://doi.org/10.1002/ep.11984
Sivamani S, Shanmugam A, Baskar R (2015) Optimization of ethanol production from mixed feedstock of cassava peel and cassava waste by coculture of Saccharomycopsis fibuligera NCIM 3161 and Zymomonas mobilis MTCC 92. Chem Bioprocess Eng. https://doi.org/10.1201/b18402-4
Sovorawet B, Kongkiattikajorn J (2012) Bioproduction of ethanol in SHF and SSF from cassava stalks. Asia-Pacific J Sci Technol 17:565–572
Srinorakutara T, Suesat C, Pitiyont B et al (2004) Utilization of waste from cassava starch plant for ethanol production. In: Proceedings of the joint international conference on sustainable energy and environment (SEE), pp 344–349
Sriroth K (2001) Outlook of biomass utilization as biofuel in Thailand. http://www.biomass-asia-workshop.jp/biomassws/01workshop/material/Klanarong%81@Sriroth.pdf. Accessed 13 Mar 2015
Suttibak S, Sriprateep K, Pattiya A (2012) Production of bio-oil via fast pyrolysis of cassava rhizome in a fluidised-bed reactor. Energy Proc 14:668–673. https://doi.org/10.1016/j.egypro.2011.12.993
Thanarak P (2012) Supply chain management of agricultural waste for biomass utilization and CO2 emission reduction in the lower northern region of Thailand. Energy Proc 14:843–848. https://doi.org/10.1016/j.egypro.2011.12.887
Thongchul N, Navankasattusas S, Yang ST (2010) Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst Eng 33:407–416. https://doi.org/10.1007/s00449-009-0341-x
Ubalua AO (2007) Cassava wastes: treatment options and value addition alternatives. Afr J Biotechnol 6:2065–2073. https://doi.org/10.5897/AJB2007.000-2319
Uchechukwu-Agua AD, Caleb OJ, Opara UL (2015) Postharvest handling and storage of fresh cassava root and products: a review. Food Bioprocess Tech 8:729–748
Ukpai PA, Nnabuchi MN (2012) Comparative study of biogas production from cow dung, cow pea and cassava peeling using 45 l biogas digester. Adv Appl Sci Res 3:1864–1869
Ukpai PA, Agbo PE, Nnabuchi MN (2015) The effect of temperature on the rate of digestion and biogas production using cow dung, cow pea, cassava pending. Int J Sci Eng Res 6:1255–1261
Wang L, Yang S-T (2007) Solid state fermentation and its applications. Bioprocess Value Added Prod Renew Resour. https://doi.org/10.1016/B978-044452114-9/50019-0
Wei M, Zhu W, Xie G et al (2015) Cassava stem wastes as potential feedstock for fuel ethanol production: a basic parameter study. Renew Energy 83:970–978. https://doi.org/10.1016/j.renene.2015.05.054
Wilkinson J, Rocha R (2008) The agro-processing sector: empirical overview, recent trends and development impact. Plenary paper for global industries forum, New Delhi, India
Woiciechowski AL, Saul N, Pandey A, Soccol CR (2002) Acid and enzymatic hydrolysis to recover reducing sugars from cassava bagasse: an economic study. Braz Arch Biol Technol 45:393–400
Wongskeo P, Rangsunvigit P, Chavadej S (2012) Production of glucose from the hydrolysis of cassava residue using bacteria isolates from thai higher termites. World Acad Sci Eng Technol 64:353–356
Yoonan K, Yowapui P, Kongkiattikajorn J (2007) Ethanol production from acid hydrolysate of cassava peels using Saccharomyces cerevisiae. KMUTT Res Dev J 30:405–418
Zhang M, Xie L, Yin Z et al (2016) Biorefinery approach for cassava-based industrial wastes: current status and opportunities. Bioresour Technol 215:50–62. https://doi.org/10.1016/j.biortech.2016.04.026
Zhu W, Lestander TA, Örberg H et al (2015) Cassava stems: a new resource to increase food and fuel production. GCB Bioenergy 7:72–83. https://doi.org/10.1111/gcbb.12112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivamani, S., Chandrasekaran, A.P., Balajii, M. et al. Evaluation of the potential of cassava-based residues for biofuels production. Rev Environ Sci Biotechnol 17, 553–570 (2018). https://doi.org/10.1007/s11157-018-9475-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-018-9475-0